Abstract
In this study, an advanced oxidation process using ozone application in a fixed-bed reactor filled with bio-rings was used for purification and disinfection of cattle wastewater (CWW) previously treated in a UASB reactor. The O3 concentration applied to the reactor was 7.8 mg L−1 (±1). Four ozonation times were tested in a batch process: T1 = 0.5 h, T2 = 1 h, T3 = 1.5 h and T4 = 2 h. The pH values tended to increase with the time of contact between liquid and gas. COD and BOD5 removal of 55 and 64% was recorded in T4. Color and turbidity were reduced by 88.5 and 93.4%, while total solids and total suspended solids were reduced by 65.7 and 93.5%, respectively. Oils and greases were completely removed from the wastewater. Electrical conductivity did not vary significantly between treatments, and its association with the presence of cations, such as Na+, Ca2+ and Mg2+, suggests good quality of the water ozonated in T4 for application in the soil, with no risk of salinization. High concentrations of nitrogen compounds were removed, but the efficiency in removing phosphate compounds was low. Coliform removal of above 99% was achieved with T3. Only the CWW ozonated in T4 is recommended for irrigation of raw-consumed crops. Based on most parameters analyzed, the CWW treated in T4 would also be suitable for discharge into water bodies according to Brazilian legislation, suggesting that this technology could be applied in the near future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The cattle breeding in Brazil has grown steadily, and the country has become self-sufficient in milk production. This has resulted in the need for an expansion of intensive livestock farming (Vale et al. 2019).
The amount of waste produced every day by livestock farming is one of the largest environmental concerns in intensive management systems. For instance, one unit with 1000 animals in confinement can generate an average organic load of 750 kg of biochemical oxygen demand (BOD5) day−1, which has a polluting potential equivalent to that of 13,890 people (Mendonça et al. 2016; Mendonça et al. 2017).
Cattle wastewater (CWW) is composed mainly of water from the washing of confinement facilities containing feces and urine, which can mix with surface water and cause undesirable environmental effects including depletion of dissolved oxygen (DO), increase in real/apparent color and turbidity, nutrient enrichment and foul odors (Silva et al. 2012; Mendonça et al. 2018).
In addition, the disposal of untreated CWW into water bodies can cause an increase in the concentration of coliforms, affecting the quality of the water for consumption and irrigation. Thus, the need for treating this type of wastewater before its discharge into rivers and lakes is urgent.
In Brazil, the discharge of treated CWW into water bodies must follow the requirements of the Brazilian National Environmental Council (CONAMA) resolutions 357/05 and 430/11 (Brasil 2005, 2011), which establish conditions and standards for the final disposal of effluents.
In recent decades, new technologies for effluent treatment have been developed and optimized by researchers. One example is advanced oxidation processes (AOP), which include treatment by ozone (O3) application (Araújo et al. 2016).
The generation of O3 for application in effluents is generally performed by passing air between two electrodes that are subjected to a high potential difference, which causes an electrochemical discharge that converts atmospheric O2 into O3 (Almeida et al. 2004).
This type of treatment has been proven to be effective, because O3 is able to react with several organic and inorganic compounds due to its high oxidation potential (E0 ≈ 2.1 V), which is greater than that of Cl2 (E0 ≈ 1.4 V) and KMnO4 (E0 ≈ 1.7 V). Another advantage of O3 application is that the product generated in its degradation is oxygen (O2), a non-polluting element (Mahmoud & Freire 2007).
When dissolved in wastewater, O3 can react with organic compounds through direct reaction (molecular ozone) or indirect reaction by the formation of secondary oxidants such as hydroxyl radicals (Subha and Muthukumar 2012), and may be more effective in the treatment of certain recalcitrant and low-biodegradability compounds (e.g., cellulose, hemicellulose and lignin) typically found in CWW, while simultaneously assisting in their disinfection.
According to Lee et al. (2008), the ozonation process associated with flotation is more cost-effective than other advanced treatments. It can satisfactorily meet the quality standards for treated wastewater in various sectors, and also holds promise as post-treatment for CWW in small cattle farms.
The ozonation process used as treatment or post-treatment has proven to be efficient for wastewater such as landfill leachate, effluents from the textile, paper and pharmaceutical industries, olive derivatives, and effluents containing pesticides/herbicides or antibiotics (Peña et al. 2003; Oller et al. 2011; Marcelino et al. 2017; Paździor et al. 2017). There are no reports in the literature on the use of O3 to treat CWW anaerobically pretreated in a UASB reactor.
Thus, the aim of this study was to evaluate the potential for the use of chemical treatment with O3 for organic matter oxidation by measuring the removal of nutrients, solids, oils and greases, coliform removal, and clarification of CWW after primary treatment in a UASB reactor. In addition, the results were compared in terms of quality for discharge into receiving water bodies based on the current Brazilian legislation, when pertinent, and also in terms of the quality of the treated water for irrigation purposes.
2 Materials and Methods
The study was conducted with CWW collected in the experimental unit “Fazendinha Agroecológica” of the Federal Rural University of Rio de Janeiro (UFRRJ), Seropédica campus, RJ, Brazil (coordinates: 22°45′21”S, 43°40′28”W). In this experimental field, cattle are reared in confinement and are fed only organic feed (without the use of agricultural pesticides) produced on the farm itself. In addition, there is no use of antibiotics or hormones and no external industrial sources of feed, bran or proteins in cattle diet.
Prior to O3 application on a bench scale, the CWW underwent preliminary treatment to remove coarse solids (decanter) and anaerobic biological treatment (UASB reactor) with hydraulic retention time (HRT) of 7 days.
The CWW characterization after treatment in the UASB reactor is presented in Table 1.
All analytical procedures were conducted in the laboratory according to APHA (2012).
An O3 generator (Ozone Generation, GL-3189A, China), with a flow rate of 1.3 L min−1, purity of 92% (±2) and average concentration of 7.8 mg L−1 (±1), was used to conduct the experiment. The ozone concentration applied was determined by the iodometric method through indirect titration (Clescerl et al. 2000). The liquid temperature during the experiments was 25 °C (±1). No procedure was performed to adjust the pH of the raw effluent for ozone application.
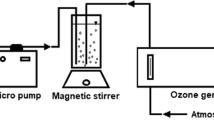
This phase of chemical treatment was carried out on a bench scale, using a fixed bed reactor (Fig. 1).
The reactor had a usable volume of 750 mL, filled with porous plastic media (bio-rings) to maximize gas–liquid transfer. At the bottom of the reactor, a diffuser (20-μm pore size) was connected to the ozone generator. Cotton was inserted in the reactor head in order to generate an internal atmosphere with higher ozone concentration, avoiding direct contact of atmospheric air with the gas/liquid surface. The cotton also served to absorb the floating particles mobilized by the drag force of the gas during the treatment process.
In the ozonation tests, CWW was exposed for different contact times (CT) for each batch, as follows: T1 – 30 min, T2 – 1 h, T3 – 1h 30min, and T4 – 2 h. Foam formation was controlled by adding 3 mL (5%) of silicone-based anti-foaming agent to the control volume (CV) immediately at the beginning of the O3 application. The experiments were carried out seven times (n = 7).
After each ozonation treatment, the treated CWW was analyzed based on different parameters including organic matter (BOD5 and COD), nitrogen compounds, total phosphorus, solids, oils and greases, coliform concentrations and clarification. The analyses were performed according to standard methods (APHA 2012).
The sodium adsorption ratio (SAR) was calculated using Eq. 1:
where Na+, Ca++ and Mg++ represent concentrations of sodium, calcium and magnesium, respectively, in millimoles per liter.
Significant differences between treatment means were checked using the Tukey test at a 95% confidence level. Before performing the parametric tests, the normality of the data was confirmed by the Shapiro–Wilk test using PAST software.
3 Results and Discussion
3.1 Hydrogen Potential (pH)
The average pH measured in the CWW after the UASB reactor was 7.2 (Table 1). As the ozone/wastewater CT gradually increased, the pH of the solution also increased, as shown in Fig. 2, although no significant differences between treatments were detected by the Tukey test (p ≥ 0.05).
The increase in pH with increasing ozonation time is justified by the oxidation (molecular/radical) of organic compounds present in the solution, where CO2 is produced. As O3 is inserted through the bottom of the reactor, CO2 stripping occurs, providing concentrations of HCO3− and CO3−2 in the solution (Lei and Li 2014).
The reason for the gradual increase in pH is that when O3 comes into contact with the effluent, hydroxide ions (OH−) are released and hence become dominant over the H+ ions. When the pH reaches 8.0, most of the ozone is decomposed into hydroxide ions, leading to a more alkaline medium (Lage Filho et al. 2008; de S. Coelho et al. 2015; Júnior et al. 2019), as observed with the longest CT applied in this study (T4). Similar behavior was reported by Bhata et al. (2015), who observed an increase in pH with increased effluent/ozone CT.
The pH ranges observed in the present study were between 5 and 9, as recommended by CONAMA resolution 430/2011 for effluent discharge into water bodies. With regard to the influence of pH on applications in the soil for irrigation purposes, notably there would be no problems of soil acidification, since the pH ranges in all treatments remained above neutrality.
3.2 Turbidity, Real Color, Solids, and Oils and Greases
A reduction in the turbidity of the treated CWW was observed, with efficiency of 32.3%, 66.9%, 84.6% and 88.5% in treatments T1, T2, T3 and T4, respectively. Turbidity values (NTU) are presented in Fig. 3a. A statistically significant difference was found between the means of all four treatments (p ≤ 0.05). The removal of turbidity from the CWW was effective, gradually increasing with the CT.
Cabrera-Díaz et al. (2016) observed higher turbidity removal, reaching 99.2%, in the treatment of vinasse previously digested by an anaerobic filter reactor. The authors used an O3 concentration of 100 mg L−1 with a CT of 3 h, which explains the greater efficiency than that obtained in the present study. However, the 88.5% turbidity removal observed in T4 (2 h) was achieved despite using a lower concentration (7.8 mg O3 L−1). This was possible because the reactor was filled with bio-rings, which promoted longer permanence/contact time of the gas with the CWW. As the gas rose, it was retained in the spaces of the bio-rings, promoting more efficient gas-to-liquid mass transfer.
Júnior et al. (2019) treated sanitary sewage with ozone (17 mg O3 L−1 for 2 h) and recorded a turbidity reduction of 81.5%, a value close to that in the present study. In this case, it should be highlighted that these authors used no filler (bio-rings or other type of media) in the reactor, which necessitated the adoption of higher O3 concentrations to achieve turbidity reductions greater than 80%.
The color removal efficiency in the present study was equal to 29.9% in T1, 67.2% in T2, 83.4% in T3 and 93.4% in T4. The behavior of color removal was similar to that of turbidity removal, with a gradual reduction in color as the gas/liquid CT increased (Fig. 3b).
Silva et al. (2004) recorded 87% efficiency in color removal after ozonation (3000 mg O3 L−1) when treating landfill leachate that had been previously coagulated and flocculated, with initial color of between 5300 and 6900 uH. Similar efficiency was found in the present study for treatments T3 and T4, but with the use of lower O3 concentration. This occurred for two reasons: the color concentration of the CWW was lower than that of the landfill effluents, which in turn had higher concentrations of recalcitrant compounds; thus lower O3 concentrations were needed for the treatment of CWW. Another factor that contributed to maximizing the color removal process was the filling of the reactor with bio-rings, which promoted a longer CT between ozone and CWW, since microbubbles were concentrated in the filler pores.
The total solids (TS) removal was equal to 27.5%, 53.3%, 65% and 65.7% in treatments T1, T2, T3 and T4, respectively. In addition, the removal of TS was gradual, reaching a value of 308 mg L−1 in T4 (Table 2).
It can be clearly observed that with T3, there was virtually no reduction in TS. According to Marcelino et al. (2017), ozone destroys the solid particles of organic matter, especially at the beginning of the reaction, which also explains the substantial reduction in turbidity. In the present study, it was also observed that particles were transported by the drag force of the O3 gas to the cotton placed on the reactor head. Ozone application provided two processes inside the reactor: (1) the separation of solids by microbubbles (flotation) and (2) oxidation of soluble organic compounds, intensifying the treatment effect. The same effect was reported by Wilinsyki and Jeremi (2012) in the treatment of sewage with O3. Therefore, the flotation process along with the adhesion of particles to the cotton filter maximized the removal of solids from the CWW.
Júnior et al. (2019) reported that although the concentrations of TS varied according to exposure time and O3 dose, in general there were no significant differences between treatments. The present study similarly found no statistically significant difference in the means between treatments T4 and T3 (p ≥ 0.05). These findings indicate that under the experimental conditions proposed here, there is no reduction of TS in the CWW with 1 h 30 min ozone exposure, indicating stabilization in the process of TS removal.
For TSS, substantial removal was achieved, ranging from 44.2% to 93.5% and reaching a value of 6 mg L−1 in T4 (Table 2). Significant differences were observed between the means of all treatments for TSS (p ≤ 0.05). The data obtained confirmed that ozonation was effective in removing TSS from the CWW under the experimental conditions established here, especially for treatments T3 and T4, which are those most recommended for plants at full scale.
With regard to the quality of the ozonated CWW for irrigation purposes, it was verified that with T2, the values of the remaining TS were very close to those found in clean surface waters commonly used for irrigation (540 mg L−1), according to Barreto et al. (2009). With regard to TSS concentration, to avoid the risk of dripper clogging, this parameter should be below 50 mg L−1 (Nakayama 1982); thus all treatments except T1 reached the recommended level. As the lowest concentration of TSS (6 mg L−1) was obtained in T4, this would be an optimal value for avoiding both dripper clogging and surface sealing of the soil.
It can therefore be concluded that in terms of the solids concentration, this treated CWW could be used in the irrigation of agricultural crops without causing clogging of sprinkler/drip nozzles or surface sealing of the soil.
As for the removal of oils and greases (O&G), complete removal was achieved with all treatments, with no levels detected in the ozonated CWW samples. This indicates that this material was easily oxidized, which also contributed to the reduction of the TS contained in the CWW. The high removal efficiency, even for the shorter CT adopted (T1 and T2), is also attributable to the low initial value of this parameter in the CWW, which was 18 mg L−1.
3.3 Chemical Oxygen Demand (COD) and Biochemical Oxygen Demand (BOD5)
The initial COD of the CWW (after anaerobic digestion, see Table 1) was equal to 1100 mg L−1, and after ozonation treatments T3 and T4, the levels were reductions to 612 and 450 mg L−1 (Fig. 4a), constituting COD reductions of 44 and 55%, respectively. Lower removal percentages were observed for treatments T1 and T2, on the order of 11% and 25%.
In a similar experiment, Hansson et al. (2015) treated effluents generated by the timber industry, also without pH adjustment of the raw wastewater, and recorded COD removal of 65%, a value closer to that of T4.
In the present study, significant COD removal was obtained, since the use of bio-ring media as filler in the reactor maximized the liquid–gas exchange, promoting oxidation, similar to the effects observed in color removal. BOD5 values also decreased throughout the treatments (Fig. 4b), with removal efficiency of 23%, 37%, 48% and 64% in treatments T1, T2, T3 and T4, respectively.
According to CONAMA resolutions 357/05 and 430/11, at least 60% BOD5 removal is necessary for the discharge of effluents into water bodies. Thus, only T4 succeeded in meeting this requirement.
Cabrera-Díaz et al. (2016) treated anaerobically digested vinasse with ozone at 70 mg L−1 and recorded BOD5 removal of only 11.1%. The higher BOD5 removal efficiency in the present study can be attributed to the 25-fold lower value of this parameter in the CWW than in the vinasse. Therefore, an O3 concentration of 7.8 mg L−1 may be considered sufficient for the treatment of CWW when treated in the reactor model proposed here with time T4. In the present study, both COD and BOD5 were satisfactorily removed, especially in T3 and T4, even without pH adjustment or the addition of additives such as H2O2 or UV radiation.
Based on the statistical test for means comparison, a significant difference between the means was found for both BOD5 and COD (p ≤ 0.05) (Fig. 4 a and b). This confirms that, in general, satisfactory efficiency was obtained for both parameters in the treatment of CWW with O3, especially for longer ozonation times (T3 and T4).
3.4 Electrical Conductivity (EC), Na+, Ca2+ and Mg2+
EC values showed little change after ozonation and between treatments. The average EC in the CWW was 1.49 dS m−1, and chemical treatment with ozone led to average values of 1.45 dS m−1 in T1, 1.56 dS m−1 in T2, 1.49 dS m−1 in T3 and 1.45 dS m−1 in T4. Thus it can be observed that, compared to the CWW without ozonation treatment (Table 1), the EC decreased in T1, increased in T2 and T3, and decreased again in T4, although the changes were very small. Bhata et al. (2015) similarly reported no change in electrical conductivity after ozonation. In order to assess whether the ozonated CWW could be used for irrigation purposes, the concentrations of Na+, Ca2+ and Mg2+ in T4 were analyzed, and values of 21, 30 and 52 mg L−1 were obtained, respectively, reflecting a sodium adsorption ratio (SAR) of 3.27. Based on a comparison of the SAR and EC values to those recommended by Ayers and Westcot (1985), it is possible to conclude that the ozonated water has the potential for use in the irrigation of crops, causing virtually no reduction in soil infiltration capacity. Notably, there was no influence of O3 on the concentrations of these elements, as they remained virtually identical to those detected in the CWW after anaerobic digestion (Table 1).
3.5 Total Kjeldahl Nitrogen (TKN), Ammoniacal Nitrogen (NH4+), Nitrate (NO3−) and Total Phosphorus (Pt)
The highest removal of ammoniacal nitrogen and nitrate was found in T4, reaching final values of 8 mg NH4+ L−1 (94.7% removal) and 0.25 mg NO3− L−1 (99.7% removal), respectively (Fig. 5 a and b). Due to the low concentrations of nitrogen compounds detected with this treatment, after ozonation for 2 h, the wastewater could be discharged into water bodies according to CONAMA resolution 430/11, which establishes a limit of 20 mg NH4+ L−1. The effects of eutrophication caused by the release of nitrogen in water bodies would be minimized, as the level would be half the limit established by federal law. However, for agricultural purposes, nitrogen deficiency makes the ozonated CWW unattractive for use in the fertigation of crops, although nothing prevents it from being used for irrigation. According to the means comparison test, a statistically significant difference was observed in all cases except between T2 and T3 in terms of the reduction of NH4+ (Fig. 5a). The NH4+ reductions were associated with the stripping process, mainly in T4, where the pH of the solution was 8.2.
The use of O3 is also a well-known method for the oxidation of ammonia. The reaction of ammonia with ozone can be expressed as: NH4+ + 4O3 → 2H+ + NO3− + H2O + 4O2 (Singer and Zilli 1975).
For NO3−, significant differences were found between the means of all treatments except T3 and T4 (Fig. 5b). In T3 and T4, nitrate was highly oxidized, presenting concentrations of 0.25 mg L−1 (Fig. 5b). According to Schroeder et al. (2011), the rapid oxidation of nitrite via ozonation in the short term is highly beneficial for removing this anion.
Removal efficiency for TKN was 92.8%, with a final value of 20 mg TKN L−1, in T4 (Fig. 5c), thus achieving a substantial reduction of 256 mg TKN L−1. According to the means comparison test, a significant difference was observed only between T1 and the other treatments. The effective removal of TKN is a consequence of the removal of NH4+ from the CWW, also associated with oxidation of nitrogen compounds in their organic form.
The removal of total phosphorus was low, at only 40.1%, which led to a final value of 22 mg Pt L−1 (Fig. 5d) in the CWW ozonated in T4. In addition, there was no statistically significant difference between the means of the treatments (p ≥ 0.05) (Fig. 5d), indicating that there was low ozone action on the phosphate compounds contained in the CWW. It is possible that the P fractions bound to particulate organic compounds either were oxidized or floated and were then retained at the top of the reactor close to the cotton mass, resulting in unsatisfactory removal. There was no reaction of O3 with soluble phosphorus, as reflected in the higher final values of Pt.
From an agronomic point of view, this wastewater, already free of much of the solids and pollutants, could be used for agricultural purposes in phosphorus-poor soils, as is the case in several areas of the Brazilian territory, especially in areas with Red-Yellow Latosol and Dystrophic Yellow Latosol.
3.6 Total Coliforms and Thermotolerant Coliforms
After anaerobic digestion, the cattle effluent still had a high level of thermotolerant coliforms, and ozonation led to suitable removal efficiency. The lowest efficiency found was 92.3% in T1, and the highest was 99.99% in T3 and T4. Removal efficiency of 99.56% was obtained in T2 (Table 3).
For the removal of total coliforms, the lowest efficiency of 80.6% was observed in T1. In treatments T2, T3 and T4, removal efficiency of 99.44%, 99.90% and 99.99% was obtained, respectively.
According to the statistical test performed, there were no significant differences (p ≥ 0.05) between treatments T2, T3 and T4 for either class of coliforms.
Based on World Health Organization guidelines (WHO 2006), for irrigation of raw-consumed crops, an effluent value lower than or equal to 103 MPN 100 mL−1 of thermotolerant coliforms is strictly recommended. Despite the high efficiency in the removal of coliforms recorded in this study, the WHO-recommended value was achieved only in T4 (200 MPN 100 mL−1). Therefore, in microbiological terms, this effluent could be used for irrigation of raw-consumed crops. For the other treatments (T1, T2 and T3), the quality of the water was not suitable for irrigation due to the high residual concentrations of thermotolerant coliforms.
4 Conclusions
In this study, removal efficiency recorded ranged from 11 to 55% for COD, from 23 to 54% for BOD5, 27.5 to 65.7% for TS, and 44.1 to 93.5% for TSS. After O3 application, O&G concentrations were not detected with any of the treatments. Regarding the removal of nitrogen and phosphate compounds, efficiency of 54–94.7% was achieved for NH4+, 83.4–99.7% for NO3− and 27.1–40.1% for Pt. With regard to clarification of the CWW, color reduction of 29.9–93.4% and turbidity reduction of 32.3–88.5% were recorded. Removal of total and thermotolerant coliforms of 80.6–99.9% and 92.3–99.9%, respectively, was achieved.
High removal efficiency was achieved in the present study relative to the results of other studies, despite the low ozone concentration used, leading to the conclusion that the differential factor in the present study was the use of bio-ring media, which promoted a longer contact time/permanence of ozone with CWW.
Considering the removal of organic matter by treatment of CWW with O3, only T4 achieved BOD5 levels that conformed to the requirements for disposal into water bodies according to CONAMA resolutions 357/05 and 430/11. In terms of nutrients, only treatment T4 reduced NH4+ to a concentration suitable for discharge into water bodies. With regard to thermotolerant coliforms, only T4 reached the WHO-recommended level for the use of this wastewater in the irrigation of raw-consumed crops. In general, the effluent treated in T4 meets all the requirements described in the literature for reuse in the irrigation of raw-consumed crops.
References
Almeida, E., Assalin, M. R., Rosa, M. A., & Durán, N. (2004). Tratamento de efluentes industriais por processos oxidativos na presença de ozônio. Química Nova, 27(5), 818–824. https://doi.org/10.1590/S0100-40422004000500023.
APHA (2012). American Public Health Association, American Public Health Association, American Water Works Association, Water Environment Federation. Standard Methods for the Examination of Water and Waste Water. 22nd edn. Washington DC.
Araújo, K. S. D., Antonielli, R., Gaydeczka, B., Granato, A. C., & Malpass, G. R. P. (2016). Advanced oxidation processes: A review of fundamentals and applications in the treatment of urban and industrial wastewaters. Revista Ambiente e Água, 11(2), 387–401. https://doi.org/10.4136/ambi-agua.1862.
Ayers, R. S., & Westcot, D. W. (1985). Water quality for agriculture. FAO irrigation and drainage paper 29 (revised 1) (p. 174). Rome: FAO.
Barreto, A. C., & Campos, C. M. M. (2009). Avaliação de um sistema de irrigação autopropelido aplicando água residuária de suinocultura.Ciência e Agrotecnologia, 33, 1752–1757. https://doi.org/10.1590/S1413-70542009000700009.
Bhatta, R., Kayastha, R., Subedi, D. P., & Joshi, R. (2015). Treatment of wastewater by ozone produced in dielectric barrier discharge. Journal of Chemistry, article ID 648162. https://doi.org/10.1155/2015/648162.
BRASIL. Resolução CONAMA 357, de 17 de março de 2005. Conselho Nacional de Meio Ambiente. Available at: http://www2.mma.gov.br/port/conama/legiabre.cfm?codlegi=459. Accessed Dec. 2019.
BRASIL. Resolução CONAMA 430, de 13 de maio de 2011. Conselho Nacional de Meio Ambiente. Available at: http://www2.mma.gov.br/port/conama/legiabre.cfm?codlegi=646. Accessed Dec. 2019.
Cabrera-Díaz, A., Pereda-Reyes, I., Dueñas-Moreno, J., Véliz-Lorenzo, E., Díaz-Marrero, M. A., Menéndez-Gutiérrez, C. L., & Zaiat, M. (2016). Combined treatment of vinasse by an upflow anaerobic filter-reactor and ozonation process. Brazilian Journal of Chemical Engineering, 33(4), 753–762. https://doi.org/10.1590/0104-6632.20160334s20150268.
Clescerl, L. S., Greenberg, A. E., & Eaton, A. D. (2000). Standard methods for the examination of water and wastewater (p. 1220). American Water Works Association: Denver.
de S. Coelho, C. C., Freitas-Silva, O., Campos, R. D. S., Bezerra, V. S., & Cabral, L. M. (2015). Ozonização como tecnologia pós-colheita na conservação de frutas e hortaliças: Uma revisão. Revista Brasileira de Engenharia Agrícola e Ambiental, 19(4), 369–375. https://doi.org/10.1590/1807-1929/agriambi.v19n4p369-375.
Hansson, H., Kaczala, F., Amaro, A., Marques, M., & Hogland, W. (2015). Advanced oxidation treatment of recalcitrant wastewater from a wood-based industry: A comparative study of O3 and O3/UV. Water, Air, & Soil Pollution, 226, 1–12. https://doi.org/10.1007/s11270-015-2468-5.
Júnior, C. B. O., Sandri, D., Alencar, E. R. D., & Hebling, L. F. (2019). Ozonation improves physical attributes in domestic sewage effluent. Revista Ambiente & Água, 14(2). https://doi.org/10.4136/ambi-agua.2328.
Lage, F. F. A. (2008). Avaliação da filtração e ozonização de efluente sanitário primário: aspectos de inativação microbiana e variáveis de ozonização. Química Nova, 31(2), 312–316. https://doi.org/10.1590/S0100-40422008000200023.
Lee, B. H., Song, W. C., Manna, B., & Ha, J. K. (2008). Dissolved ozone flotation (DOF) – A promising technology in municipal wastewater treatment. Desalination, 225, 260–273. https://doi.org/10.1016/j.desal.2007.07.011.
Lei, L., & Li, Y. (2014). Effect of ozonation on recalcitrant chemical oxygen demand (COD), color and biodegradability of hardwood Kraft pulp (KP) bleaching effluent. BioResources, 9(1), 1236–1245.
Mahmoud, A., & Freire, R. S. (2007). Métodos emergentes para aumentar a eficiência do ozônio no tratamento de águas contaminadas. Química Nova, 30(1), 198–205. https://doi.org/10.1590/S0100-40422007000100032.
Marcelino, R. B., Leão, M. M., Lago, R. M., & Amorim, C. C. (2017). Multistage ozone and biological treatment system for real wastewater containing antibiotics. Journal of Environmental Management, 195, 110–116. https://doi.org/10.1016/j.jenvman.2016.04.041.
Mendonça, H. V., Ometto, J. P. H. B., & Otenio, M. H. (2017). Production of energy and biofertilizer from cattle wastewater in farms with intensive cattle breeding. Water, Air, & Soil Pollution, 228(2), 1–14. https://doi.org/10.1007/s11270-017-3264-1.
Mendonça, H. V., Ometto, J. P. H. B., Rocha, W. S. D., Martins, C. E., Otenio, M. H., & Borges, C. A. V. (2016). Crescimento de cana-de-açúcar sob aplicação de biofertilizante da bovinocultura e ureia. Revista Agronegócio e Meio Ambiente, 9(4), 973–987. https://doi.org/10.17765/2176-9168.2016v9n4p973-987.
Mendonça, H. V., Ometto, J. P. H. B., Otenio, M. H., Marques, I. P., & Reis, A. D. (2018). Microalgae-mediated bioremediation and valorization of cattle wastewater previously digested in a hybrid anaerobic reactor using a photobioreactor: Comparison between batch and continuous operation. Sci Total Environ, 633, 1–11. https://doi.org/10.1016/J.SCITOTENV.2018.03.157.
Nakayama, F. S. (1982). Water analysis and treatment techniques to control emitter plugging. Portland, Oregon: Proc. Irrigation Association Conference.
Oller, I., Malato, S., & Sánchez-Pérez, J. (2011). Combination of advanced oxidation processes and biological treatments for wastewater decontamination—A review. Science of the Total Environment, 409(20), 4141–4166. https://doi.org/10.1016/j.scitotenv.2010.08.061.
Paździor, K., Wrębiak, J., Klepacz-Smółka, A., Gmurek, M., Bilińska, L., Kos, L., Sójka-Ledakowicz, J., & Ledakowicz, S. (2017). Influence of ozonation and biodegradation on toxicity of industrial textile wastewater. Journal of Environmental Management, 195, 166–173. https://doi.org/10.1016/j.jenvman.2016.06.055.
Peña, M., Coca, M., González, G., Rioja, R. and García, M. T. (2003). Chemical oxidation of wastewater from molasses fermentation with ozone. Chemosphere, 51, 893-900. 10.1016/S0045-6535(03)00159-0.
Schroeder, J. P., Croot, P. L., Von Dewitz, B., Waller, U., & Hanel, R. (2011). Potential and limitations of ozone for the removal of ammonia, nitrite, and yellow substances in marine recirculating aquaculture systems. Aquacultural Engineering, 45, 35–41 https://doi.org/10.1016/j.aquaeng.2011.06.001.
Silva, A. C., Dezotti, M., & Sant’Anna Jr., G. L. (2004). Treatment and detoxification of a sanitary landfill leachate. Chemosphere, 55(2), 207–214 https://doi.org/10.1016/j.chemosphere.2003.10.013.
Silva, J. B. G., Martinez, M. A., Pires, C. P., Andrade, I. P. S., & Silva, G. T. (2012). Avaliação da condutividade elétrica e pH da solução do solo em uma área fertirrigada com água residuária de bovinocultura de leite. Revista Irriga - Botucatu, special edition, 250–263. https://doi.org/10.15809/irriga.2012v1n01p250
Singer, P. C., & Zilli, W. B. (1975). Ozonation of Ammonia in wastewater. Water Research, 9, 127–134. https://doi.org/10.1016/0043-1354(75)90001-9.
Subha, B, & Muthukumar, M. (2012). Optimization of ozonation process for the reduction of excess sludge production from activated sludge process of sago industry wastewater using central composite design. The Scientific World Journal, Article ID 460 239271. https://doi.org/10.1100/2012/239271.
Vale P., Gibbs H., Vale R., Christie M., Florence E., Munger J., Sabaini, D. (2019). The expansion of intensive beef farming to the Brazilian Amazon. Global Environmental Change Human and Policy Dimensions, 57, article ID: UNSP 101922. https://doi.org/10.1016/j.gloenvcha.2019.05.006.
Wilinski, P. & Jeremi, N. (2012). Dissolved ozone flotation as innovative and prospect method for treatment of micro pollutants and wastewater treatment costs reduction. 12th edition of the worldwide workshop for young environmental scientists (WWW-YES-2012) - urban waters: Resource or risks? France. Available in: https://hal.inria.fr/hal-00709736
World Health Organization (WHO). (2006). Guidelines for the safe use of wastewater, excreta and greywater: Wastewater use in agriculture (Volume II). Available in: http://www.who.int/water_ sanitation_health/wastewater/gsuweg2/en/index.html.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Souza, D.S., Maciel, A.M., Otenio, M.H. et al. Optimization of Ozone Application in Post-Treatment of Cattle Wastewater from Organic Farms. Water Air Soil Pollut 231, 362 (2020). https://doi.org/10.1007/s11270-020-04736-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04736-2