Abstract
Acrocomia is one of the most complex genera to understand in the Neotropical Arecaceae, and there is no consensus on the number of species in the genus. A comparative study of leaf anatomy was conducted on seven species of Acrocomia: one with a wide distribution in the Americas (A. aculeata), five endemic to different regions of Brazil, Bolivia and Paraguay (A. emensis, A. glaucescens, A. hassleri, A. intumescens and A. totai) and one endemic to Cuba (A. crispa). Characters that unify the species of Acrocomia include the following: epidermis covered with cuticle, hypodermis on both sides of the leaflets, non-vascular fiber bundles, and primary and secondary vascular bundles. The shape of the leaflet margin, the distribution of the primary vascular bundles and fiber bundles, the number of idioblasts with raphides and the size of the vascular bundles, along with other characteristics, were used to distinguish these species in Acrocomia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family Arecaceae has 240 genera and approximately 2700 species predominantly concentrated in tropical and subtropical regions (Dransfield et al. 2008; Govaerts et al. 2016). In Brazil, there are approximately 200 species from 40 genera (Lorenzi et al. 2010).
Acrocomia species are commonly used for subsistence by native people throughout tropical America (Morcote-Ríos and Bernal 2001) in addition to commercial exploitation, primarily for oil extraction, as occurs mostly in Brazil and Paraguay (Janick and Paull 2008). In Brazil, the species A. aculeata, A. intumescens and A. totai are used for various purposes, including using the stems for construction, leaves for feeding livestock and horses, fibers for making fishing lines, the endocarps or nuts as a substitute for crushed stone, the endosperm for extracting edible oil or for cosmetics and the fruit pulp for fresh or processed consumption and production of biofuel oil (Pott and Pott 1994; Salis and Mattos 2009; Damasceno-Jr and Souza 2010; Amaral et al. 2011; Vianna et al. 2013).
The genus Acrocomia is one of the most taxonomically complex genera in the Neotropical Arecaceae. The genus is in the subfamily Arecoideae, tribe Cocoseae and subtribe Bactridinae, with the latter represented by the genera of spiny palms (Acrocomia, Aiphanes, Astrocaryum, Bactris and Desmoncus), and the distribution is restricted to the Americas, particularly South America (Henderson et al. 1995; Dransfield et al. 2008). According to Henderson et al. (1995), the genus has two species: Acrocomia aculeata (Jacq.) Lodd. ex Mart., which is tree-sized and widely distributed throughout the Americas, and Acrocomia hassleri (Barb.Rodr.) W.J.Hahn, which is an acaulescent species (species having a rhizome or a short subterranean stem) restricted to Cerrado areas of Brazil and Paraguay. However, more recent references accept a greater number of species for the genus. Lorenzi et al. (2010) recognize five species in addition to the two described above, citing the existence of Acrocomia emensis (Toledo) Lorenzi, an acaulescent species distributed in Cerrado areas of Brazil in the states of Goiás, Mato Grosso do Sul, Minas Gerais, São Paulo and Paraná; Acrocomia glaucescens Lorenzi, a dwarf species with a distribution in Brazilian Cerrado areas that grows in sandy soils in the states of Mato Grosso and Goiás; Acrocomia intumescens Drude, a tree-sized palm occurring in a small region of the northeast associated with the Atlantic Forest; Acrocomia totai Mart., a tree-sized palm with a distribution restricted to the state of Mato Grosso do Sul and Acrocomia crispa (Kunth) C.F.Baker ex Becc., a tree-sized palm endemic to Cuba.
Acrocomia aculeata, A. crispa, A. intumescens and A. totai are tree-sized and can be mainly distinguished by their stem features, in addition to other characteristics. Acrocomia aculeata has a spiny stem and is usually covered by persistent leaf bases from fallen leaves, especially in the southern portion of its distribution and its fruits are 3.0–5.0 cm in diameter. The spiny stems of A. crispa are slightly swollen toward the apex, with small fruits 1.0–2.5 cm in diameter, and white spines on the leaf rachis. Acrocomia intumescens has a swollen stem that is spiny when young, becoming smooth at adult stage, with fruits 3.8–5.4 cm in diameter. Acrocomia totai usually has a smooth stem, which is sometimes spiny, and is rarely covered with the persistent leaf bases of fallen leaves, its fruits are 2.5–3.5 cm in diameter (Lorenzi et al. 2010).
The species A. emensis and A. hassleri do not have a visible stem but a rhizome or a short subterranean stem, termed acaulescent, according to the descriptions of Lorenzi et al. (2010). Acrocomia emensis is 40–60 cm tall, with a long rhizome (30–80 cm in length), spiny peduncular bract and leaf, and fruit 1.8–2.2 cm in diameter. Acrocomia hassleri is shorter, 30–40 cm tall, with a short rhizome (10–12 cm length), the peduncular bract and leaves are only slightly spiny, and the fruit is 1.6–2.0 cm in diameter. The species A. glaucescens occurs in both an acaulescent form or with a visible stem up to 6.5 m tall, which lack spines and with visible leaf base scars (Lorenzi et al. 2010).
The classifications published for Acrocomia are based only on morphological characters; therefore, using other analyses or techniques that may complement such data can greatly improve our understanding of the taxonomic relationships.
Leaf anatomy has been very useful taxonomically for the differentiation of species. According to Parthasaranthy (1968) and Tomlinson (1961, 2011), the anatomical characters of vegetative organs in the family Arecaceae have diagnostic value for inter-specific differentiation. Silva and Potiguara (2008) distinguish some species of Oenocarpus using morphological and anatomical analysis of the leaflets, and Millán and Kahn (2010) differentiate species of Astrocaryum and Hexopetion. Guevara et al. (2011) conducted a comparative study of leaf anatomy of the subtribe Mauritiinae to differentiate genera within the group. Additionally, Henderson (2013) differentiated species of Leopoldinia and Noblick (2013) differentiated those of Syagrus, based on leaf anatomy.
In this context, to demonstrate the species-level diversity of leaf anatomy and contribute to the taxonomy of the genus, this study aimed to characterize the leaf anatomy of seven species of Acrocomia.
Materials and methods
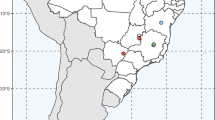
As a reference for the classification of the species that we analyzed, we used the taxonomic circumscription of Acrocomia accepted by Lorenzi et al. (2010); therefore, the following species were analyzed: Acrocomia aculeata, Acrocomia crispa, Acrocomia emensis, Acrocomia hassleri, Acrocomia glaucescens, Acrocomia intumescens and Acrocomia totai (Fig. 1).
Collection and preparation of plant material
The botanical material was collected from the living collections of the Montgomery Botanical Center (MBC), Coral Gables, FL, USA, with a tropical climate, in a coastal area, classified as Am, according to the climatic classification of Köppen–Geiger, at sea level, with predominantly shallow to deep sandy soils and calcareous bedrock. Collections were also made at the Jardim Botânico do Instituto Plantarum (IP), Nova Odessa, SP, Brazil, with a tropical savanna climate, Aw type according to the Köppen–Geiger classification, at an elevation of 570 m, flat terrain, and with deep clayey soils. Samples of dried specimens from the Fairchild Tropical Botanic Garden Herbarium (FTG), Coral Gables, FL, USA were also used. Some individuals were collected in situ (Table 1).
The material was fixed in FAA (5% formaldehyde, 5% glacial acetic acid and 90% of 70% ethanol) for 48 h (Johansen 1940) and stored in ethanol 70%. The herbarium samples were rehydrated in a 5% solution of Contrad 70® for 24 h and then fixed and stored as previously described.
Optical microscopy and scanning electron microscopy
Three leaflets from the middle third of three leaves of each species were analyzed. Samples removed from the margin of the leaflet, and the midrib were dehydrated through an ethanol series followed by infiltration and embedment in synthetic resin (Historesin® Leica), according to the manufacturer’s recommendations. These samples were sectioned with a rotary microtome and stained with blue toluidine 0.05% in a 0.1 M acetate buffer (pH 4.7) (O’Brien et al. 1964). Specimens were mounted in water on temporary slides, and the images were captured with a digital camera (Olympus DP71) coupled to an optical microscope (Olympus BX51). The description of the anatomical data obtained followed the terminology adopted by Tomlinson (1961) and Tomlinson et al. (2011).
For analyses with a scanning electron microscopy, samples of a set of leaflets 0.5 cm square were preserved in 70% alcohol, dehydrated in an ethanol series, and then subjected to further dehydration by drying to a critical point using carbon dioxide (Horridge and Tamm 1969). Thereafter, samples were mounted on aluminum brackets (stubs) and passed through the sputtering process to be covered by a layer of gold (Castro 2002). The electron micrographs were taken on a scanning electron microscope Jeol model JSM 5800LV operated at 10 kV, with the scale printed directly on the images. The terminology adopted for the type of epicuticular wax deposition was that of Barthlott et al. (1998).
Data analyses
To quantitatively differentiate the tissues and structures found in each species of the genus under study, 19 characters were measured with the program Image J as follows: (1) thickness of the mesophyll, (2) thickness of the epidermis + the hypodermis on the adaxial surface, (3) thickness of the epidermis + the hypodermis on the abaxial surface, (4) number of primary vascular bundles, (5) length of the primary vascular bundles, (6) width of the primary vascular bundles, (7) thickness of the sclerenchymatous sheath of the primary vascular bundles, (8) number of secondary vascular bundles, (9) length of the secondary vascular bundles, (10) width of the secondary vascular bundles, (11) number of non-vascular fiber bundles, (12) length of the non-vascular fiber bundles, (13) width of the non-vascular fiber bundles, (14) length of the marginal vascular bundle, (15) width of the marginal vascular bundle, (16) thickness of the sheath around the marginal vascular bundle, (17) number of idioblasts, (18) length of the idioblasts and (19) width of the idioblasts. The statistical description of the data was completed, followed by an analysis of variance with Tukey’s posttests to identify significant differences among the species.
The leaf anatomy of the species of Acrocomia was compared using cluster analysis to determine the potential taxonomic diagnostic value of different characters. We analyzed 77 anatomical characters, which included descriptive or qualitative characters (i.e., type of deposition of epicuticular wax) and quantitative characters (i.e., width of the primary vascular bundle). We tabulated the information in the form of a binary matrix and described each character as present (1) or absent (0) and then subjected the matrix to cluster analysis using Euclidean distance and UPGMA as an algorithm (Mantovani et al. 2010; Millán and Kahn 2010); the analyses were performed using the program PAST (Hammer et al. 2001). A character was considered significant with a cophenetic value >0.7 (Valentin 2000). The stability of the groups was tested using the resampling procedure to 10,000 randomizations.
Results
In Acrocomia, significant differences were observed among the morphoanatomical characters of the individuals of the different species studied. In addition to species descriptions, the differences in morphoanatomical characters were demonstrated and confirmed numerically with statistical analyses.
Anatomical description
The epidermis and hypodermis were similar in all species of Acrocomia. The epidermis was one-layered on both the adaxial and the abaxial surfaces, and in frontal or surface view, the cells were hexagonal with thickened, straight anticlinal walls and covered with cuticle (Figs. 2, 3b). Immediately below the epidermis on both surfaces, the hypodermis was a single layer of cells, which were larger than those of the epidermis, without wall thickening, usually without chloroplasts, and whose greatest cell length formed a right angle to the greatest length of an epidermal cell (Figs. 2, 3).
Leaflet blade in cross section: a Acrocomia aculeata, b A. crispa, c A. emensis, d A. glaucescens, e A. hassleri, f A. intumescens and g A. totai. ep epidermis, hy hypodermis, snvfb small non-vascular fiber bundle, nvfb non-vascular fiber bundles, st stomata, pvb principal vascular bundle, sf sclerenchymatic fiber, svb secondary vascular bundle and idb idioblast (see Fig. 3c for close up). Bar 200 µm
Leaflets with stomata that occurred mostly on the abaxial surface were hypostomatic; however, in the species A. emensis, A. glaucescens and A. hassleri, the leaflets were amphistomatic, and stomata commonly occurred on both sides, although they were more numerous on the abaxial surface (Fig. 3a). In the genus under study, the stomata were tetracytic and were organized in parallel longitudinal, rows to the midribs.
The number of trichomes that occurred predominantly on the abaxial surface was variable depending on the species of Acrocomia. Trichomes were more abundant on A. crispa, with trichomes being fewer or absent on A. emensis, A. hassleri and A. glaucescens. Trichomes were multicellular and 1–5 branched (Fig. 3b).
The leaflets of the tree-sized species, A. aculeata, A. crispa, A. intumescens and A. totai, were dorsiventrally symmetric, with 3–5 layers of more elongated parenchyma cells near the adaxial surface and 5–8 layers of rounded parenchyma cells closer to the abaxial surface (Fig. 2). The leaflets of the acaulescent species A. hassleri and A. emensis and the small-sized A. glaucescens were isolaterally symmetric, with parenchyma cells of similar shape throughout the mesophyll (Fig. 2).
Idioblasts were observed with raphides that were surrounded by a mucilaginous material (Fig. 3c), and the number of idioblasts was variable, with most observed in A. emensis (2.10 ± 1.17) and A. hassleri (1.23 ± 0.98).
Vascular bundles were distributed throughout the mesophyll of all species and were divided into primary, secondary and even tertiary bundles (Fig. 2b, e). The primary vascular bundles varied in number and size among the species studied and were arranged parallel to the longitudinal axis of the leaf featuring a parallel venation pattern (Fig. 3d). These bundles were completely or partially enveloped by one or more layers of sclerenchyma fibers, which were surrounded by a layer of parenchymal cells. Acrocomia emensis had the most primary vascular bundles (5.70 ± 0.57) and A. totai had the fewest (1.80 ± 0.79).
Secondary vascular bundles were usually enveloped by a parenchymatic sheath and rarely also by sclerenchymatic tissue (Fig. 2a, d), varying in number and size with most secondary bundles being found in A. totai (8.40 ± 0.97).
In Acrocomia, non-vascular fiber bundles were distributed in the mesophyll, with high diversity in the number, size, distribution and location depending on the species (Fig. 2). In the acaulescent species A. emensis, A. hassleri and A. glaucescens, many of these bundles occurred, which were of varying sizes and randomly and unevenly distributed in the mesophyll. In the tree-sized species A. aculeata, A. intumescens and A. totai, the fiber bundles were similar in size (within each species) and with a distribution at almost regular intervals near the abaxial surface (A. aculeata and A. intumescens) or the adaxial surface (A. totai). The most non-vascular fiber bundles (30.33 ± 6.37) were observed in A. crispa, because this species was the only one with many small non-vascular fiber strands that were regularly distributed immediately underneath the adaxial hypodermis in addition to the larger non-vascular fiber bundles regularly distributed near the abaxial surface.
All species of Acrocomia had silica bodies with an extended, straight base and a conical upper portion, called stegmata, which occurred in continuous or discontinuous rows associated with non-vascular fiber bundles. Each stegmata contained one silica body. Although their observation was possible under a light microscope, scanning electron microscopy provided greater detail of the stegmata arranged in longitudinal rows parallel to the associated non-vascular fiber bundles, with thickened walls, rounded, and with a wider base and a central concavity in which the silica body was found, which was globular and with a spike-like surface similar to a druse (Fig. 4).
Epicuticular wax deposition patterns
Both sides of the leaflets in all species studied had epicuticular wax coverage, ranging from a thin layer to a dense covering, and a depositional pattern of wax that was different among the species.
On the adaxial surface of A. aculeata, streaks or bands formed the pattern of epicuticular wax deposition (Fig. 5a), and on A. crispa, wax was deposited as small granules that outlined epidermal cells (Fig. 5b). The only species of the genus with wax deposition in the form of threads forming a tangle on the leaf surface and around the ostioles (exterior opening of a stomata) was A. emensis, with the form of deposition similar in shape longitudinally aggregated rodlets (Fig. 5c). In A. glaucescens, wax deposition was of two types: in the form of band over the veins and in between the veins as longitudinally aggregated rodlets (Fig. 5d). In A. hassleri, the wax was deposited in the form of bands and also as longitudinally aggregated rodlets in the ostiole; moreover, scattered deposits were observed in the form of wax crystals (Fig. 5e). In A. intumescens, wax deposition occurred in the form of band marks and small granules (Fig. 5f), and in A. totai, wax deposits were in the form of overlapping scales (Fig. 5g).
Based on observations of the abaxial surface, we noted that all species of Acrocomia had deposits of epicuticular wax and wire-like longitudinally aggregated rodlets. The amount of wax deposited was sufficient to block the ostioles.
Midrib
In Acrocomia, the midrib cross section appeared more prominent on the abaxial surface. Underlying the abaxial epidermis in most species of Acrocomia between the mesophyll and the midrib were parenchyma palisade cells with thickened walls, known in palms as expansion tissue (Fig. 6).
In the midrib of A. aculeata, two opposing vascular bundles with the xylem turned inward and the phloem turned toward the outer surface were surrounded by 3 or more layers of sclerenchyma fibers, with 2–3 layers of parenchyma cells. At the boundary between the midrib and the mesophyll, on the abaxial surface, 1 or 2 layers of expansion cells were observed (Fig. 6a).
The midrib of A. crispa had a larger vascular bundle near the abaxial surface and two smaller, parallel ones closer to the adaxial surface. The vascular bundles had the xylem turned inward and the phloem facing toward the outer surface. These bundles were surrounded by 3–6 layers of sclerenchyma fibers. Between the vascular bundles parenchyma cells with thick walls were near the vascular bundles, and the rest were without thickening. Two layers of expansion cells were observed in the region between the mesophyll and the midrib on the abaxial surface, with the first layer composed of elongated cells and the second layer of smaller cells (Fig. 6b).
In A. emensis, 3–5 bundles were wrapped externally by 3–8 layers of sclerenchyma fibers and 3–10 layers of cells were distributed between the parenchymatic cells. Various, randomly dispersed non-vascular fiber bundles were also observed. Between the midrib and mesophyll, on the adaxial surface, 2–3 layers of expansion cells occurred (Fig. 6c).
The midrib of A. glaucescens consisted of one central vascular bundle, partially covered externally by 5–7 layers of sclerenchyma fibers and internally by 2–3 non-thickened cell layers. At the boundary between the midrib and the mesophyll on the adaxial surface, two layers of elongated expansion cells were observed (Fig. 6d).
Acrocomia hassleri had a midrib with various, randomly distributed vascular bundles, with some surrounded bundles by 1–2 individual layers of sclerenchyma fibers and a group or set of bundles surrounded by more than 10 layers of sclerenchyma fibers. Between the vascular bundles, lignified parenchyma cells were randomly distributed. Between the midrib and the mesophyll on the adaxial surface, three layers of expansion cells were observed, although somewhat shorter and wider than those in the other species (Fig. 6e).
In the midrib of A. intumescens, three vascular bundles were arranged in a semicircle closest to the abaxial side, and two parallel vascular bundles were located closest to adaxial side that were partially wrapped by 1 to more than 10 layers of sclerenchyma fibers. Between the bundles, non-thickened parenchyma cells occurred. The vascular bundles had the xylem turned inward and the phloem turned toward the outer surface. Below the epidermis, 1–6 layers of parenchyma cells were observed, and between the midrib and the mesophyll on the abaxial surface, one or two layers of expansion cells were observed (Fig. 6f).
The midrib of A. totai had 3–4 vascular bundles and two primary vascular bundles arranged in the central region. Each individual bundle was partially surrounded by 2–5 layers of sclerenchyma fibers, and the bundles together were surrounded by more than 5–10 layers of sclerenchyma fibers. The parenchyma cells that occurred between the vascular bundles showed no thickening. Below the epidermis 2 to more than 10 layers of parenchyma cells were observed, in addition to secondary vascular bundles and non-vascular fiber bundles. At the boundary between the midrib and mesophyll on the abaxial surface, two layers of elongated expansion cells occurred (Fig. 6g).
Margin
In A. aculeata, the margin of the leaflet was rounded and usually without a vascular bundle, although when present, the bundle was small. The other species of the genus had a square edge or margin and a large vascular bundle in the margin (Fig. 7), and the size of the bundles and the amount of surrounding sclerenchyma fibers were variable. The largest average length and width (μm) of a marginal vascular bundle, in cross section, was observed in A. emensis (154.45 ± 11.62 and 101.45 ± 6.49, respectively). The mesophyll, epidermis and other structures found in the margin were similar to those in rest of the leaflet.
Analysis of anatomical data
Statistical tests were performed as a complement to the anatomical descriptions, identifying significant differences for most characters and numerically demonstrating the differences in leaf anatomy among the species of Acrocomia. Significant differences (p < 0.05) were detected among the averages of the 19 characters analyzed by ANOVA, with Tukey’s posttests to separate species (Table 2).
Among the species examined, A. emensis had the highest number of primary vascular bundles (5.70 ± 0.57) and the highest values for the, length of the primary vascular bundles (149.00 ± 20.94), width of the primary vascular bundles (90.30 ± 25.34), sclerenchymatic thickness of the sheath of the primary vascular bundles (38.00 ± 11.96), length of the secondary vascular bundles (40.30 ± 11.36), width of the secondary vascular bundles (35.50 ± 5.80), length of the marginal vascular bundle (154.45 ± 11.62), width of the marginal vascular bundle (101.45 ± 6.49), thickness of the sclerenchymatic sheath of the marginal vascular bundle (80.25 ± 11.97) and number of idioblasts (2.10 ± 1.17).
The cluster analysis of the 77 characters of leaflet morphoanatomy resulted in the separation of species of Acrocomia as shown in Fig. 8, with the cophenetic value of 0.8.
The evaluation of Acrocomia revealed anatomically distinct species, which reinforced the taxonomic distinctions adopted as the morphological basis for this work. Cluster analysis divided the species into two groups, tree-sized species (A. aculeata, A. crispa, A. intumescens and A. totai) and acaulescent species (A. emensis, A. hassleri and A. glaucescens). This result was notable because the analysis used only characters of leaflet anatomy combined with the observations and the descriptions of the species accepted by Lorenzi et al. (2010), which also used only morphological data (Fig. 8).
Discussion
The leaflets of the species A. emensis, A. glaucescens and A. hassleri were amphistomatic, whereas those of A. aculeata, A. crispa, A. intumescens and A. totai were hypostomatic. This feature separated the species of Acrocomia into two large groups: acaulescent and tree-sized species. According to Henderson (2006), stomata on one or both surfaces of the mesophyll and stomatal location in relation to the other epidermal cells, at or above, can be used as taxonomic characters.
The leaves of the acaulescent species were isolaterally symmetric with non-vascular bundles of fibers randomly distributed throughout the mesophyll, whereas the leaves of the tree-sized species were dorsiventrally symmetric and had non-vascular fiber bundles distributed in an organized manner in the mesophyll. Although a dorsiventral mesophyll is considered rare in Arecaceae (Henderson 2006), articles cite the occurrence, for example, in Oenocarpus spp. and Cocos nucifera L. (Araújo et al. 2013; Silva and Potiguara 2008). However, although presenting different symmetries, according to Tomlinson et al. (2011) the most rounded cells near the abaxial face cannot be termed spongy parenchyma, because they do not have conspicuous intercellular spaces except near the substomatic cameras. In this work, the symmetry referred to layers of cells with different size and shape that may occur in the mesophyll of the studied species. In tree-sized species, two types of cell layers were observed, which identified a dorsiventral parenchyma in this study: one nearest to the adaxial face with elongated cells and hexagonal format and the other nearest to the abaxial face with rounded cells.
In acaulescent species, the distribution of non-vascular fiber bundles, of varying size and in greater numbers was random, which most likely provided the greater rigidity and less flexibility of the leaflets of acaulescent species compared with those of tree-sized species. In the tree-sized species, A. aculeata, A. intumescens and A. totai, the fiber bundles were similar in size (within species) and were distributed at almost regular intervals, near the abaxial surface (A. aculeata and A. intumescens) or the adaxial surface (A. totai). Acrocomia crispa was clearly differentiated from other species as the only one with many small non-vascular fiber bundles regularly distributed immediately below the adaxial hypodermis. Therefore, the differences in the amount, size and distribution pattern of the secondary vascular bundles and non-vascular fiber bundles in the Acrocomia species examined in this study were used to differentiate the genus. According to Cutler (1978), the arrangement patterns of vascular tissue in a leaf, with the exception of the number, which is under genetic control, do not exhibit any environmental variance, and thereby, constitute a useful taxonomic character to separate taxa.
The species with isolateral symmetry were amphistomatic and with non-vascular fiber bundles randomly distributed throughout the mesophyll, whereas the species with dorsiventral symmetry were hypostomatic and with non-vascular fiber bundles distributed in an organized manner in the mesophyll.
The numerous idioblasts in acaulescent species might be related to protection against herbivory, because their small-sized leaves are in direct contact with or near to the ground, which favors herbivore attack. According to Franceschi and Horner (1980) and Franceschi and Nakata (2005), these structures act as a protective mechanism for plants against herbivores, because the idioblasts with raphides decrease the palatability of the leaves and other organs. No distinct differences in the standard distribution of idioblasts in the mesophyll were observed.
We observed stegmata with similar morphology and distribution in all species that were associated with non-vascular fiber bundles. According Moller and Rasmussen (1985), the occurrence and morphology of stegmata are genetically determined; therefore, because the environment has little influence, stegmata have taxonomical value. The presence of stegmata in the group Liliopsidas, in the Arecaceae, is a plesiomorphic character (Chase et al. 2000), and the shape and location of those observed in this study was a unifying character for the subfamily Arecoideae, which contains the genus under study (Dransfield et al. 2008; Silva and Potiguara 2008, 2009; Tomlinson et al. 2011).
Silica in tissues is related to the mechanical protection against attack by insects and pathogens (Paviani 1972; Kikuchi et al. 2007; Silva and Potiguara 2009), control of excessive transpiration and water retention (Hanberlandt 1925; Sangster 1977), preventing tissue breakdown in dry conditions (Metcalfe and Chalk 1983) and the balance of thermal exchanges with the external environment (Adatia and Besford 1986). The functions of transpiration control and water retention are important to all species of the genus because of the distributions in hotter and drier areas (Lorenzi et al. 2010). The protective function against pathogens and pest attack is also important, particularly for acaulescent species, which because of their size are more susceptible.
Leaflets in all species had a large deposition of epicuticular wax, except for A. crispa, which had scattered granules. The different species had different patterns of deposition or ornamentation of the epicuticular wax, and therefore, epicuticular wax served as an additional character that could be used in the taxonomic differentiation of Acrocomia. According to Metcalfe and Chalk (1979) and Stace (1965), environmental factors may or may not influence the type of wax deposition. Some studies find similar patterns of the ornamentation of epicuticular wax in plants from different environments (Metcalfe and Chalk 1979; Mantovani et al. 1995; Vieira and Gomes 1995). Therefore, based on these studies, the differentiating factor for the type of wax deposition is under genetic control of the species and not the environment.
In Acrocomia, the distribution pattern of tissues and anatomical structures in the midrib region is used to differentiate the species. Based on other work (Glassman 1972; D’Arcy and Keating 1979; Silva and Potiguara 2008), the anatomy of the midrib is a useful character for the identification and separation of species within this genus. Although the tissues and structures in the midrib were identical, the number, size and distribution of these were different in each Acrocomia species examined, in addition to the differences in the position of the expansion tissue found adjacent to the midrib. For example, the highest number of vascular bundles was in the midrib of A. hassleri and A. totai, and the thickest layer of sclerenchyma fibers surrounding the vascular bundles was in A. hassleri. In the tree species of Acrocomia (A. aculeata, A. crispa, A. intumescens and A. totai), the expansion tissue occurred on the abaxial surface of the leaflets, whereas in acaulescent species (A. emensis and A. hassleri) and the smaller-sized A. glaucescens, the expansion tissues occurred on the adaxial surface. In other studies on the leaf anatomy of palm species, the position of the expansion tissue is a useful character in the taxonomy. Millán and Kahn (2010) found in Astrocaryum spp. and Hexopetion spp. that it is the species that determines whether the position of the expansion tissue is on the adaxial or abaxial surface. Sant’Anna-Santos et al. (2015), for two species of Butia, found that the expansion tissue in both species occurs on the abaxial surface. According Tomlinson et al. (2011), the location of the expansion tissue in Arecaceae on the abaxial or adaxial surface varies with the type of leaf. Furthermore, according to Tomlinson (1961), as cited by Silva and Potiguara (2008), the expansion tissue is associated with the folding and unfolding mechanisms of leaflets resulting from changes in the turgor pressure of cells.
For leaflets in the species studied, we observed two types of margin that that enabled the differentiation of the species: rounded and without a vascular bundle or a small one (A. aculeata), and square (in other species) and with variation in the vascular bundle size and the number of layers of sclerenchyma fibers. This character might be an additional taxonomic delimitation within Acrocomia. Bieras and Sajo (2004), Scatena et al. (2004) and Noblick (2013) demonstrated that the morphoanatomy of the leaflet margin could differentiate species.
The differences in leaf anatomy among the Acrocomia species reinforced the taxonomic distinction of these seven species in the genus based on the morphological descriptions of Lorenzi et al. (2010), in addition to the formation of two groups, one with the tree-sized species (A. aculeata, A. crispa, A. intumescens and A. totai) and one with acaulescent species (A. emensis, A. hassleri and A. glaucescens). The acaulescent species were primarily characterized by amphistomatic leaflets (similar on both surfaces), numerous non-vascular fiber bundles (23–31.8 μm−1) randomly distributed in the mesophyll and expansion tissue between the midrib and the mesophyll on the adaxial surface. By contrast, the tree-sized species group had hypostomatic leaflets, fewer non-vascular fiber bundles distributed in a pattern and expansion tissue between the midrib and the mesophyll on the abaxial surface.
The origins of the samples used in this work were different, with most coming from the living collections of two gardens in different environments. Additionally, when possible, samples were collected in native areas, which were also different types of environment. However, the anatomical variation found among the species was highly significant, which demonstrated that the traits that were examined were under genetic control with little influence of the environment.
Several papers published on the Acrocomia palms of Mato Grosso do Sul, Brazil, a region in which A. totai occurs, consider this species a synonym of A. aculeata (Pott and Pott 1994; Scariot 1998; Hiane et al. 2005; Ciconini et al. 2013). Additionally, based on work conducted on A. intumescens, this species, distributed only in some areas of northeastern Brazil, is also considered synonymous with A. aculeata. However, in addition to the previously recognized morphological differences, the data of leaflet anatomy supported the distinction of three species: A. aculeata, A. totai, and A. intumescens.
Based on the foregoing discussion, there are likely more species of Acrocomia than only the two accepted by Henderson et al. (1995), at least three more: A. crispa, A. intumescens and A. totai. The acaulescent species, A. emensis and A. hassleri, are apparently actually in the genus Acanthococos, because of the many morphological and anatomical differences. More taxonomic work is required on Acrocomia. Work especially on samples from various localities of Central America and islands of the Caribbean are needed to determine if any of the numerous species of Acrocomia described by Bailey (1858–1955), but later transferred to A. acuteata, are valid. In addition to classical taxonomy, other techniques must also be used, including studies of leaf anatomy, floral and fruit and pollen characterizations, and genetic diversity and phylogeny. In this study, seven species were differentiated, representing an important contribution to the taxonomic resolution of Acrocomia; however, we emphasize that more studies should be conducted on this genus, primarily, because of the great economic importance. Additionally, without a proper understanding of the different species and their geographical distributions, research conducted to improve the genus could harm the conservation of native populations of each species in their native habitat.
Taxonomic treatment
To identify the species of Acrocomia, complementing the morphological data, an identification key was constructed based on anatomical characters and graphical representations of the distribution of structures and tissues (Fig. 9).
Anatomical identification key for the species of Acrocomia Mart. in this study
-
1a
Amphistomatic leaflet; non-vascular fiber bundles distributed throughout the mesophyll … 2
-
1b
Hypostomatic leaflet; non-vascular fiber bundles distributed near one or both surfaces of the leaflets … 4
-
2a
Surface of the leaflets straight or smooth … A. emensis
-
2b
Surface of the leaflets undulate … 3
-
3a
Surface of the leaflets very undulate due to constrictions next to the primary vascular bundles; secondary vascular bundles distributed near the middle portion of the mesophyll; many stomata, often occurring in pairs … A. hassleri
-
3b
Surface of the leaflets only slightly undulate, secondary vascular bundles distributed closer to the abaxial surface; few stomata irregularly distributed … A. glaucescens
-
4a
Non-vascular fiber bundles distributed only near the adaxial surface; small non-vascular fiber strands absent … A. totai
-
4b
Non-vascular fiber bundles distributed only near abaxial surface; small non-vascular fiber strands, when present, immediately below the hypodermis … 5
-
5a
Small non-vascular fiber strands of non-vascular fibers beneath hypodermis of the adaxial surface … A. crispa
-
5b
Small non-vascular fiber strands of non-vascular fibers absent; non-vascular fiber bundles interspersed with 1–2 secondary vascular bundles … 6
-
6a
Margin round with no or only a small marginal vascular bundle; non-vascular fiber bundles interspersed by a single secondary vascular bundle … A. aculeata
-
6b
Margin squared with a large marginal vascular bundle; non-vascular fiber bundles interspersed with 1–2 secondary vascular bundles … A. intumescens
Conclusions
The seven species studied showed great variability at both morphological and anatomical levels, with significant differences in the organization of tissues and in the size and number of different structures in the leaflets. The small-sized species (A. emensis, A. hassleri and A. glaucescens) were amphistomatic and had non-vascular fiber bundles distributed throughout the mesophyll, whereas the tree-sized species (A. aculeata, A. crispa, A. intumescens and A. totai) were hypostomatic and had non-vascular fiber bundles close to one or two leaflet surfaces. Each species showed a particular organization of anatomical tissues, which was sufficient to differentiate among species. These unpublished results and discussions are a pioneer contribution to easily recognize the taxonomic differences among the species of Acrocomia, using the identification key and the graphic representations provided.
References
Adatia MH, Besford RT (1986) The effects of silicon on Cucumber plants grown in recirculating nutrient solution. Ann Bot (Oxford) 58:351–353
Amaral FP, Broetto F, Batistella CB, Jorge SMA (2011) Extração e caracterização qualitativa do óleo da polpa e amêndoas de frutos da Macaúba (Acrocomia aculeata (Jacq.) Lodd. ex Mart.) coletada na região de Botucatu, SP. Botucatu 26:12–20
Araújo KL, Silveira SF, Bianchini E, Medri ME, Gilio TAS, Miguens F (2013) Caracterização anatômica e histoquímica de folíolos de coqueiro. Revista Bras Ci Agrár 8:251–256. doi:10.5039/agraria.v8i2a2796
Barthlott W, Neinhuis C, Cutler D, Ditsch F, Meusel I, Theisen I, Wilhelmi H (1998) Classification and terminology of plant epicuticular waxes. Bot J Linn Soc 126:237–260
Bieras AC, Sajo MG (2004) Anatomia foliar de Erythroxylum P. Browne (Erythroxylaceae) do cerrado do estado de São Paulo, Brasil. Acta Bot Brasil 18:601–612
Castro LAS (2002) Processamento de amostras para Microscopia Eletrônica de Varredura. Embrapa Clima Temperado, Documentos 93
Chase MW, Soltis DE, Soltis OS, Rudall PJ, Fay ME, Hahn WH, Sullivan S, Joseph J, Molvray M, Kores PJ, Givnish T, Sytsma KJ, Pires C (2000) Higher-level systematics of the monocotyledons: an assessment of current knowledge and a new classification. In: Wilson KL, Morrison DA (eds) Monocots: systematics and evolution, vol 1. CSIRO, Melbourne. Avaliable at: https://www.researchgate.net/publication/255708721_Higherlevel_systematics_of_the_monocotyledons_an_assessment_of_current_knowledge_and_a_new_classification
Ciconini G, Favaro SP, Roscoe R, Miranda CHB, Tapeti CF, Miyahira MAM, Bearari L, Galvani F, Borsato AV, Colnago LA, Naka MH (2013) Biometry and oil contents of Acrocomia aculeata fruits from the Cerrados and Pantanal biomes in Mato Grosso do Sul, Brazil. Ind Crops Prod 45:208–214. doi:10.1016/j.indcrop.2012.12.008
Cutler DF (1978) Applied plant anatomy. Longman, London
Damasceno-Jr GA, Souza PR (2010) Sabores do Cerrado and Pantanal: Receitas e boas práticas de aproveitamento. Editora UFMS, Campo Grande
D’Arcy WG, Keating RC (1979) Anatomical support for the taxonomy of Calophyllum L. (Clusiaceae) in Panama. Ann Missouri Bot Gard 66:557–571
Dransfield J, Uhl NW, Asmussen CB, Baker WJ, Harley MM, Lewis CE (2008) Genera Palmarum: The evolution and classification of palms. Royal Botanic Gardens, Kew
Franceschi VR, Horner HT Jr (1980) Calcium oxalate crystals in plants. Bot Rev 56:361–527
Franceschi VR, Nakata PA (2005) Calcium oxalate in plants: formation and function. Annual Rev Pl Biol 56:51–71. doi:10.1146/annurev.arplant.56.032604.144106
Glassman SF (1972) Systematic studies in the leaf anatomy of palm genus Syagrus. Amer J Bot 59:775–788
Govaerts R, Dransfield J, Zona SF, Hodel DR, Henderson A (2016) World checklist of arecaceae. Royal Botanic Gardens, Kew. Available at: http://apps.kew.org/wcsp/
Guevara L, Stauffer FW, Jauregui D (2011) Anatomía comparativa de la lámina foliar y sistemática en la subtribu neotropical Mauritiinae (Arecaceae, Calamoideae). Brittonia 63:379–395. doi:10.1007/s12228-010-9176-7
Hammer O, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics Software Package for education and data analysis. Palaeontol Electronica 4:1–9
Hanberlandt G (1925) Physiological plant anatomy. Today and Tomorrow`s, Delhi
Henderson FM (2006) Morphology and anatomy of palm seedlings. Bot Rev 72:273–329. doi:10.1663/0006-8101(2006)72[273:MAAOPS]2.0.CO;2
Henderson F (2013) Leaf anatomy of the genus Leopoldinia (Arecaceae). J Torrey Bot Soc 140:369–372. doi:10.3159/TORREY-D-12-00027.1
Henderson A, Galeano G, Bernal R (1995) Field guide to the Palms of the Americas. Princeton University Press, New Jersey
Hiane PA, Filho MMR, Ramos MIL, Macedo MLR (2005) Óleo da polpa e amêndoa de bocaiúva, Acrocomia aculeata (Jacq.) Lodd. ex Mart. caracterização e composição em ácido graxos. Braz J Food Technol 8:256–259
Horridge GA, Tamm SL (1969) Critical point drying for scanning electron microscopy study of ciliary motion. Science 163:817–818
Janick J, Paull RE (2008) The encyclopedia of fruit and nuts. CAB International, London
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book Company, New York
Kikuchi TYP, Potiguara RCV, Santos PP (2007) Caracterização histoquímica e ultra-estrutural do estipe de Socratea exorrhiza (Mart.) H. Wendl. (Arecaceae). Bol Mus Paraense “Emilio Goeldi” 2:61–68
Lorenzi H, Kahn F, Noblick LR, Ferreira E (2010) Flora Brasileira: Arecaceae (Palmeiras). Instituto Plantarum, Nova Odessa
Mantovani A, Gomes M, Gomes DMS, Vieira RC (1995) Anatomia foliar de Rudgea decipiens Müll. Arg. (Rubiaceae) e R. macrophylla Benth. (Rubiaceae). Acta Bot Brasil 9:247–261
Mantovani A, Filartiga ALP, Coelho MAN (2010) Anatomia comparada da folha e espata de espécies de Anthurium (Araceae) ocorrentes na Mata Atlântica. Revista Brasil Bot 33:185–200. doi:10.1590/S0100-84042010000100016
Metcalfe CR, Chalk L (1979) Anatomy of the dicotyledons, vol. 1, 2nd edn. Claredon Press, Oxford
Metcalfe CR, Chalk L (1983) Anatomy of the dicotyledons: Wood structure and conclusion of the general introduction, 2nd edn. Claredon Press, Oxford
Millán B, Kahn F (2010) Characterization of leaf anatomy in species of Astrocaryum and Hexopetion (Arecaceae). Revista Peru Biol 17:81–94
Moller JD, Rasmussen FLS (1985) Stegmata in Orchidales: character state distribution and polarity. Bot J Linn Soc 89:53–76
Morcote-Ríos G, Bernal R (2001) Remains of palms (Palmae) at archaeological sites in the new world: a review. Bot Rev 67:309–350
Noblick LR (2013) Leaflet anatomy verifies relationships within Syagrus (Arecaceae) and aids in identification. PhytoKeys 26:75–99. doi:10.3897/phytokeys.26.5436
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373. doi:10.1007/BF01248568
Parthasaranthy MV (1968) Observation on metaphloem in the vegetative parts of palms. Amer J Bot 55:1140–1168
Paviani TI (1972) Estudos morfológico e anatômico de Brasilia sickii G.M. Barroso. Revista Brasil Biol 32:551–572
Pott A, Pott VJ (1994) Plantas do Pantanal. Embrapa, Corumbá
Salis SM, Mattos PP (2009) Floração e Frutificação da Bocaiúva (Acrocomia aculeata) e do Carandá (Copernicia alba) no Pantanal. Embrapa, Corumbá. Available at: http://www.cpap.embrapa.br/publicacoes/online/COT78.pdf
Sangster AG (1977) Characteristics of the silica deposition in Digitaria sanguinalis (L.) Scop. (Crabgrass). Ann Bot (Oxford) 51:350–351
Sant’Anna-Santos BF, Carvalho-Júnior WGO, Amaral VB (2015) Butia capitata (Mart.) Becc. lamina anatomy as a tool for taxonomic distinction from B. odorata (Barb. Rodr.) Noblick comb. nov (Arecaceae). Anais Acad Brasil Ci 87:71–81. doi:10.1590/0001-3765201520130457
Scariot A (1998) Seed dispersal and predation of the palm Acrocomia aculeata. Principes 42:5–8
Scatena VL, Vich DV, Parra LR (2004) Anatomia de escapos, folhas e brácteas de Syngonanthus sect. Eulepis (Bong. ex Koern.) Ruhland (Eriocaulaceae). Acta Bot Brasil 18:825–837
Silva RJF, Potiguara RCV (2008) Aplicações taxonômicas da anatomia foliar de espécies amazônicas de Oneocarpus Mart. (Arecaceae). Acta Bot Brasil 22:999–1014
Silva RJF, Potiguara RCV (2009) Substâncias ergásticas foliares de espécies amazônicas de Oenocarpus Mart. (Arecaceae): caracterização histoquímica e ultra-estrutural. Acta Amazonica 39:793–798. doi:10.1590/S0044-59672009000400007
Stace CA (1965) Cuticular studies as an aid to plant anatomy. Bull Brit Mus Nat Hist Bot 4:1–83
Tomlinson PB (1961) Anatomy of the monocotyledons-II. Palmae. Oxford University Press, New York
Tomlinson PB, Horn JW, Fisher JB (2011) The anatomy of palms, Arecaceae—Palmae. Oxford University Press, New York
Valentin JL (2000) Ecologia numérica: uma introdução à análise multivariada de dados ecológicos. Editora Interciência, Rio de Janeiro
Vianna SA, Pott A, Silva RH, Moura EB, Maranhão HL, Silva NDC, Borsato AV (2013) Phenotypical characterization of Acrocomia aculeata fruits in natural populations in the Pantanal region, Mato Grosso do Sul, Brazil. Acta Hort 1003:169–172. doi:10.17660/ActaHortic.2013.1003.23
Vieira RC, Gomes DMS (1995) Superfície da lâmina foliar de Psychotria nuda (Cham. and Schltdl.) Wawra, P. leiocarpa Cham. and Schltdl., P. stenocalyx Müll. Arg. e P. tenuinervis Müll. Arg. (Rubiaceae). Acta Bot Brasil 9:263–270
Acknowledgements
We acknowledge the Fundação de Apoio à Pesquisa do Estado de São Paulo (FAPESP) for funding the project and the scholarship of the first author. We thank the Instituto Plantarum for the collection of botanical material in their garden, the Montgomery Botanical Center for financial and technical support, permission to collect from their living collection of palms and for use of their garden facilities. To the Fairchild Tropical Botanic Garden (FTG) for the use of their plant anatomy laboratory and for samples of their herbarium specimens. We thank the Universidade Estadual de Campinas (UNICAMP) for the use of their laboratory and Dr. Luis Carlos Bernacci (Herbarium IAC) for assistance in the preparation of the species identification key.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Jürg Schönenberger.
Rights and permissions
About this article
Cite this article
Vianna, S.A., Carmelo-Guerreiro, S.M., Noblick, L.R. et al. Leaf anatomy of Acrocomia (Arecaceae): an additional contribution to the taxonomic resolution of a genus with great economic potential. Plant Syst Evol 303, 233–248 (2017). https://doi.org/10.1007/s00606-016-1369-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-016-1369-4