Abstract
Planted Eucalyptus forests are the largest potential energy source in Brazil. They supply energy for several industrial sectors, so a detailed quality assessment of this biomass is required. Although many important parameters have been evaluated, few studies have considered the effect of lignin composition in firewood, as the amount of syringyl (S) and guaiacyl (G) units on thermogravimetric characteristics. The aim of this research was to evaluate the energetic characteristics of Eucalyptus clones and identify the effect of chemical composition and lignin quality on the resistance to thermal degradation of firewood. The study was performed with 14 clones of Eucalyptus from a planted forest. Plant material was analyzed for energy productivity, heating value, ash content, proximate and elemental compositions, and thermal characteristics. Methods to determine chemical composition were proximate analysis, soluble extractives in acetone and quantification of lignin contents. Chemical composition and the quantity of G units influenced the combustion performance. The soluble extractives in acetone and the higher proportion of G units in lignin resulted in an increase in thermal stability and prolonged the combustion time. The mass of the structural unit G per kilogram of dry wood is a good parameter to classify Eucalyptus clones for heat generation. G units ranged from 19.61 to 25.68 g kg−1 (dry wood). The clone 1037 (Eucalyptus sp.) had superior combustion performance and the highest energetic productivity (2921.61 MJ tree−1) of the 14 clones studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The requirement for reducing fossil fuel usage, especially petroleum, natural gas, and coal, has been discussed by Moya and Tenorio [1] and Gonçalves et al. [2], and the replacement with renewable energy sources has been suggested by Benavente and Fullana [3] and Mehmood et al. [4]. Production of energy from wood species with short rotation is highlighted in Gonçalves et al. [2]. Wood may be a good alternative to replace the energy generated by fossil fuels since it would emit less greenhouse gases upon combustion [5].
The internal energy supply within Brazil consists of 42.9% renewable sources (about the equivalent of 125,327,000 tons oil), of which firewood and charcoal corresponds to 8.0% [6]. The numerous species and clones of Eucalyptus and Corymbia are important potential sources of firewood. In order to increase the quality and quantity of the firewood produced, it is necessary to obtain clonal plantations of Eucalyptus and Corymbia with higher productivity, developmental uniformity, and satisfactory energetic characteristics. Understanding the thermogravimetric behavior of wood during the combustion process is needed so that industries can make full use of the wood [1].
Wood used as an energy source needs to be evaluated for its performance during combustion in order to determine its suitability for bioenergy production [7]. Knowing how plant growth conditions, tree age, and chemical composition effect wood combustibility in conjunction with more efficient conversion technologies will help to better utilize wood as fuel [1, 4, 7]. The most important parameters to evaluate include heating value, moisture content, bulk density, ash content, and chemical composition [1, 2, 7,8,9].
Extractives and lignin are the chemical components of the lignocellulosic materials which influence the combustion process [10,11,12,13,14]. Higher extractive contents may facilitate the wood combustibility at lower temperatures as they show high volatility, which consequently, accelerate the process of thermal degradation [8, 11]. Additionally, lignin chemistry plays a major role in thermal degradation. The lignin monomers, p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S), have different numbers of active sites in the phenylpropane due to variable methoxyl groups (–O–CH3) [15, 16]. The G unit has one less methoxyl group on the aromatic ring than the S unit, and this allows the occurrence of more stable and thermally resistant chemical bonds [15,16,17]. Lignin with more methoxyl groups produces less charcoal during the pyrolysis process, presenting less resistance to thermal degradation [16]. Lignin with greater proportions of G units may be more resistant to thermal degradation, and it may influence the thermogravimetric characteristics. Hardwood and softwood lignin macromolecules differ depending on the relative abundance of three basic units; lignin can be classified as type-G (softwood lignin), type-G-S (hardwood lignin) and type-H-G-S (grass lignin) [16]. The S/G ratio of lignin in Eucalyptus wood ranges from 2.6 to 3.3 [18].
Studies on the quantity of S and G units in Eucalyptus lignin with respect to the resistance of thermal degradation and combustibility are limited. Combustion and thermal stability of fast-growth species were affected by moisture content [13], but other factors may also have some degree of relationship with these processes such as extractives, porosity, and some chemical characteristics. The aim of this research was to evaluate the energetic characteristics of Eucalyptus clone wood from homogeneous plantations in Brazil, to identify the effect of the chemical composition and lignin quality on the resistance to thermal degradation, and to assess how these chemical parameters influence thermogravimetric characteristics of the firewood.
Material and Methods
Material Sampling
Samples were taken for evaluation from three trees of each of 14 Eucalyptus spp. clones (81 months; Table 1). The plantation was located in Curvelo, State of Minas Gerais, Brazil (spacing 3 × 3 m; Fig. 1). Individual tree volume was calculated using the Smalian method. Discs (2.5 cm thick) were obtained from the following five longitudinal positions: 2, 10, 30, 50, and 75% of the commercial height of the tree, considering a minimum commercial diameter of 4.0 cm with bark. All five longitudinal samples were assessed for the physical, chemical, and thermal characteristics.
Physical and Chemical Characterization of the Wood
The wood basic density was determined according to the ABNT NBR 11941 [19] standard. The higher heating value (HHV) (dry mass basis) was determined by a digital calorimeter IKA C-200® developed by LabControl Scientific Instruments (São Paulo, Brazil) according to the E711-87 [20] standard. The materials were dried at room temperature and ground into sawdust by a Wiley mill. Two sieves of 40 (0.420 mm) and 60 (0.250 mm) mesh were used to retain the materials used in this analysis. The lower heating value (LHV) (dry mass basis) was calculated according to Eq. 1:
where LHV is the lower heating value (kJ kg−1); HHV is the higher heating value (kJ kg−1); and H is the hydrogen amount (%).
The unitary energetic density was calculated by multiplying the mean basic density by HHV by LHV. The estimated dry mass of wood per tree was obtained by multiplying the individual tree volume by the mean basic density. The possible energy produced per tree via combustion (heat energy expressed as MJ tree−1) on a dry weight basis was calculated by the multiplication of the parameters individual dry mass and LHV.
The quantification of carbon (C), hydrogen (H), nitrogen (N), and sulfur (S) was performed in an Elementar Universal Analyzer developed by Biovera (Rio de Janeiro, Brazil). The oxygen percentage was calculated by the subtraction of C, H, N, S, and ash percentages from 100 [O (%) = 100 − C (%) − H (%) − N (%) − S (%) − Ash (%)]. The fraction of sample which was retained between the sieves of 60 (0.250 mm) and 200 mesh (0.074 mm) was used in this analysis. The ratios O/C, H/C, N/C, and the empirical formulas of the studied woods were calculated according to Pereira et al. [15]. Additionally, the molecular chemical characterization of the wood was performed (Table 2).
Soluble and insoluble lignin contents (Klason lignin) were determined using methods from Goldschimid [24] and Gomide and Demuner [23] respectively. Briefly, two acid hydrolysis reactions were initiated in order to extract lignin. First, 3 ml of 72% sulfuric acid were mixed with 0.3 g of sawdust and were left to react for 1 h at 30 °C. Next, the mixture was diluted to 3% and allowed to react for 1 h at 2 atm and 121 °C. The mass of insoluble lignin was determined by filtration, and the soluble lignin content was determined by UV spectroscopy. The Klason lignin content was obtained from the ratio between the insoluble lignin mass and the dry mass of the sample free of the extractives. Total lignin was obtained by the sum of insoluble and soluble lignin.
The quantification of the syringyl and guaiacyl units was performed by alkaline oxidation of wood with nitrobenzene followed by high efficiency liquid chromatography (HELC) according to Lin and Dence [25] with some adaptations described in Araújo et al. [26]. The sawdust fraction retained in 40 (0.420 mm) and 60 (0.250 mm) mesh were used. The estimates of the syringaldehyde mass (representative molecule of the syringyl unit—S) and vanillin (representative molecule of the guaiacyl unit—G) were performed according to the Eq. 2:
where U is the mass of the structural unit (S or G) per kg of dry wood, C is the concentration of the referred structural unit in the analytical solution (mol L−1), Vf is the final volume of the analytical solution (L), M is the molar mass of the syringaldehyde or vanillin compound (g mol−1), and Md is the wood dry mass (kg) used for the oxidation.
Thermal Characterization of the Wood
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were performed with samples from all longitudinal positions of the tree. Particles used for this analysis were sieved through 200 mesh (0.074 mm). The TGA was performed in an atmosphere of oxygen using the automatic thermal analyzer DTG-60H developed by SHIMADZU (Barueri, Brazil). The flow was 50 ml min−1, and the temperature ranged from room temperature (room temperature was 20 to 35 °C) to 550 °C, with a heating rate of 5 °C min−1. The graph of first derivative of the TGA curve allowed the identification of the mass loss rate per minute and the combustion stages. This graph was then used to relate these parameters with the chemical composition of the wood.
The following parameters were used to evaluate the combustion of the wood: the ignition temperature (Ti), the burnout temperature (TB), the combustion index (S), the ignition index (Di), the time corresponding to the maximum combustion rate (tm), the time of ignition (tig), the maximum rate of combustion, and the mean rate of combustion. The ignition and burnout temperatures were suggested by Sahu et al. [27], Wang et al. [28], Wang et al. [29], and Moon et al. [30]. The ignition temperature was defined as the temperature at which the combustion rate increases 1% min-1; this indicates the starting point of the main combustion process. The burnout temperature was defined as the temperature at which the combustion rate decreases by 1% min−1; this designates the end of the combustion process. The combustion index (S) was obtained by Eq. 3, cited by Moon et al. [30], Qian et al. [31], and Liu et al. [32]. The ignition index (Di) was obtained by Eq. 4 and was determined by Xiang-Guo et al. [33].
where (dm/dt)max is the maximum combustion rate (% min−1), (dm/dt)me is the mean combustion rate, Ti is the ignition temperature (°C), and TB is the burnout temperature (°C).
where (dm/dt)max is the maximum combustion rate (% min−1), tm is the time corresponding to the maximum combustion rate (min), and tig is the ignition time (min).
The DSC was performed in a differential scanning calorimeter model DSC-60 developed by Shimadzu (Barueri, Brazil). Analysis was performed using the same heating rate, initial and final temperature, and oxygen flow from the TGA analysis. Particles used for this analysis were sieved through 200 mesh (0.074 mm).
Statistical Analysis
Analysis of variance was used to evaluate the physical-chemical and thermal characteristics of the wood. Differences between clones were determined using Scott-Knott test (p ≤ 0.05). Pearson linear correlations were established between the chemical characteristics and the combustion profile of the studied woods. A t test was performed at 5% and 10% significance levels to determine correlations between wood properties and thermal characteristics.
Results and Discussion
Combustion Energy and Chemical Composition of the Wood
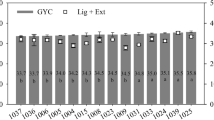
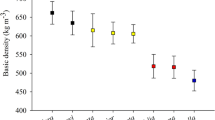
The unitary energetic density of wood (Fig. 2), which was calculated by multiplying the basic density by the heating value, varied significantly among the clones. Clones 1025 (E. camaldulensis hybrid), 1039 (E. grandis hybrid), and 1009 (E. urophylla) had highest unitary energetic density values of those studied; this result may be due to the higher values of wood basic density of these clones (Table 3). The clone 1004 (E. urophylla x E. camaldulensis hybrid) had a significantly lower value in comparison to the other previously mentioned clones, and it is a member of the group of clones with the highest basic density. Generally, the clones coming from crosses with E. camaldulensis present a higher wood density and a higher unitary energetic density, as observed with the clone 1025.
Castro et al. [34] observed that in 81-month-old clones of E. camaldulensis, E. grandis, and E. urophylla, high values of energetic density obtained the best ranking, forming a single group, confirming the results found in this work. These results highlight the importance of studying basic density, heating value, and unitary energetic density for classification and selection of superior genetic material for generating heat energy.
Transportation cost, in terms of energy released upon combustion, is lower when the wood density is higher. Moreover, energy conversion equipment, such as ovens and boilers burn more efficiently with higher density materials. The improved cost of transportation and equipment use efficiency is valid for species/clones with similar values of HHV/LHV, that were observed in this study, since in these cases, the unitary energetic density will only depends on the wood density.
Other considerations beyond transportation costs, such as energy productivity (individual or per area), should also be considered for the genetic breeding program of Eucalyptus for clones with enhanced energetic characteristics. Clone 1037 of Eucalyptus sp. produced the highest amount of heat energy upon combustion. This could be explained by the individual dry mass (161.28 kg tree−1), the greatest DBH (19.6 cm), and highest total height (25.1 m) of clone 1037 (Fig. 3). Greater diameter and total height were related to higher dry mass of trees. Clone 1037 produced 94% higher heat energy in comparison with clone 1023 (E. urophylla hybrid), 1025 (E. camaldulensis hybrid), 1036 (E. urophylla), and 1024 (E. urophylla hybrid), which together formed a group with the lowest heat energy (Table 3). Clones 1039 and 1009 were classified in the third lowest of four group based on heat energy per tree.
Results of the heat energy (MJ tree−1) disqualify the clones 1039, 1009, and 1025 for generation of thermal energy and highlight the need to consider the energetic productivity as a more predictive parameter for heat generation. Considering the energy productivity, only the clones 1037, 1033, 1031, and 1006 are viable for firewood production. No significant effect of clone was found for the heating values. The mean values were 19.40 MJ kg−1 (HHV) and 18.00 MJ kg−1 (LHV), which are similar to the reported results in the literature for Eucalyptus clones [15, 17]. These results may be explained by the elemental chemical composition of the Eucalyptus clones (Table 4). The results show that the productivity of the individual dry mass is the most important energetic parameter, since no significant effect of clone was found for elemental chemical composition and heating value (HHV and LHV).
There was no clonal effect for C, H, O, and N wood content. Thus, the empirical formula of the different clones was similar. It is known that the wood combustion heat is directly related to the C and H contents which show combustible characteristics. On the other hand, high proportions of O and N result in a decrease of the calorific value, i.e., LHV or HHV [9, 35]. S does not affect the energetic characteristics of the wood but was evaluated in this study. Only traces of it were detected (up to 0.07%).
The elemental chemical composition of Eucalyptus wood does not vary among clones, sites, and ages. The mean values reported in the literature ranged from 44 to 47% C, 5.6 to 6.0% H, 45 to 50% O, 0.10 to 0.12% N, and 0.003 to 0.009% S [15], which are close to the values observed in this study. Thus, Eucalyptus wood could be less polluting than coal due to low content of N and S [36]. Based on these results, the heating value and the elemental chemical composition might not be suitable parameters for selection of Eucalyptus clones for heat generation. However, they are required to estimate the energy stored in the tree and the energy densities.
For total lignin content, a significant effect of clones was observed (Table 5). The clones 1039, 1025, 1008, 1033, 1006, 1009, and 1005 were similar and formed the group highlighted in the table with the highest mean percentage of lignin (31%). With exception of the clone 1005, the other members of the group showed the statistically lowest values for S/G ratio, which is a favorable property for the energetic application of the wood, especially for charcoal production. It is necessary to confirm the effect of S/G ratio on the combustibility of wood. Therefore, the differences in the amount and composition of the lignin macromolecule may influence the wood combustion as well as the duration of the different combustion stages.
The correlation of proximate composition is modified by the relative proportions of holocellulose, lignin, and extractives, and it influences wood combustion [37]. A higher ratio of volatile matter/fixed carbon results in faster oxidation, resulting in higher combustion intensity and biomass combustibility [30, 38, 39]. It is expected that clones 1039 (E. grandis hybrid), 1025 (E. camaldulensis hybrid), 1033 (E. urophylla hybrid), and 1006 (E. urophylla) have lower ratios of volatile matter/fixed carbon, and thus burn more slowly with higher ignition temperatures in comparison with the other selected Eucalyptus clones. These clones had more lignin, low S/G ratio, and more G units per kg of wood.
Regarding the estimate of G units per kg of dry wood, clones 1039, 1025, 1033, 1006, 1036, 1004, and 1031 were not different and had a mean value of 25.62 g G units per kg of dry wood. Araújo et al. [26] reported a mean value of 21.96 g of G per kg of dry wood. This difference between the findings of Araújo et al. [26] and this research may be due to younger clones used in their study (72 vs 81 months). Age is inversely related to the S/G ratio which may be due to an increase of the G units in the juvenile wood of Eucalyptus clones [17, 34].
For the content of soluble extractives in acetone, there was significant variability among clones which formed four distinct groups. Clones 1039 (E. grandis hybrid), 1036 (E. urophylla) and 1037 (Eucalyptus sp.) were highlighted with higher mean values (Table 5). The mean value observed for this group (2.5%) differs from that reported by Gouvêa et al. [40] for a hybrid E. grandis x E. urophylla (3-year old) and had a soluble extractive mean value of 1.75%. This may be due to the distinct ages of the clones, since the extractive content tends to increase with age [17]. Depending on the extractives thermoresistance, higher proportions may result in higher thermal stability [13, 40].
The clones 1033, 1009, 1006, 1036, 1005, 1025, and 1039, highlighted in Table 6, had lower levels of volatile matter and higher levels of fixed carbon; this is probably associated with the more lignin, low S/G ratio, and more G units per kg of wood (Table 5). The clones 1036 and 1039 also had high levels of soluble extractives in acetone compared to all other clones. Clones 1009 and 1005, which belong to the group with the highest proportions of fixed carbon (Table 6), also have higher total lignin (Table 5) than the other clones. The clones 1036 and 1004 had low S/G ratios and high levels of G units per kg of dry wood. The clone 1036 was highlighted by the amount of soluble extractives in acetone. The reactivity of the lignin linkages is influenced by the functional groups (phenolic hydroxyl group and methoxyl group). Furthermore, the lignin with higher levels of methoxyl groups (high S/G ratio and lower G units per kg of dry wood) produces less charcoal during the pyrolysis process [16], which implies less resistance to thermal degradation and lower fixed carbon content.
Seventy-one percent of the Eucalyptus clones with high levels of fixed carbon were also in the group with the highest estimate of G units per kg of dry wood. Clone 1008 (E. urophylla) was classified in the group with higher total lignin content and lower S/G ratio; however, it had a higher proportion of volatile matter/fixed carbon. This was assumed to be due to fewer G units per kg of wood and less extractives. The clone 1024, despite less lignin and higher S/G ratio, had intermediate amounts of fixed carbon and lower volatile matter. Wood is a heterogeneous material, and this may explain the results obtained, for example, extractives composition and differences in the cellulose crystallinity and crystallite size. According to Poletto et al. [11], more extractives are associated with less crystallinity; lower cellulose crystallite size can accelerate the degradation process and reduce the wood thermal stability. This may be related to the formation of volatile matter during the thermal decomposition process.
Wood composed of high amounts of lignin and low proportions of S/G units may contribute to the resistance to thermal degradation which increases the amount of fixed carbon or charcoal during thermal degradation [41]. In this context, it is expected that Eucalyptus clones 1033, 1009, 1006, 1036, 1005, 1025, and 1039 will lose less mass in the initial stages of combustion and reduce the maximum ratio of mass loss, thus the residence time will increase in the energy conversion equipment as a result of the low volatile matter to fixed carbon ratio [37].
In comparison to the other lignocellulosic materials, such as rice husk [37], Eucalyptus wood has low ash content, lower than 0.5%, and better combustibility [15]. Therefore, the ash values found in this work did not harm the energetic use of Eucalyptus wood even though a clonal effect was observed.
Thermogravimetric Analysis
The TGA and DTG curves (Figs. 4 and 5) suggest that wood combustion occurs in two main stages, similar to the results reported by Li et al. [42] and Fernandes et al. [38] for lignocellulosic materials. The slope in TGA curves indicates a significant mass loss due to bond dissociation and decomposition [43]. The different stages of combustion (Table 7) may be attributed to the substantial differences in the thermal behavior of the molecular constituents of lignocellulosic biomass [12], mainly due to the structures and chemical bonds which occur in cellulose, hemicelluloses, lignin, and extractives.
The derivative of the TGA curve (DTG) exhibits two thermal decomposition peaks and allows the division of the characteristic stages of wood oxidation. The first phase ranged from approximately 200 to 360 °C and is attributed to the thermal degradation of the main molecular constituents of wood (hemicelluloses, cellulose, and part of the lignin), resulting in the emission, ignition, and homogeneous combustion of the volatile matter [12, 38, 44, 45]. Lignocellulosic biomass is predominantly composed of carbohydrates resulting in low resistance to thermal degradation and in an accentuated rate of mass loss. In contrast, the thermal degradation of lignin occurs in a large range compared with cellulose [16].
The xylans constitute the main fraction of the hemicelluloses of Eucalyptus wood [16] and are the less thermally stable components of biomass compared with cellulose and lignin [12]. These molecules start degrading at 187 °C with the maximum mass loss occurring at 264 °C [12], which is similar to results in this study (Fig. 5). According to the mentioned authors, the thermal decomposition of cellulose starts at 266 °C and has maximum mass loss ratio at 354 °C. In this context, it can be inferred that high levels of holocellulose promotes greater volatilization and increases the rate of wood thermal decomposition at lower temperatures. Consequently, this increases the combustion intensity due to the greater release of volatile matter. López-González et al. [12] observed a maximum degradation peak for Eucalyptus wood at 290 °C which differs from that found in this study. It should be noted that the authors used a synthetic air atmosphere and a 10 °C min−1 heating ratio. The authors attributed the maximum mass loss to the thermal degradation of the cellulose molecule.
A significant clonal effect was found for mass loss in the first stage of combustion. Clones 1004, 1009, 1025, 1005, 1033, 1036, 1024, 1006, and 1039, highlighted in Table 7, had the lowest mean values. This fact may be attributed to the lower volatile matter/fixed carbon ratio found for these clones and was likely influenced by the molecular chemical composition. Clones 1008, 1015, 1037, 1023, and 1031 were considered similar and had more mass loss in the first combustion stage (mean value of 75.5%; Table 7). These clones had less lignin among those studied. Clone 1008, despite higher total lignin, had fewer G units per kg of dry wood (Table 5).
The second phase of Eucalyptus wood combustion occurred approximately between 360 and 460 °C (Figs. 4 and 5) due to the thermal decomposition of the remaining lignin and the fixed carbon formed during the first stage [30]. The combustion of lignin occurs in a wide thermal range (152 to 700 °C), but the mass loss at low temperatures is minor [12]. López-González et al. [12] observed two peaks at 397 °C and 518 °C in the lignin DTG curve, and they noted that the lignin is the component with higher thermal stability and contributes mainly to the energy release in the second combustion stage since it has a positive correlation with solid carbon or charcoal [10, 46].
Hardwood lignin contains both G and S units, therefore lignin of hardwood species can be classified as type-G-S [47]. These units form the lignin matrix through various linkages and different functional groups attach to the propyl side chain leading to extremely complicated lignin structures [16]. It results in low mass loss in the later combustion phases due to slow combustion of fixed carbon which is characterized by the incandescent burning of the fuel [13]. The solid carbon is strongly bound to other carbon atoms by single or double bonds which have higher chemical bonding enthalpy in comparison to the C–O bond, for example [48]. The combustion reaction between the O and the fixed carbon is complex, slow, and heterogeneous [49]. In addition, the content of methoxyl groups in lignin is correlated with the lignin pyrolysis and charcoal production [16], or fixed carbon. Therefore, lignin with high levels of methoxyl groups, i.e., high S and low G units, produce less charcoal during the pyrolysis process [16].
During the second phase of combustion, clone 1039 lost the most mass while clones 1006, 1024, 1036, 1033, 1025, and 1009 lost the second greatest amount of mass. These results may be due to the content and composition of the lignin macromolecule. Specifically, the clones with greater mass loss had higher contents of soluble extractives in acetone (during the second phase of combustion), which demonstrates the greater resistance to thermal degradation of these molecules. In addition, the clones 1039, 1006, 1024, 1036, 1033, 1025, and 1009 had more fixed carbon in relation to volatile matter; this is important as the second stage of combustion is characterized by the burning of solid carbon.
Clones 1008, 1015, 1037, 1023, and 1031 were characterized by having higher mass loss in the first combustion stage but had a low thermal decomposition ratio in the second stage. The wood of these clones presented a high proportion of volatile matter, which is oxidized in the initial phase of combustion. In the second combustion stage, the temperature of maximum mass loss for clones 1039, 1033, and 1024 was higher and was highlighted by low percentages of ash with the exception of 1039 (Table 6). The clones 1004 and 1005, with low peak temperatures in the second stage, had high ash content. These results indicate that wood with high ash content may have greater energetic losses from heating the mineral oxides resulting in a low peak temperature in the second stage of wood oxidation.
Regarding the ignition temperature (Ti), a significant clonal effect was found, in which the clones 1006 (E. urophylla), 1024 (E. urophylla hybrid), and 1005 (E. urophylla) had low mean values (Table 8). Low Ti suggests that the wood may burn more easily [44].
The volatile matter of the biomass is a complex mixture of gases, such as: H2, CO2, CO, CH4, hydrocarbons, and water vapor which are produced from the thermal decomposition of lignin, cellulose, hemicelluloses, and extractives [46, 50]. The devolatilization of lignocellulosic biomass occurs at low temperatures, which indicates that these materials are more easily ignited. The high volatile matter/fixed carbon ratio is related to fuel reactivity [39]. In this sense, high levels of volatile matter in relation to the fixed carbon results in a decrease of the biomass ignition temperature [37]. However, despite the statistical differences in the proximate composition of the Eucalyptus clones, there was no relationship between the volatile matter, the temperature and the ignition time.
Clones 1008, 1031, 1015, 1023, and 1037, with high levels of volatile matter (Table 6) did not have low ignition temperatures. All clones had practically the same ignition time (Table 8). Volatile matter percentage between the clones mentioned above ranged from 83.1 to 84.0%, and so there may not have been enough variability to observe the impact of volatile matter on the time to ignition. Sahu et al. [27] observed an ignition temperature of 243.0 °C for wood sawdust and 251.7 °C for rice husk, which differs considerably from the ignition temperature of the Eucalyptus clones from this study which was on average 236.1 °C. The sawdust and rice husk analyzed by the authors had volatile matter percentage of 69.5% and 58.6%, respectively, justifying the higher ignition temperatures in comparison with the wood of the studied clones.
Burnout temperatures varied among clones with the highest values for clones 1033, 1025, 1039, and 1024 (Table 8). In a similar study, Tenorio and Moya [13] reported higher burnout temperature corresponded with high concentration of soluble extractives in acetone stating that the extractives may increase wood thermal stability and extend the time of combustion. But the present study shows mixed results with respect to this relationship. For example, clones 1025, 1039, and 1024 (three of the four with the highest burnout temperatures) had higher levels of extractives in general (2.12, 2.60, and 1.85% respectively) whereas clone 1005 had one of the lowest extractive levels (0.82%) and the lowest burnout temperature of 433.0 °C. However, this relationship breaks down when considering clones 1033 and 1006 which did not have different extractive concentrations, yet 1033 had a significantly higher burnout temperature than 1006 (443.2 vs 435.0 °C). The burnout range for this study was 433.0–445.7 °C, whereas the range for the Tenorio and Moya [13] study was 365 °C for the Bombacopsis quinata to 400 °C for the Vochysia guatemalensis wood.
Combustion rate (mean 0.877 ± 0.02% min−1) and time of maximum mass loss (mean 52 ± 0.7 min) were not affected by clone so these are inappropriate characteristics for classification and selection of Eucalyptus clones for generation of heat energy; however, the maximum rate of mass loss and the combustion index are important to be quantified. For the maximum rate of mass loss and the combustion index, clones 1008, 1015, 1033, 1031, 1023, and 1037 had higher values and formed one of two distinct groups. These clones had a higher volatile matter/fixed carbon ratio; higher ratios result in higher combustion intensity [37, 39, 51] and greater mass loss on the initial combustion stage. The higher quantity and the fast emission of volatile matter are factors which contribute to acceleration of fuel ignition [30].
Clones 1008, 1037, 1033, 1015, 1031, and 1023 had a higher ignition index compared with all other clones (Table 8) which reflects a shorter time of the biomass to begin burning. Combustion index expresses the wood combustion reactivity. Higher combustion index values represent better fuel performance when burned [31, 52]. The lignocellulosic biomass with higher ignition index starts burning earlier than the other clones [27, 31]. These results reinforce the importance of volatile matter (the gases generated from the biomolecules thermal decomposition and compose the lignocellulosic biomass). These gases mix with O in the air and promote the homogeneous combustion reactions resulting in higher combustion intensity and wood flammability at lower temperature and less time [44]. In addition, among the studied clones, 1037 of Eucalyptus sp. had higher energetic productivity, and it belonged to the group with the best combustion performance and had earlier burning start expressed by the ignition index. Therefore, clone 1037 is recommended for heat generation or bioelectricity production in Brazil.
The lignin, soluble extractives in acetone, and G units per kg of dry wood had negative and positive correlations with the mass loss on the first and second combustion stages, respectively (Table 9). The extractives also had positive correlations with the temperature of maximum mass loss in the second combustion stage.
More extractives may increase the flammability of the wood at lower temperatures as a result of their intense volatility and consequently accelerate the thermal degradation process [8, 11, 14]. However, in this research, the extractives contributed to an increase of thermal stability with pronounced burning in the second stage of combustion (fixed carbon oxidation). The extractives are heterogeneous chemicals so depending on their chemical composition and thermoresistance, higher levels of extractives may result in higher thermal stability of the wood [13, 40]. The soluble extractives in hot water positively correlated with the temperature corresponding to the end of the combustion and with the remaining mass after the maximum thermal decomposition [13].
The molecular chemical composition of the wood, as well as lignin, influenced the combustion performance. The quantity of G units within the lignin and the soluble extractives in acetone are more thermally resistant and contribute to the second combustion stage. Although high ratios of volatile matter/fixed carbon result in more mass loss and higher peak temperature during the first combustion stage; the second combustion stage is characterized by the burning of solid carbon, and so lower ratios result in more mass loss during this stage.
Hardwood lignin has G and S units and exhibits weak thermal stability due to its high proportion of β–O–4 and low proportion of 5–5′ bonds than softwood lignin [53]. The reactivity of the linkages within the lignin macromolecule is influenced by the substituted functional groups (phenolic hydroxyl group and methoxyl group) [16]. Lignin with more methoxyl groups (more S units) are less resistant to thermal degradation as observed in this research. The G unit does not have a methoxyl group (–OCH3) on carbon 5 of the phenylpropane unit. This enables the occurrence of bonds between aromatic rings, for example C=C and C–C, which are more thermally resistant [15]. More G units may be related to more lignin condensation, which results in more thermal resistance and less reactivity by occurrence of chemical bonds with higher bonding enthalpy between the phenylpropane units [17, 34, 40]. This consequently results in less mass loss in the initial stages of combustion (Table 9).
Volatile matter/fixed carbon relative proportions are essential for recommending species for heat generation since they influence the combustibility and ease the biofuel ignition. Volatile matter correlated positively with the combustion index (r = 0.79) and the ignition index (r = 0.75). The reverse was observed for the fixed carbon content of the wood clones, that is, negative correlation with the combustion index (r = − 0.78) and the ignition index (r = − 0.75). The higher volatile matter/fixed carbon ratio is related to higher combustion reactivity and with ease of ignition [37, 39]. Higher ratios of volatile matter/fixed carbon resulted in a higher burning rate of Eucalyptus wood. In addition, the S and Di could also be used for selection of genetically superior clones for generation of thermal energy since they relate the wood reactivity during combustion and the lower energy required to initiate the combustion.
Differential Scanning Calorimetry of the Wood
There were two peaks of maximum temperature which characterized two stages of complete oxidation during combustion of the Eucalyptus wood (Fig. 6). The first stage occurred between 200 and 360 °C, while the second started at 400 °C and extended up to approximately 460 °C (Table 10). The first peak of the DSC curve may be attributed to the thermal degradation of the main molecular components of wood (hemicelluloses, cellulose, and part of the lignin), resulting in the emission, ignition, and homogeneous combustion of the volatile matter [49, 51]. The decomposition of the three main molecular constituents (hemicelluloses, cellulose, and lignin) and oxidation of solid carbon [54] occurs in parallel reactions and accounts for mass loss and release of forest fuels energy.
During the second combustion stage the thermal decomposition of the remaining lignin and fixed carbon formed in the first stage occurs [30, 49, 51]. The peaks of maximum energy released correspond to the maximum mass loss temperatures observed in the derivative of the TGA curve (Figs. 4 and 5).
Independent of the studied clones, the first combustion peak was higher, which indicates greater energy was released during the initial phase. This result may be explained by the proximate composition of the wood (see Table 6). As previously mentioned, the volatile matter from the thermal decomposition of the molecules is the major component. Consequently, in the first combustion stage, there is higher fuel mass loss and higher release of heat due to the volatilization reactions of the wood chemical compounds, especially the carbohydrates (Fig. 6). The volatile gases from the wood thermal decomposition are composed mainly of water, carbon monoxide, carbon dioxide, formaldehyde, H, methane, and hydrocarbons [49, 55].
There was a clonal effect of the temperature of maximum energy release during the first stage of combustion. The clones 1015, 1008, 1036, 1037, and 1031 had statistically the highest values. About 80% of the clones of this group formed the cluster with the highest contents of volatile matter, greater mass loss in this stage of the combustion, and less fixed carbon. Alternatively, the clones 1039 and 1024, with the lowest peak temperatures in the initial combustion stage, had less volatile matter, less mass loss in this stage, and more fixed carbon, explaining the peak temperature in the DSC curve.
Clones 1024, 1033, and 1039 had the highest temperature of maximum energy released during the second combustion stage, and they formed a single group that were statistically equal (Table 7). With the exception of 1023, the other clones had lower relation volatile matter/fixed carbon, and consequently, they had greater mass loss in the second combustion stage (Tables 6 and 7). These results support the trends observed in the DSC analysis since the second stage of combustion is characterized by the incandescent burning of solid carbon. Moreover, there is a declining trend of the peaks in the second combustion stage with the increase of ash content (Table 6) which indicates that a higher proportion of mineral oxides may result in energetic losses.
Clone 1037 released the most energy during the first combustion stage (Fig. 6), as well as had a higher ignition index and combustibility index than the other clones evaluated. The lignin composition, level of extractives, and the S/G ratio had low correlation with maximum energy released temperatures (Table 11).
The proximate composition considerably influenced the heat released from the wood during combustion. Thus, the ratio between volatile matter, ash, and fixed carbon must be considered when selecting Eucalyptus clones for heat generation because it influences the release of thermal energy and the maximum temperatures of the combustion stages. However, the contents of lignin and extractives present in the biomass influence the proximate composition. Based on these results, higher peaks of maximum heat released are expected when fuel has more volatile matter which results in greater mass loss during the first combustion stage. The amount of fixed carbon was negatively correlated with the maximum temperature during the first combustion stage. The greater mass loss in the second combustion stage is associated with higher temperature of maximum energy release at this stage.
Relative proportions of ash to fixed carbon influence energy losses and burn time. Ash was negatively correlated with the temperature of maximum energy released in the second combustion stage. This suggests that more ash compared with fixed carbon results in decreased burn time and higher energy losses. Greater ash content resulted in more energetic losses due to the heating of mineral oxides, and consequently, the temperature of the solid carbon combustion was lower. These correlations may be explained by the reactions which occur during wood combustion. The initial stage is characterized by the homogeneous combustion of the volatile gases and O in the air, while the second stage is characterized by heterogeneous combustion between the solid carbon and O [46, 49, 51].
Conclusion
Volatile matter, fixed carbon, extractive levels, and the quantity of G units in the lignin influenced the performance of Eucalyptus wood during combustion in TGA curves. The more content of soluble extractives in acetone and the higher proportion of G units in the lignin macromolecule resulted in greater thermal stability and prolonged the combustion time of Eucalyptus firewood.
The ratio of volatile matter/fixed carbon correlated with the chemical composition of the wood, influenced the release of thermal energy, and consequently, the maximum temperatures of the combustion stages of Eucalyptus wood.
The best parameters for selecting Eucalyptus clones for firewood are energetic productivity and chemical composition (lignin composition, proximate composition, and extractive content). We recommend using the G unit mass per kilogram of dry wood to classify Eucalyptus clones for heat generation.
The results obtained here provide a baseline for further studies on the factors that influence the process of wood combustion and fuel characteristics for energetic sustainability. These types of studies will be important to design and select for developing production systems for Eucalyptus firewood.
References
Moya R, Tenorio C (2013) Fuelwood characteristics and its relation with extractives and chemical properties of ten fast-growth species in Costa Rica. Biomass Bioenergy 56:14–21
Gonçalves B, Dustin T, Oladiran F, Bijay T, Tom G (2015) Influence of bark on the physical and thermal decomposition properties of short-rotation eucalyptus. BioEnerg Res 8:1414–1423
Benavente V, Fullana A (2015) Torrefaction of olive mill waste. Biomass Bioenergy 73:186–194
Mehmood MA, Ibrahim M, Rashid U, Nawaz M, Ali S, Hussain A, Gull M (2017) Biomass production for bioenergy using marginal lands. Sustain Prod Consum 9:3–21
Skeva T, Swinton SM, Hayden NJ (2014) What type of landowner would supply marginal land for energy crops? Biomass Bioenergy 67:252–259
Empresa de Pesquisa Energética (Brasil) (2018) Brazilian energy balance: year 2017. EPE, Rio de Janeiro 292p
Lachowicz H, Sajdak M, Paschalis-Jakubowicz P, Cichy W, Wojtan R, Witczak M (2018) The influence of location, tree age and forest habitat type on basic fuel properties of the wood of the silver birch (Betula pendula Roth.) in Poland. BioEnerg Res 11:638–651
Guo X, Wang S, Wang K, Liu Q, Luo Z (2010) Influence of extractives on mechanism of biomass pyrolysis. J Fuel Chem Technol 38:42–46
Choi HL, Sudiarto SIA, Renggaman A (2014) Prediction of livestock manure and mixture higher heating value based on fundamental analysis. Fuel 116:772–780
Sanchez-Silva L, López-Gonzalez D, Villaseñor J, Sánches P, Valverde JL (2012) Thermogravimetric–mass spectrometric analysis of lignocellulosic and marine biomass pyrolysis. Bioresour Technol 109:163–172
Poletto M, Zattera AJ, Forte MMC, Santana RMC (2012) Thermal decomposition of wood: influence of wood components and cellulose crystallite size. Bioresour Technol 109:148–153
López-González D, Fernandez-Lopez M, Valverde JL, Sanches-Silva L (2013) Thermogravimetric-mass spectrometric analysis on combustion of lignocellulosic biomass. Bioresour Technol 143:562–574
Tenorio C, Moya R (2013) Thermogravimetric characteristics, its relation with extractives and chemical properties and combustion characteristics of ten fast-growth species in Costa Rica. Thermochim Acta 563:12–21
Poletto M (2016) Effect of extractive content on the thermal stability of two wood species from Brazil. Maderas-Cienc Tecnol 18:435–442
Pereira BLC, Carneiro ACO, Carvalho AMML, Colodette JL, Oliveira AC, Fontes MPF (2013) Influence of chemical composition of eucalyptus wood on gravimetric yield and charcoal properties. Bioresources 8:4574–4592
Wang S, Dai G, Yang H, Luo Z (2017) Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog Energy Combust 62:33–86
Soares VC, Bianchi ML, Trugilho PF, Höfler J, Pereira AJ (2015) Properties of Eucalyptus wood hybrids and charcoal at three ages. Cerne 21:191–197
Santos RC, Carneiro ACO, Vital BR, Castro RVO, Vidaurre GB, Trugilho PF, Castro AFM (2016) Effect of properties chemical and syringyl/guaiacyl relation wood clones of Eucalyptus in the production of charcoal. Cienc Florest 26:657–669
Brazilian Association of Technical Standards - ABNT NBR 11941 (2003) Wood - determination of basic density. Rio de Janeiro. 6p
American Society for Testing Materials – ASTM E711-87 (2004) Standard test method for gross calorific value of refuse-derived fuel by the bomb calorimeter. ASTM International, Philadelphia 8p
Technical Association of the Pulp and Paper Industry – TAPPI. T 280 pm-99 (1999) Acetone extractives of wood and pulp. TAPPI, Atlanta 3p
American Society for Testing Materials – ASTM D1762- 84 (2007) Standard test method for chemical analysis of wood charcoal. ASTM International, Philadelphia 2p
Gomide JL, Demuner BJ (1986) Determinação do teor de lignina em material lenhoso: método Klason modificado. O Papel 47:36–38 (In Portuguese)
Goldschimid O (1971) Ultraviolet spectra. In: Sarkanen KV, Ludwig CH (eds) Lignins: occurrence, formation, structure and reactions. J. Wiley, New York, pp 241–298
Lin SY, Dence CW (1992) Methods in lignin chemistry. Springer-Verlag, Berlin 578 p
Araújo AC, Trugilho PF, Napoli A, Braga PPC, Lima RV, Protásio TP (2016) Effects of the syringyl/guaiacyl ratio and of lignin-derived phenols on the wood and charcoal characteristics in eucalyptus spp. Sci Forums 44:405–414
Sahu SG, Sarkar P, Chakraborty N, Adak AK (2010) Thermogravimetric assessment of combustion characteristics of blends of a coal with different biomass chars. Fuel Process Technol 91:369–378
Wang C, Liu Y, Zhang X, Che D (2011) A study on coal properties and combustion characteristics of blended coals in northwestern China. Energy Fuel 25:3634–3645
Wang C, Zhang X, Liu Y, Che D (2012) Pyrolysis and combustion characteristics of coals in oxyfuel combustion. Appl Energy 97:264–273
Moon C, Sung Y, Ahn S, Kim T, Choi G, Kim D (2013) Effect of blending ratio on combustion performance in blends of biomass and coals of different ranks. Exp Thermal Fluid Sci 47:232–240
Qian W, Xie Q, Huang Y, Dang j SK, Yang Q, Wang J (2012) Combustion characteristics of semicokes derived from pyrolysis of low rank bituminous coal. Int J Min Sci Technol 22:645–650
Liu X, Chen M, Yu D (2013) Oxygen enriched co-combustion characteristics of herbaceous biomass and bituminous coal. Thermochim Acta 569:17–24
Xiang-Guo L, Bao-Guo M, Li X, Zhen-Wu H, Xin-Gang W (2006) Thermogravimetric analysis of the co-combustion of the blends with high ash coal and waste tyres. Thermochim Acta 441:79–83
Castro AFNM, Castro RVO, Carneiro ACO, Lima JE, Santos RC, Pereira BLC, Alves ICN (2013) Multivariate analysis for the selection of Eucalyptus clones destined for charcoal production. Pesq Agrop Brasileira 48:627–635
Velázquez-Martí B, Sajdak M, López-Cortés I, Callejón-Ferre AJ (2014) Wood characterization for energy application proceeding from pruning Morus alba L., Platanus hispanica Münchh. and Sophora japonica L. in urban areas. Renew Energy 62:478–483
Musule R, Acuña E, Romero-Hermoso Osorio LS, Domínguez Z, Bárcenas-Pazos GM, Pineda-López MR, Teixeira Mendonça R, González ME, Sánchez-Velásquez LR (2018) Growing up at different altitudes: changes in energy content of the Abies religiosa wood. Bioenerg Res 11:209–218
García R, Pizarro C, Lavín AG, Bueno JL (2014) Spanish biofuels heating value estimation. Part II: proximate analysis data. Fuel 117:1139–1147
Fernandes ERK, Marangoni C, Souza O, Sellin N (2013) Thermochemical characterization of banana leaves as a potential energy source. Energy Convers Manag 75:603–608
García R, Pizarro C, Lavín AG, Bueno JL (2013) Biomass proximate analysis using thermogravimetry. Bioresour Technol 139:1–4
Gouvêa AFG, Trugilho PF, Assis CO, Assis MR, Colodette JL, Gomes CM (2015) Evaluation of syringyl/guaiacyl ratio of eucalypt lignin in the charcoal production. Brazil J Wood Sci 6:71–78
Collard F-X, Blin J (2014) A review on pyrolysis of biomass constituents: mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew Sust Energ Rev 38:594–608
Li L, Zhao N, Fu X, Shao M, Qin S (2013) Thermogravimetric and kinetic analysis of Spirulina wastes under nitrogen and air atmospheres. Bioresour Technol 140:152–157
Singh YD, Mahanta P, Bora U (2017) Comprehensive characterization of lignocellulosic biomass through proximate, ultimate and compositional analysis for bioenergy production. Renew Energy 103:490–500
Riaza J, Gibbins J, Chalmers H (2017) Ignition and combustion of single particles of coal and biomass. Fuel 202:650–655
Shan L, Kong M, Bennet TD, Sarroza AC, Eastwick C, Sun D, Lu G, Yan Y, Liu H (2018) Studies on combustion behaviours of single biomass particles using a visualization method. Biomass Bioenergy 109:54–60
Yeo JY, Chin BLF, Tan JK, Loh YS (2019) Comparative studies on the pyrolysis of cellulose, hemicellulose, and lignin based on combined kinetics. J Energy Inst 92:27–37
Li CZ, Zhao XC, Wang AQ, Huber GW, Zhang T (2015) Catalytic transformation of lignin for the production of chemicals and fuels. Chem Rev 115:11559–11624
Atkins P, Jones L (2006) Princípios de química: questionado a vida moderna e o meio ambiente, 3rd edn. Bookman, Porto Alegre 968p (in Portuguese)
Magdziarz A, Wilk M (2013) Thermogravimetric study of biomass, sewage sludge and coal combustion. Energy Convers Manag 75:425–430
Saldarriaga JF, Aguado R, Pablos A, Amutio M, Olazar M, Bilbao J (2015) Fast characterization of biomass fuels by thermogravimetric analysis (TGA). Fuel 140:744–751
Liu Y, Cao X, Duan X, Wang Y, Che D (2018) Thermal analysis on combustion characteristics of predried dyeing sludge. Appl Therm Eng 140:158–165
Xiong S, Zhang S, Wu Q, Dong A, Chen C (2014) Investigation on cotton stalk and bamboo sawdust carbonization for barbecue charcoal preparation. Bioresour Technol 152:86–92
Zhao J, Xiuwen W, Hu J, Liu Q, Shen D, Xiao R (2014) Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS. Polym Degrad Stab 108:133–138
Bach Q-V, Tran K-Q, Skreiberg Ø (2017) Combustion kinetics of wet-torrefied forest residues using the distributed activation energy model (DAEM). Appl Energy 185:1059–1066
Magdziarz A, Werle S (2014) Analysis of the combustion and pyrolysis of dried sewage sludge by TGA and MS. Waste Manag 34:174–179
Acknowledgments
English editing services were provided by PhD Camila Magalhães Lameiras Alves and Ilse Renner.
Funding
The authors sincerely thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for scholarships for masters (process 132431/2013-0) and doctorate (process 141439/2014-9) courses for the first author, the Plantar Group for donating the clones used for this study, the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and Coordenacão de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Paula Protásio, T., Scatolino, M.V., de Araújo, A.C.C. et al. Assessing Proximate Composition, Extractive Concentration, and Lignin Quality to Determine Appropriate Parameters for Selection of Superior Eucalyptus Firewood. Bioenerg. Res. 12, 626–641 (2019). https://doi.org/10.1007/s12155-019-10004-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-019-10004-x