Abstract
During tequila production, up to 75 % w/w of the Agave plant is discarded when leaves are removed from the stem. The discarded leaves represent an extensive amount of unexploited biomass that was used here for bioethanol production in no-input fermentations, where no acid or enzymatic hydrolysis, supplementation of nutrients or standardization of carbohydrate content occur. Ethanol yield from Agave leaf juice is unaffected by sterilization but reduced if fermentation is reliant solely on endogenous microorganisms. Non-Saccharomyces yeasts, including Kluyveromyces marxianus and Candida akabanensis, proved to be more robust than standard Saccharomyces spp. and yielded up to 88 % of the theoretical maximum ethanol from leaf juice. Combining leaf and stem juice, as from a whole plant, was predicted to maximize yield at up to 19,439 L/ha of ethanol from mature plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The advent of plant-based ethanol production using fermentation can be traced back to as early as 6000 BC [1]. Alcoholic fermentation involves the release of energy from carbohydrates by microorganisms, usually under low oxygen conditions, and yields ethanol and carbon dioxide. Historically, the production of alcoholic beverages has been reliant on a consortium of commensal microflora to convert the plant-derived carbohydrates into ethanol [2]. However, these spontaneous (uninoculated) fermentations often yield unpredictable end-products. In more recent times, the industry has optimized the fermentation process to generate more predictable and consistent high value end-products, such as wine, beer and spirits.
In principle, the same techniques apply for the production of alcohol for transportation fuel; however, the goal is not to produce a high value end-product but rather a cheaply produced “drop-in” fuel, which is both profitable and cost competitive with fossil fuels. In recent years, waste from feedstocks used for the manufacture of various beverages has been attracting attention as potential biomass sources for bioethanol and biochemical production [3]. One example of agro-industrial waste attracting attention is Agave tequilana plants, which are traditionally used for making tequila in Mexico. In tequila making, only part of this feedstock is converted to alcohol, rendering a substantial amount of plant material being underutilized. The highest producing region in Mexico, Jalisco, alone yields 6.59 × 105 tons of biomass per year [4].
For tequila making, the leaves are discarded and extensive processing is performed upon the stem biomass to generate a fermentable juice. Such processing steps include cooking the stem for extended periods to hydrolyse polymers, milling the cooked stems to extract the juice or “honey”, converting the sugars in the honey to alcohol by fermentation and finally double distilling the fermentation broth to produce pure tequila, which may be aged for a further 3 to 12 months in oak barrels [5]. The fibrous biomass that remains following milling of the stems is commonly referred to as the bagasse. This discarded bagasse contributes 40 % (wet w/w) of the stem mass [5]. Thus, under this processing regime, only about 25 % of the above-ground biomass of the Agave plants is utilized. In a biofuel context, this processing scheme provides opportunities for converting a substantial amount of aggregated vegetative tissue to ethanol or other low molecular mass alcohols.
To date, available information is related to the fermentation of juice extracted from cooked Agave stems, which is reflective of the processes used in the tequila industry (Table 1). Agave stem tissue is enriched with fructans, which are soluble polymers of fructose with mainly fructosyl-fructose linkages linked to a terminal glucose molecule that acts as storage polymers in 15 % of flowering plant species [6]. In these published studies, the effects of a range of parameters, including incubation temperatures and differing fermenting microorganisms, have been investigated (Table 1). Standard methods for Agave fermentation include sterilizing the juice, spiking it with monosaccharides and nutrients (usually to boost nitrogen content) and/or adding polysaccharide-specific enzymes to optimize fermentation conditions. However, the costs incurred by optimizing the nutrient content of the juice are frequently overlooked [7] and may not be economically viable for the production of cheap biofuel. In addition, little is known about the composition and fermentation of juice extracted from the discarded Agave leaves. To obtain a realistic, base-line view of utilizing novel fructan-enriched biomass such as Agave for potential large-scale bioethanol production, information about fermentations using no or minimal inputs other than leaf and stem juice is required.
In the present study, ethanol yields achieved from Agave leaf juice on a small scale were investigated using different processing methods and fermenting microorganisms. Four different fermentation schemes were used for Agave leaf juice, none of which included supplementation or standardization of the carbohydrate or nutrient content. The production of ethanol by native or commercially available fermenting organisms was assayed from raw and sterilized leaf juice substrates and compared with fermentation rates of either pure stem juice or combined stem and leaf juice. The ethanol (g/L) produced is then compared to the theoretical maximum ethanol production to determine the efficiency achieved. The theoretical maximum ethanol production was calculated by measuring the total sugar content in each substrate and assuming 100 % conversion of monosaccharides to ethanol [8]. This fermentation data was then extrapolated to predict the ethanol that could be produced using Agave waste on a large scale. Thus, a toolset of methods and information specific to the small-scale fermentation of Agave juice has been developed and is likely to be useful in a commercial context for forecasting the potential yields of bioethanol from novel biomass sources such as Agave leaves.
Methods
Collection of A. tequilana Plants
A. tequilana plants (n = 3) were harvested in Ayr, Queensland, Australia (19° 31′ 49.9″ S; 147° 24′ 51.5″ E). At the time of harvest, the 4.5-year-old plants were separated into leaves, roots, stem and offshoots, and the fractions were weighed. A commercial shredder (Cutter-Grinder CG03; South Australia, Australia) was used to extract the juice from a subset of each fraction. The juice was collected and transported to the University of Adelaide on dry ice and stored at −20 °C.
Total Carbohydrate Content in Agave Juice
The carbohydrate content in raw and pre-treated (autoclaved, trifluoroacetic acid (TFA) or fructanase-treated) A. tequilana juice from leaves, stem and offshoots was quantified by hydrophilic interaction chromatography (HILIC) [9]. Briefly, glucose, fructose and sucrose were quantified using a Prevail Carbohydrate ES column (150 × 4.6 mm) (Alltech; IL, USA) on an Agilent 1200 series liquid chromatography instrument equipped with an evaporative light scattering detector (Alltech ELSD 800). The mobile phase was 10 % water (A) and 90 % acetonitrile (B) at a flow rate of 1.0 mL/min. Sample peak areas were compared to calibration curves of standard solutions.
For pre-treatment, juice samples were autoclaved at 121 °C (Tuttnauer 3850 ELC Benchtop Sterilizer; Brinkmann Instruments, NY, USA) or hydrolysed using either TFA or fructanase (Fructan HK-Megazyme: AOAC Method 999.03; International Ireland Ltd., Wicklow, Ireland) as previously described [10]. For acid hydrolysis, juice and acid were mixed in equal proportions to a final concentration of 0.2 M TFA and heated at 80 °C for 1 h. For enzymatic hydrolysis, juice and enzyme were combined 1:1 and incubated at room temperature for 30 min. The enzyme was deactivated by heating 100 °C for 15 min. A sugar recovery standard was carried through the acid hydrolysis to calculate the degradation rates of released monosaccharides, which were used to correct HILIC data.
pH, Brix, Total Soluble Solids and Mineral Content
The pH of the juice was determined using a 900-P pH-mv-Temperature metre (TPS Pty Ltd, Australia). Brix readings of the juice samples were measured with a refractometer (Atago Co., Japan). The total soluble solids (TSS) and mineral content of juice samples were determined according to Corbin et al. [9].
Selection of Microorganisms
Fermenting microorganisms were obtained from the United States Department of Agriculture Agricultural Research Service (ARS) Culture Collection, National Center for Agricultural Utilization Research (Peoria, IL, USA) [11]. The yeast strains selected were Kluyveromyces marxianus (NRRL 1598), Pichia kluyveri (NRRL 17228), Candida akabanensis (NRRL 7846) and Saccharomyces cerevisiae (NRRL 636 and NRRL 139). The strains were streaked on 2 % w/v agar (yeast extract-peptone-dextrose, YPD) plates containing 1 % w/v yeast extract, 2 % w/v peptone and 2 % w/v d-glucose, and incubated at 28 °C for 48 h. Single colonies were picked and inoculated into the YPD medium and shaken (120 rpm) overnight at 28 °C. Cultures were diluted to an OD600 of 0.5 to determine the optimal growth temperature. Serial 5-fold dilutions were spotted on YPD plates and incubated for 24 h at 28, 32, 37 or 42 °C (n = 2).
Fermentation Conditions
Juice samples were centrifuged at 5000 rpm for 5 min to remove excess leaf tissue. Autoclaving of juice was completed at 121 °C at a maximum pressure of 210 kPa (Tuttnauer 3850 ELC Benchtop Sterilizer; Brinkmann Instruments, NY, USA). Leaf and stem juice were combined in a volume ratio of 3:1 (leaf/stem), to a final volume of 100 mL, representative of the whole plant being fermented simultaneously. At least two replicates were used for each of the inoculated fermentations and three replicates for spontaneous fermentations.
Native microorganisms in raw Agave leaf and stem juice were streaked (0.05 mL aliquots) on solid YPD plates and grown at 28 °C. A representative microbial culture of the endogenous species found within the Agave juice was made by harvesting these plates using a sterile loop. This formed an inoculum that was used to re-inoculate 100 mL of autoclaved juice samples, representative of juice samples that had become contaminated following sterilization.
The yeasts obtained from the ARS Culture Collection were grown overnight at 28 °C on YPD and a single colony was picked. The single colony was grown in YPD liquid broth (28 °C) in a shaker incubator (120 rpm) and used to inoculate 100 mL of raw juice samples at a cell density of 5 × 106 cells/mL. All fermentations were completed in sterile Erlenmeyer flasks (250 mL in size) with side arm sampling ports sealed with water-filled airlocks. The fermentation flasks were shaken at 150 rpm and at 28 °C. The yeast cells were removed from the fermentation broth by centrifugation (1 min/10,000×g), and the supernatant was stored at −20 °C until analysis.
Analysis of Substrate and Fermentation Products
The concentrations of organic acids (acetic, citric, lactic and malic acid), glycerol and ethanol in the fermentation broth were quantified by high-performance liquid chromatography (HPLC) as previously described [12]. Analysis was completed using an Aminex HPX-87H cation exchange column (Bio-Rad Laboratories) on a HPLC 1100 series (Agilent Technologies, Germany) with a mobile phase of 2.5 mM sulphuric acid (H2SO4). Calibration curves relating concentration to optical density or refractive index were fitted using ChemStation software (Agilent, CA, USA).
Theoretical Maximum Ethanol Production
Theoretical maximum ethanol production was calculated using the assumption that 0.511 kg of ethanol is produced from 1 kg of completely hydrolysed substrate comprising glucose and fructose monomers only [8, 10]. The following mathematical formulae were used to calculate the theoretical maximum ethanol production and conversion efficiency. a = total sugar in TFA-treated juice (monosaccharides g/L); b = ethanol yield coefficient 0.511; c = theoretical maximum ethanol production (g/L); d = observed ethanol yield from fermentations (g/L); and e = % of predicted ethanol production.
Results and Discussion
Composition of A. tequilana
A whole Agave plant, including the main unit (mother plant) and its attached offshoots, generated 360 kg of raw biomass that could be used for biofuel production. The harvested biomass was separated into different anatomical fractions (leaves, stem and offshoots) to create multiple production streams from one feedstock (Fig. 1). For 4.5-year-old A. tequilana plants, 73 % w/w of the above-ground biomass was attributable to leaves and 27 % w/w to the stem (Table 2). By crushing the leaves, 68 % w/w of the leaf mass could be extracted as a weakly acidic fermentable juice (Table 2).
Diagram outlining the fractions that can be generated from processing of Agave plants. Whole units of Agave plants (a) were harvested and divided into offshoots (b), leaves (c) and stem (d). Each of these fractions can be further processed to generate a cellulose-enriched fibrous bagasse (e), a fermentable juice (f) and residual biomass (g)
The acidity of Agave juice (pH 4.6–5.0; Online Resource 1) is a characteristic of plants that use the Crassulacean acid metabolism (CAM) photosynthesis pathway, in which organic acids such as malic acid are stored in the vacuoles of cells during the night [13]. The two abundant organic acids measured by HPLC in the leaf juice were acetic and malic acid (Online Resource 2). High concentrations of weak acids such as acetic acid or low concentrations in the presence of other compounds (i.e. furfural) have been shown to reduce biomass accumulation and production of ethanol, resulting in a need for superior strain selection [14]. A small percentage of the total mass of the juice was attributed to minerals at 1–2 % w/w (Online Resource 1).
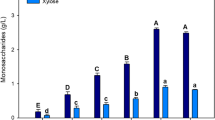
TSS and °Brix have previously been used to estimate sugar content in raw and fermented Agave juice [15–18]. The measured carbohydrate content for A. tequilana leaf (∼9.6 % w/v) and stem juice (∼17.3 % w/v) using the two methods, TSS and °Brix, were comparable (Table 2). However, when juice was analysed using HPLC, it was clear that these methods overestimated the amount of carbohydrates in leaf and offshoot juice (Table 2). This overestimation is likely due to the presence of solutes other than carbohydrates such as calcium oxalate crystals. To quantify the real carbohydrate content and subsequently predict ethanol yields, it is essential that accurate methods are used.
Juice samples were treated with TFA or fructanase to cleave higher molecular weight carbohydrates, predominantly fructans, into monosaccharides for quantification. A. tequilana plants are considered to be high accumulators of branched fructans and neo-fructans [19]. However, only 10–15 g/L of the carbohydrates in A. tequilana leaf and offshoot juice is attributed to fructans (Table 3). Whilst over 85 % of the carbohydrates in stem juice is complex fructans, many microorganisms cannot ferment these in their native branched forms and thus a hydrolysis or cooking step is required [20]. Although such methods are considered to be efficient for the complete hydrolysis of fructans in both leaf and stem tissue, they can be costly and unfavourable for downstream processing [20, 21]. In addition, fructose is highly unstable relative to other monosaccharides such as glucose, even under moderate pre-treatment conditions, and is converted into compounds, such as hydroxymethylfurfural, which are toxic to fermenting microorganisms [21]. As a result, acid and enzymatic pre-treatments were not further explored in this study.
Alternatively, autoclaving is considered a low-cost, low-input pre-treatment for Agave juice. For example, autoclaving increased the measurable monosaccharides in stem juice by 50 g/L (Table 3). In addition, higher levels of the intermediate hydrolysis product sucrose were present, indicating that partial breakdown of the complex fructans was achieved where one sucrose moiety is liberated per molecule of fructan [21].
In the tequila industry, cooking the stem at elevated temperatures for an extended period is the standard method for fructan hydrolysis, for production of aromatic compounds and for softening of the recalcitrant tissue before milling [5]. Autoclaving can similarly be used to treat the juice prior to fermentation (Table 1), but there are inconsistent reports in the literature regarding the efficiency or necessity of the autoclaving step. Some studies indicate that autoclaving Agave juice partially hydrolyses fructans [15]. Other studies imply that autoclaving is employed strictly as a sterilization step and does not modify the structure or composition of carbohydrates in the juice [16], whilst others claim that for complete hydrolysis (98 %) of fructans to occur, the samples must be heated at about 80 °C for more than 25 h [19]. Findings herein indicate that the efficiency of fructan hydrolysis by a simple thermal pre-treatment (autoclaving) is substrate-dependent, as there was no difference in the monosaccharide content between raw and autoclaved leaf juice, but that the amount of monosaccharides in the stem juice increased following autoclaving.
Although immature offshoots contributed a substantial amount of the total biomass (38 % w/w; Table 2), they were at varying stages of development, potentially rendering biomass weights, carbohydrate content and ultimately ethanol yields unpredictable. As a result, it may be more advantageous to harvest only the mother plant and to leave the offshoots to grow for future harvests; thus, offshoots were not further analysed in this study.
Spontaneous Fermentation of Agave Juice Is Induced by Endogenous Species
In commercial tequila production, spontaneous (uninoculated) fermentation is a common practice [22]. However, in a biofuel context, spontaneous fermentations are unlikely to be favourable and partial fermentation of the carbohydrates can occur prematurely during transport of feedstocks to processing facilities, thus reducing ethanol yields (Online Resource 3). Here, the spontaneous fermentation of A. tequilana leaf and stem juice was tested by allowing raw leaf and stem juice to ferment for 120 h without the addition of any external microorganisms. Both juice sources gave similar, albeit low, yields of ethanol: 9.0 g/L for leaf juice and 14.6 g/L for stem juice (Fig. 2a). However, the fermentation profiles of these two substrates over 120 h were quite different (Fig. 2a).
Spontaneous fermentation of A. tequilana juice. Raw Agave leaf and stem juice was fermented using only the native organisms present within the substrate; n = 3. The production of ethanol (g/L; a) was measured over 120 h. The actual ethanol yield achieved was compared to the maximum yield to determine fermentation efficiency (%; b)
The proportion of sugar converted to ethanol was higher in leaf samples (Fig. 2b), which may be attributed to the structural differences of the carbohydrates in the two substrates compared. For example, the majority of the carbohydrates in Agave leaf juice are fermentable mono- or oligosaccharides, whereas in stem juice, they are complex fructans (Table 3). The final ethanol yield for both spontaneous fermentations was similar (9–15 g/L), which may be indicative of similarities between the membership of the microbial mixture both at the start and throughout the fermentation. The endogenous microorganisms found within these Agave samples may have a low tolerance to ethanol resulting in incomplete fermentation (only 32 and 17 % of the theoretical ethanol maximum was produced for leaf and stem juice, respectively), although other factors may be hindering the conversion of carbohydrates to ethanol.
Spontaneous fermentations are heterogeneous microbiological and biochemical processes in which populations increase and decrease as selective pressures are induced, ultimately resulting in the dominance of species with superior catabolism rates and/or tolerance to the alcoholic end-products [2]. Microorganisms isolated from the early stages of Agave stem fermentations have been shown to contain a rich mixture of yeast species. For example, 192 yeasts were identified from alcoholic fermentation of Agave salmiana juice when it was streaked on nutrient agar plates [23]. In this study, Saccharomyces was found to be the predominant strain at the onset of fermentation (94–98 % occurrence) and remained the most abundant species. However, as fermentations progress, the number of species and their abundance can change. Some studies indicate that the diversity diminishes over the course of the fermentation [24], whereas in other studies, a more diverse mixture of microorganisms was observed due to the introduction of contaminants [23]. A negative interaction between S. cerevisiae and non-Saccharomyces yeast strains has been proposed, which may be one of the factors contributing to the reduced efficiency of spontaneous fermentations [25].
In addition, active killer yeast (and bacterial) genera, such as K. marxianus var. drosophilarum and Pichia membranaefaciens, are frequently present in the spontaneous fermentation of plant biomass, including Agave, resulting in sluggish or stuck fermentations [26, 27]. Strains that exhibit these killer properties tend to colonize fermentations early and produce zymocidal substances that can reduce beneficial yeast populations [28]. The rise and fall of populations during the course of the fermentation could explain the sporadic changes in ethanol content observed in the fermentation profiles of unmodified Agave leaf and stem juice. Prolonged spontaneous fermentations of Agave juice, if not monitored, could therefore inadvertently result in reduced ethanol yields. Such information will be instructive when considering the processing, transport, handling and storage of Agave biomass and juice.
Selection of Microorganisms
As microorganisms exhibit a narrow range of tolerances to environmental conditions, identifying fermenting organisms that are particularly adapted to the substrate is crucial. In this work, five novel yeast strains were obtained from the ARS Culture (NRRL) Collection [11] for fermentation studies (Table 4). The selected yeast had been isolated directly from Agave, other succulents or from biomass rich with carbohydrates found in Agave such as grape (glucose and fructose) or sugarcane molasses (sucrose). The use of novel microorganisms isolated from a biomass source has been shown to have great potential for the biofuel industry [29]. A recent study showed that fungi growing on a feedstock had higher enzymatic activity and was more efficient at degrading the cell wall components compared to commercial fungus [29]. Furthermore, in this study, it was determined that the most superior fungi identified had not previously been exploited for bioconversion processing, highlighting the importance of trialling novel strains.
All genera of yeast selected have been identified as dominant or secondary yeasts in tequila fermentations or tequila processing facilities [24]. The fermentation performance of three strains had previously been tested (CBS-KNAW Fungal Biodiversity Centre [30]; Table 4). For the other two yeasts, data specific to the strains were not available, but species-level information [30] regarding fermentation performance was considered (Table 4). Of the yeast selected, one strain is known to ferment the primary carbohydrates in Agave juice, glucose, sucrose and fructans, K. marxianus (Km1598) (CBS-KNAW Fungal Biodiversity Centre id: CBS 745 [30]; Table 4). In addition, isolates of K. marxianus have been shown to produce fructan-hydrolysing enzymes [31]. The yeast P. kluyveri was also selected as it has been reported to display high fermentation efficiency in tequila making [32].
To determine the optimal growth temperature, each yeast was diluted to a standardized cell concentration and spotted on YPD plates incubated at four different temperatures (28, 32, 37 and 42 °C). All yeast strains grew similarly at temperatures at or below 32 °C (Online Resource 4), and thus, 28 °C was chosen as the optimal temperature for the fermentation experiments. Interestingly, it was found that K. marxianus (Km1598) tolerated temperatures above 37 °C. This strain was isolated from Agave that is able to thrive in arid regions and which can tolerate temperatures above 60 °C [33]. Such information suggests that further analysis of the metagenome of Agave may identify novel, superior microorganisms for the production of bioethanol. For example, although less than 1 % of microorganisms present in many natural environments can be cultured in vitro [34], endogenous microorganisms from Agave juice exhibited growth over a wide range of temperatures (28–42 °C; data not shown). Yeasts isolated from Agave also have the advantage of being tolerant to toxic compounds (i.e. furans) present in the juice and are less likely to become inactive during fermentation as they are more adaptive to the substrate [35].
Comparison of Microbial Strains and Treatment of A. tequilana Leaf Juice
In large-scale biofuel production, where rapid and reliable fermentations are essential, the use of robust and reliable pure yeast inocula of known performance is common practice, rather than spontaneous fermentation [2]. Here, five yeasts (Table 4) were selected for the fermentation of raw and autoclaved Agave leaf juice. Subsequently, yeast strains with the highest fermentation performance were cultured in autoclaved leaf juice after the re-introduction of endogenous microbes (Table 5).
S. cerevisiae is one of the most widely utilized yeasts for alcoholic fermentations such as wine making and brewing, yet the ethanol yields achieved from Agave leaf juice (raw or autoclaved) using two Saccharomyces isolates (Sc636 and Sc139) were lower compared with the ethanol yields achieved using the less commonly studied yeasts Ca7846, Km1598 and Pk17228 (Table 5). The poorer fermentation performance of the Saccharomyces isolates is likely to be partially attributable to the high levels of fructose present in the substrate, as Saccharomyces yeast species preferentially use glucose (although the level of preference varies) when in a heterogeneous culture with other monosaccharides such as fructose [36]. When glucose is transported across the plasma membrane in these mixed cultures and ethanol is accumulated, the tautomeric equilibrium of fructose is shifted, converting it from fructopyranose to fructofuranose [37]. This shift in conformation has been suggested to lower the rate of fructose transport, further limiting its uptake by the yeast [36]. The accumulation of carbohydrates that are not metabolized by the yeast results in slowed or arrested fermentations, ultimately decreasing ethanol yields. In another report, reduced ethanol yields were reported for Saccharomyces inocula when used in Agave fermentations if the yeast did not originate from Agave [38]. In another fermentation study, it was observed that S. cerevisiae was unable to grow in either thermo acid or enzymatically treated Agave juice [20]. The authors correlated this inhibition of growth to toxic levels of phytochemicals such as saponins which are produced by Agave plants. Interestingly, the saponins did not inhibit the growth or fermentation performance of the yeast K. marxianus.
In this study, higher ethanol yields were achieved using non-Saccharomyces yeasts than Saccharomyces yeasts. The increase in ethanol yields using these strains may be attributed to their superior ability to degrade fructose and fructan polymers, or possibly their tolerance to compounds inherent to the substrate (i.e. malic acid) or the production of ethanol and inhibitory by-products. For example, K. marxianus is known to produce fructan-specific enzymes, which enables the yeast to simultaneously hydrolyse fructans and ferment the liberated monosaccharides [31], resulting in higher ethanol yields (Table 5). Alcohol yields achieved using the non-Saccharomyces yeasts P. kluyveri and K. marxianus were similar, consistent with previous reports [32]. The most efficient isolate used was Ca7846 (88 % conversion; Table 5). Candida species have previously been isolated from Agave fermentations [23, 24]; however, this is the first study to investigate its use for bioethanol production and little is known about its fermentation capabilities.
There is no benefit to autoclaving Agave leaf juice prior to fermentation. The modest increase in ethanol yields achieved using autoclaved juice (Table 5) is likely due to the slight increase in available monosaccharides following autoclaving (Table 3). No differences between ethanol yields (g/L) or conversion efficiency were observed between non-Saccharomyces strains when cultured in raw or autoclaved Agave leaf juice (Table 5). In addition, the accumulation and production of organic acids (acetic, citric, acetic and malic acid) and glycerol in non-Saccharomyces fermentations of raw and autoclaved juice was similar (Online Resource 2), indicating that acid production and consumption patterns of the yeast are not influenced by autoclaving the juice. However, when endogenous microorganisms were re-introduced into autoclaved juice substrates, the ethanol yields were lower for all non-Saccharomyces yeasts tested than yields from fermentations where endogenous microorganisms were not re-introduced. The strain Km1598, which was originally isolated from Agave sisalana (Table 4), had the lowest ethanol yield (g/L) in the presence of native microbes (raw and re-introduced) compared with Ca7846 and Pk17228. Overall, the efficiency of the fermentations were influenced more by the yeast species present (Table 5) than by the treatment of the juice. However, there was a clear distinction between the fermentation performance of Saccharomyces and non-Saccharomyces yeast in both raw and autoclaved leaf juice (Table 5).
The current research has revealed that autoclaving is nonetheless important, but as a means of eliminating contamination. Ethanol yields from fermentations containing native organisms in addition to the selected yeast strains were adversely affected compared to the yeast mono-culture experiments. The highest ethanol conversion was observed in the presence of native organisms, 61 % using Pk17228 (Table 5). However, this value is lower than the rate observed when culturing raw leaf juice (77 % conversion for Pk17228) and autoclaved juice without native organisms (84 % of maximum for Pk17228).
It is interesting that autoclaved juice supplemented with native organisms and the non-autoclaved juice produced different effects. This reduction in ethanol production is likely due to the compositional and chemical changes that occur as a result of autoclaving, creating an environment in which native microorganisms are not adapted. One means by which autoclaving changes the composition of Agave juice is by degrading vitamins and thereby inducing a nutrient-limited environment [16]. Deficient vitamin levels can reduce cell growth rate and biomass production, decrease viability and ultimately affect fermentation performance [39]. In addition, it can result in the degradation of nitrogen-containing compounds (i.e. ammonium), which is an essential macronutrient required by microorganisms for the maintenance of stationary phase fermentation [39].
Current studies suggest that less than 1 % of microorganisms present in many natural environments can be cultured in vitro [34]. Thus, by culturing the endogenous microbes present in Agave juice on plates, only a small proportion of the microbial population was represented, and therefore, this inoculum was not identical to the breadth of microorganisms found in raw Agave juice. However, as in the tequila industry, even a small diversity of microbial contaminants can have a profound effect on the fermentation performance and end-products generated [24]. Contaminants from Agave will inevitably be present in Agave processing facilities. The current study provides founding data from which strategies to manage Agave storage and handling may be developed.
Fermentation of Agave Stem Juice
Microbes that naturally deconstruct plant walls may provide the best enzymes for bioconversion of energy crops [40]. To explore this concept, the fermentation of autoclaved Agave stem juice was investigated using Km1598, a strain which was originally isolated from Agave. A maximum conversion rate of 64 % was achieved by 48 h (Fig. 3a). This conversion rate was lower than previously reported for K. marxianus (94–96 % efficiency after 72 h; Table 1), but the stem juice used for this study was not cooked for an extended period to hydrolyse the fructans [22].
Conversion rates of total carbohydrates to ethanol using Agave stem juice. Kinetic profile of the fermentation of Kluyveromyces marxianus (Km1598) in autoclaved A. tequilana stem juice over time, 96 h, n = 3 (a) and in combined leaf and stem juice (b). The autoclaved leaf and stem juice were combined in volumes equivalent to the mass distribution of a whole plant, namely 75 % leaves and 25 % stem, and fermented using K. marxianus (b). The fermentation of combined Agave juice from stem and leaf yielded 85 % of the predicted maximum ethanol yield
More recently, the potential of producing biofuel from whole Agave plants has been considered [41]. However, these experiments are still reliant on the use of costly chemical pre-treatments and enzymatic cocktails for hydrolysis of fructans. In our study, leaf and stem juice were combined in a volume leaf/stem ratio of 3:1, which was representative of a scenario where whole plants are crushed as one unit, without the leaves and stem being separated. When stem juice and leaf juice were combined, 85 % conversion to ethanol was achieved (Fig. 3b). This approach is advantageous as it reduces the time and labour required for processing and harvesting of Agave plants, potentially reducing production costs.
Ethanol Yield Predictions
The theoretical ethanol yields for A. tequilana rival other currently studied lignocellulosic feedstocks [42]. Juice derived from A. tequilana stems had a higher proportion of carbohydrates than leaf juice (Table 2), but in total, the juice from the leaves contributed more total carbohydrates (6.6 kg per plant) than the stem (4.4 kg per plant). Therefore, a higher theoretical maximum ethanol production was calculated for A. tequilana leaf juice, 3053 L/ha/year relative to stem juice, 2029 L/ha/year (Table 6). Fermentation of juice from whole mother plants in an Agave plantation could yield up to 5082 L/ha/year ethanol, leaving the offshoots to keep growing in the field for future harvests. However, the conversion of carbohydrates into ethanol is mediated via a complex chemical and enzymatic pathway, and actual fermentation data is required to validate the estimates of the value of this biomass as a substrate for fermentation.
There was a range in the conversion of carbohydrates to ethanol in the fermentation of Agave leaf juice, 45–88 % (Table 5). This correlates to the predicted production of up to 2687 L/ha/year ethanol (Table 6). Therefore, the fermentation of A. tequilana leaves, which are currently discarded in the beverage industry, could add significant value to existing Agave industries. Agave production specifically for biofuel may also be profitable, particularly as Agave plants are highly drought tolerant and can be produced in agriculturally marginal regions with limited rainfall [42]. Based on fermentation data from this study, if grown specifically for biofuel production at a density of 4000 plants/ha, the juice derived from 4.5-year-old A. tequilana plants (stem and leaf) could produce ethanol yields of up to 19,439 L/ha. Optimizing processing methods or fermenting microorganisms could increase ethanol yields to their theoretical maximum, 22,870 L/ha.
Conclusion
The data generated in this study challenges current practices in bioethanol production of supplementing and pre-treating Agave juice prior to fermentation. Leaf juice substrates do not benefit from autoclaving prior to inoculation, and the selection of superior fermenting organisms is essential for generating high ethanol yields. The best microorganisms studied were isolated from succulents. A third species, C. akabanensis, was used for the first time in bioethanol fermentation studies of Agave juice, suggesting that further exploration of non-traditional Saccharomyces species could improve bioethanol yields. Actual and extrapolated ethanol yields from Agave leaf juice confirm that this biomass has significant potential for bioethanol production.
Abbreviations
- CAM:
-
Crassulacean acid metabolism
- TSS:
-
Total soluble solids
- YPD:
-
Yeast extract-peptone-dextrose
References
Robinson J, Harding J (1999) The Oxford companion to wine. Oxford University Press.
Pretorius IS (2000) Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast 16:675–729
Zhang Z, O’Hara IM, Mundree S, Gao B, Ball AS, Zhu N, Bai Z, Jin B (2016) Biofuels from food processing wastes. Curr Opin Biotechnol 38:97–105
Nava-Cruz NY, Medina-Morales MA, Martinez JL, Rodriguez R, Aguilar CN (2015) Agave biotechnology: an overview. Crit Rev Biotechnol 35(4):546–559
Cedeño MC (1995) Tequila production. Crit Rev Biotechnol 15:1–11. doi:10.3109/07388559509150529
Vijn I, Smeekens S (1999) Fructan: more than a reserve carbohydrate? Plant Physiol 120:351–360. doi:10.1104/pp.120.2.351
Kadam K, Newman M (1997) Development of a low-cost fermentation medium for ethanol production from biomass. Appl Microbiol Biotechnol 47:625–629
Boulton R, Singleton V, Bisson L, Kunkee R (1998) Principles and practices of winemaking. Chapman & Hall, New York
Corbin KR, Hsieh YSY, Betts NS, Byrt CS, Henderson M, Stork J, DeBolt S, Fincher GB, Burton RA (2015) Grape marc as a source of carbohydrates for bioethanol: chemical composition, pre-treatment and saccharification. Biores Technol 193:76–83. doi:10.1016/j.biortech.2015.06.030
Corbin KR, Byrt CS, Bauer S, DeBolt S, Chambers D, Holtum JA, Karem G, Henderson M, Lahnstein J, Beahan CT (2015) Prospecting for energy-rich renewable raw materials: Agave leaf case study. PLoS One 10(8):e0135382
ARS (NRRL) Culture Collection (2015) http://nrrl.ncaur.usda.gov/. Last accessed 5 May 2015
Frayne R (1986) Direct analysis of the major organic components in grape must and wine using high performance liquid chromatography. Am J Enol Vitic 37:281–287
Nobel PS, Valenzuela AG (1987) Environmental responses and productivity of the CAM plant, Agave tequilana. Agr For Meteorol 39:319–334. doi:10.1016/0168-1923(87)90024-4
Palmqvist E, Grage H, Meinander NQ, Hahn-Haegerdal B (1999) Main and interaction effects of acetic acid, furfural, and p-hydroxybenzoic acid on growth and ethanol productivity of yeasts. Biotechnol Bioeng 63:46–55. doi:10.1002/(SICI)1097-0290(19990405)63:1
Cáceres-Farfán M, Lappe P, Larqué-Saavedra A, Magdub-Méndez A, Barahona-Pérez L (2008) Ethanol production from henequen (Agave fourcroydes Lem.) juice and molasses by a mixture of two yeasts. Biores Technol 99:9036–9039. doi:10.1016/j.biortech.2008.04.063
Díaz-Montaño DM, Délia ML, Estarrón-Espinosa M, Strehaiano P (2008) Fermentative capability and aroma compound production by yeast strains isolated from Agave tequilana Weber juice. Enz Microb Technol 42:608–616. doi:10.1016/j.enzmictec.2007.12.007
Díaz‐Montaño DM, Favela‐Torres E, Cordova J (2010) Improvement of growth, fermentative efficiency and ethanol tolerance of Kloeckera africana during the fermentation of Agave tequilana juice by addition of yeast extract. J Sci Food Agric 90(2):321–328
Martínez-Torres J, Barahona-Perez F, Lappe-Oliveras P, Colunga Garcia-Marin P, Magdub-Mendez A, Vergara-Yoisura S, Largue-Saavedra A (2011) Ethanol production from two varieties of henequen (Agave fourcroydes Lem). GCB Bioenergy 3(1):37–42
Waleckx E, Gschaedler A, Colonna-Ceccaldi B, Monsan P (2008) Hydrolysis of fructans from Agave tequilana Weber var. azul during the cooking step in a traditional tequila elaboration process. Food Chem 108:40–48. doi:10.1016/j.foodchem.2007.10.028
Villegas-Silva PA, Toledano-Thompson T, Canto-Canché BB, Larqué-Saavedra A, Barahona-Pérez LF (2014) Hydrolysis of Agave fourcroydes Lemaire (henequen) leaf juice and fermentation with Kluyveromyces marxianus for ethanol production. BMC Biotechnol 14:14. doi:10.1186/1472-6750-14-14
Nguyen S, Sophonputtanaphoca S, Kim E, Penner M (2009) Hydrolytic methods for the quantification of fructose equivalents in herbaceous biomass. Appl Biochem Biotechnol 158:352–361. doi:10.1007/s12010-009-8596-x
López-Alvarez A, Díaz-Pérez AL, Sosa-Aguirre C, Macías-Rodríguez L, Campos-García J (2012) Ethanol yield and volatile compound content in fermentation of Agave must by Kluyveromyces marxianus UMPe-1 comparing with Saccharomyces cerevisiae baker’s yeast used in tequila production. J Biosci Bioeng 113:614–618. doi:10.1016/j.jbiosc.2011.12.015
Verdugo Valdez A, Segura Garcia L, Kirchmayr M, Ramírez Rodríguez P, González Esquinca A, Coria R, Gschaedler Mathis A (2011) Yeast communities associated with artisanal mezcal fermentations from Agave salmiana. Ant Van Leeuw 100:497–506. doi:10.1007/s10482-011-9605-y
Lachance MA (1995) Yeast communities in a natural tequila fermentation. Ant Van Leeuw 68:151–160
Zott K, Miot-Sertier C, Claisse O, Lonvaud-Funel A, Masneuf-Pomarede I (2008) Dynamics and diversity of non-Saccharomyces yeasts during the early stages in winemaking. Int J Food Microbiol 125:197–203. doi:10.1016/j.ijfoodmicro.2008.04.001
Estrada-Godina AR, Cruz-Guerrero AE, Lappe P, Ulloa M, García-Garibay M, Gómez-Ruiz L (2001) Isolation and identification of killer yeasts from Agave sap (aguamiel) and pulque. World J Microb Biotechnol 17:557–560
Marquina D, Peres C, Caldas FV, Marques JF, Peinado JM, Spencer-Martins I (1992) Characterization of the yeast population in olive brines. Lett Appl Microbiol 14:279–283. doi:10.1111/j.1472-765X.1992.tb00705.x
Morais PB, Rosa CA, Linardi VR, Pataro C, Maia ABRA (1997) Short communication: Characterization and succession of yeast populations associated with spontaneous fermentations during the production of Brazilian sugar-cane aguardente. World J Microbiol Biotechnol 13:241–243
Shrestha P, Ibáñez AB, Bauer S, Glassman SI, Szaro TM, Bruns TD, Taylor JW (2015) Fungi isolated from Miscanthus and sugarcane: biomass conversion, fungal enzymes, and hydrolysis of plant cell wall polymers. Biotechnol Biofuels 8(1):1
CBS-KNAW Fungal Biodiversity Centre (2015) http://www.cbs.knaw.nl/Collections/. Last accessed 22 April 2015
Arrizon J, Morel S, Gschaedler A, Monsan P (2012) Fructanase and fructosyltransferase activity of non-Saccharomyces yeasts isolated from fermenting musts of Mezcal. Bioresour Technol 110:560–565. doi:10.1016/j.biortech.2012.01.112
Amaya-Delgado L, Herrera-López EJ, Arrizon J, Arellano-Plaza M, Gschaedler A (2013) Performance evaluation of Pichia kluyveri, Kluyveromyces marxianus and Saccharomyces cerevisiae in industrial tequila fermentation. World J Microb Biotechnol 29:875–881. doi:10.1007/s11274-012-1242-8
Garcia-Moya E, Romero-Manzanares A, Nobel PS (2011) Highlights for Agave productivity. GCB Bioenergy 3:4–14. doi:10.1111/j.1757-1707.2010.01078.x
Streit WR, Schmitz RA (2004) Metagenomics—the key to the uncultured microbes. Cur Opin Microbiol 7:492–498. doi:10.1016/j.mib.2004.08.002
Aguilar-Uscanga B, Arrizon J, Ramirez J, Solis-Pacheco J (2007) Effect of Agave tequilana juice on cell wall polysaccharides of three Saccharomyces cerevisiae strains from different origins. Ant van Leeuw 91:151–157. doi:10.1007/s10482-006-9106-6
Berthels NJ, Cordero Otero RR, Bauer FF, Thevelein JM, Pretorius IS (2004) Discrepancy in glucose and fructose utilisation during fermentation by Saccharomyces cerevisiae wine yeast strains. FEMS Yst Res 4:683–689. doi:10.1016/j.femsyr.2004.02.005
Flood AE, Johns MR, White ET (1996) Mutarotation of D-fructose in aqueous-ethanolic solutions and its influence on crystallisation. Carbohydr Res 288:45–56. doi:10.1016/S0008-6215(96)90775-2
Arrizon J, Fiore C, Acosta G, Romano P, Gschaedler A (2006) Fermentation behaviour and volatile compound production by agave and grape must yeasts in high sugar Agave tequilana and grape must fermentations. Ant Van Leeuw 89:181–189. doi:10.1007/s10482-005-9022-1
Bisson LF (1999) Stuck and sluggish fermentations. Am J Enol Vitic 50:107–119
Shrestha P, Ibáñez A, Bauer S, Glassman S, Szaro TM, Bruns TD, Taylor JW (2015) Fungi isolated from Miscanthus and sugarcane: biomass conversion, fungal enzymes, and hydrolysis of plant cell wall polymers. Biotechnol Biofuels 8:38. doi:10.1186/s13068-015-0221-3
Mielenz JR, Rodriguez M, Thompson OA, Yang X, Yin H (2015) Development of Agave as a dedicated biomass source: production of biofuels from whole plants. Biotechnol Biofuels 8(1):1
Somerville C, Youngs H, Taylor C, Davis SC, Long SP (2010) Feedstocks for lignocellulosic biofuels. Science 329:790–792. doi:10.1126/science.1189268
Valle-Rodríguez J, Hernández-Cortés G, Córdova J, Estarrón-Espinosa M, Díaz-Montaño D (2012) Fermentation of Agave tequilana juice by Kloeckera africana: influence of amino-acid supplementations. Ant Van Leeuw 101:195–204. doi:10.1007/s10482-011-9622-x
Hernández‐Cortés G, Córdova‐López JA, Herrera‐López EJ, Morán‐Marroquín GA, Valle‐Rodríguez JO, Díaz‐Montaño DM (2010) Effect of pH, aeration and feeding non‐sterilized Agave juice in a continuous Agave juice fermentation. J Sci Food Agr 90:1423–1428. doi:10.1002/jsfa.3957
Acknowledgments
This work was supported by grants from the Australian Research Council (ARC). We would like to thank Professor Joseph Holtum of James Cook University for assistance with Agave sample collection and transport of materials. Our thanks are also due to Associate Professor Paul Grbin (University of Adelaide) for providing access to his laboratory facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corbin, K.R., Betts, N.S., van Holst, N. et al. Low-Input Fermentations of Agave tequilana Leaf Juice Generate High Returns on Ethanol Yields. Bioenerg. Res. 9, 1142–1154 (2016). https://doi.org/10.1007/s12155-016-9755-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-016-9755-x