Abstract
Background
A new image reconstruction process termed the MUS method (masking process on unsmoothed images) was developed to eliminate artifacts, especially those in the inferior wall. We compared diagnostic performance between the MUS and conventional method in stress myocardial perfusion SPECT (MPS).
Methods
Enrolled were 126 patients who underwent stress-rest MPS with 99 m Tc-MIBI. Patients were divided into two groups: 91 with < 50% stenosis in the RCA or LCX (non-ischemia group) and 35 patients with ≥ 90% stenosis or FFR-positive in the RCA (ischemia group), according to coronary CT or coronary angiography within 3 months of MPS. Ischemic heart disease (IHD) was considered positive when the summed difference score of five segments corresponding to the inferior wall region was ≥ 2.
Results
Sensitivity was comparable between the MUS method and the conventional method (ordered subset expectation maximization; OSEM) (51% vs 54%, respectively; (p = 0.366), specificity was significantly higher using the MUS method (87% vs 77%, respectively; p < 0.05), and diagnostic performance was higher using the MUS method (area under curve [AUC], conventional 0.61 vs. MUS 0.69, p = 0.138). In evaluation of 87 patients after excluding 39 who received additional prone imaging, sensitivity using the MUS method was 44%, which was comparable to 44% using the conventional method but specificity was 90%, which was significantly higher than 77% using the conventional method (p < 0.05). The diagnostic performance of the MUS method was higher (AUC, conventional 0.60 vs. MUS 0.67, p = 0.185).
Conclusion
Use of the MUS method improved specificity in diagnosis of IHD while maintaining sensitivity, compared with the conventional method. The MUS method can achieve an improvement in diagnostic accuracy equivalent to the supine position, particularly in patients who have difficulty performing the prone position, without increasing the patient burden.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stress myocardial perfusion scintigraphy (MPS) permits non-invasive assessment of ischemia and is widely used for the diagnosis of coronary artery disease. Western guidelines recommend MPS as a class I first-line examination for patients with suspected chronic coronary artery disease [1, 2]. As redistribution of technetium (Tc) myocardial perfusion scintigraphy preparations is not prominent [3], the radioisotope (RI) preparation is generally administered twice during stress and rest, and scintigraphy is started after an interval of about 30 min after administration to eliminate the effect of extracardiac accumulation. Extracardiac accumulation of the administered RI preparation in abdominal organs such as the liver and gallbladder at this time affects the myocardial count and causes artifact. This phenomenon is observed relatively frequently with high-energy Tc preparations and often causes a decline in diagnostic performance, particularly in the inferior wall [4, 5].
To prevent this artifact, additional imaging in the prone position [6] and attenuation correction (AC) have been implemented with single photon emission computerized tomography/computed tomography (SPECT/CT) [7], in addition to image reconstruction using the ordered subset expectation maximization (OSEM) method. Several studies have reported improvements in diagnostic accuracy by the addition of prone imaging [4, 8, 9]. However, additional prone imaging can only be applied in limited cases because it prolongs the examination time and it can be difficult to perform depending on the patient’s condition.
CardioMUSk (masking process on unsmoothed images; MUS method, PDRadiopharma Inc., Tokyo, Japan) has recently been developed as a new image processing flow [10]. The processing flow in this method is characterized by first extracting the myocardium alone by masking, after which smoothing is performed, which suppresses the effects of subdiaphragmatic high activity (Fig. 1). Accordingly, no new imaging needs to be added in adoption of this method. Although suppression of subdiaphragmatic effects on the myocardium has been reported in phantom experiments [10, 11], there are no reports regarding the usefulness of this image processing flow for improving clinical diagnostic performance. Therefore, the aim of the present study was to compare diagnostic performance of inferior wall imaging between the MUS method and the conventional method in patients with no stenosis ≥ 50% or with ischemia in the inferior wall region.
Materials and methods
Patient population
The subjects of this retrospective study were 126 consecutive patients who underwent stress myocardial perfusion scintigraphy using a Tc-sestamibi (MIBI, PDRadiopharma) preparation between 1 April 2017 and 30 June 2021. Of the 126 patients, 91 had no stenosis ≥ 50% in either the right coronary artery (RCA) or the left circumflex artery (LCX) on coronary computed tomography angiography (CCTA) or coronary angiography (CAG) performed within 3 months before or after the examination (ischemia-negative group) and 35 had ≥ 90% stenosis or were positive for fractional flow reserve (FFR) in the RCA (ischemia-positive group) (Fig. 2). FFR refers only to invasive FFR performed during coronary angiography and does not include FFRct. Patients who had non-dominant RCA were excluded. Image reconstruction was performed using either the conventional method, with data reconstructed using either the filtered back projection (FBP) method as originally employed in our hospital or by the OSEM method (with the reconstructed data listed separately) or using the MUS method. Diagnostic performance in the inferior wall region was compared among the methods.
Flow chart of target patients. There were 661 patients who underwent stress myocardial perfusion scintigraphy with Tc-MIBI preparations between April 1, 2017 and June 30, 2021. Of these, 200 patients had coronary computed tomography angiography (CCTA) or angiography within 3 months before or after the examination date. Of these, 15 cases with missing data or difficult image evaluation were excluded. 91 patients without more than 50% stenosis in the right coronary artery (RCA) or left circumflex coronary artery (LCX) were defined as ischemia-negative group. 35 patients with more than 90% stenosis in the RCA or positive fractional flow reserve (FFR) were defined as ischemia-positive group
The present study was approved by the Institutional Medical Ethics Committee and complies with the Declaration of Helsinki. The need for informed consent was waived due to the retrospective nature of the analysis.
MPS acquisition
The normal stress-first one-day protocol was adopted. As the RI preparation, 99 m Tc-MIBI was administered at 260–370 MBq during stress and at 520–590 MBq during rest. The stress test was performed using an ergometer in compliance with the modified Bruce protocol with the RI preparation injected about 2 min before the end of exercise. If stress loading was judged not to be tolerated, it was substituted by pharmacological loading by intravenous administration of adenosine (120 μg/kg/m). Imaging was performed approximately 30 min after the RI injection. Whether additional imaging in the prone position should be performed was judged by a cardiologist and a nuclear medicine technologist according to the degree of extracardiac accumulation in the stress scintigrams and whether the patient could maintain the prone position. Images obtained in the prone position were acquired immediately after the normal stress image acquisition, and the images taken in the supine position were also reconstructed using the MUS method.
Images were acquired using the Symbia S (Siemens Healthcare, Erlangen, Germany) SPECT system with a low-energy, high-resolution collimator. Data were collected with a matrix size of 64 × 6 pixels (enlargement rate: 1.45, pixel size: 6.6 mm) using the step and shoot method (6°/step, 60 view) with a radius of rotation of 25 cm. Imaging was performed at 28 s/view during loading and at 21 s/view during rest.
MPS processing
Images were processed by enlarged reconstruction (pixel size: 3.3 mm) using FBP with a pre-treatment Butterworth filter (cutoff: 0.42 cycles/cm, order: 8) in the FBP method; by enlarged reconstruction (pixel size: 3.3 mm) with collimator aperture correction using OSEM (Flash3D) (OSEM with 3D collimator beam modeling and optional AC) with a post-treatment Gaussian filter (FWHM: 13 mm) in the OSEM method; and by enlarged reconstruction (pixel size: 3.3 mm) with collimator aperture correction using OSEM (Flash3D) with a post-treatment Gaussian filter (FWHM: 13 mm) in the MUS method. Post-treatment filtration was performed after oblique imaging.
MUS method
The masking process on unsmoothed images (MUS method) is a new image reconstruction flow devised by Komuro et al. for the purpose of improving image quality, and is based on suppression of artifacts caused by subdiaphragmatic accumulation. It is characterized by a masking process in which the myocardium alone is extracted prior to the smoothing process [10, 11]. In the masking process of the MUS method, the myocardial region is extracted by placement of a three-dimensional ellipsoid that surrounds the myocardium alone (not including subdiaphragmatic high activity) on reformed but unsmoothed horizontal long axis, vertical long axis, and short axis images. The effect of subdiaphragmatic accumulation on the inferior wall of the myocardium is suppressed by smoothing the extracted image. As a technical note, we adapted the MUS method by first manually changing the program order from the usual analysis flow and set the smoothing process to be performed last. No new equipment or additional processing was required.
Image analyses
An experienced reader not informed of the results of CCTA or CAG interpreted the MPS images according to the American Society of Nuclear Cardiology Guidelines for Reporting [12]. Segment analysis was performed by visual evaluation of five segments (Areas 3, 4, 9, 10, and 15 in the 17-segment model shown in Fig. 3) in the inferior wall region that are prone to artifact due to extracardiac accumulation. The severity of perfusion abnormalities was evaluated visually using a 5-point scale. Ischemia was judged to be positive when the summed difference score (SDS) was ≥ 2.
Artifacts
To check the degree of artifact due to scattered radiation from extracardiac accumulations or the effect of partial volume effect (PVE) caused by blurring, artifact was evaluated using a 3-point scale, as follows. No artifact or minimal unassessable artifact was graded as 0, extracardiac accumulation that had no contact with the myocardium was graded as 1, and accumulation in contact with and inseparable from the myocardium was graded as 2 (Fig. 4).
Coronary angiography
The degree of stenosis detected by CCTA or CAG performed within 3 months of MPS was evaluated visually by an experienced cardiologist who was blinded to the results of scintigraphy. Ischemia was judged to be negative when the degree of stenosis was ≤ 50% in both the RCA and LCX. Ischemia was judged to be positive if there was ≥ 90% stenosis or if the FFR was ≤ 0.8 in the RCA.
Reproducibility analysis
To evaluate inter-observer reproducibility of MPS, images of all 126 patients were rated by two experienced readers using a 5-point scale concerning five segments corresponding to the RCA. Images reconstructed using the conventional and the MUS methods were rated. Both readers performed the evaluation using the same method, and the degree of coincidence in SDS score was examined.
Statistical analysis
The numerical data of variables showing normal distribution are expressed as the mean ± standard deviation, those of variables showing non-normal distribution as the median and inter-quartile range, and categorical variables as the number (and percentage) of patients in each group. Diagnostic performance was evaluated by calculating the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the receiver-operating characteristic curve (AUC) by receiver-operating characteristic (ROC) analysis. Differences in sensitivity and specificity between the conventional and MUS methods were compared using the McNemar test [13, 14]. AUC was compared using the DeLong test [15]. The diagnostic coincidence rate between the two raters was evaluated using the κ coefficient. All statistical tests were two-tailed, and P < 0.05 was regarded as significant. Calculations were performed using JMP Pro 14.2 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
Prone imaging was added in 39 (31%) of the 126 patients.
The mean age of all subjects was 69 ± 12 years, and 70% were males (Table 1). Coronary risk factors are shown in Table 1. Pretest probability according to the 2019 ESC Guidelines was 21.4 ± 12.3% [1]. Extracardiac accumulation was observed in 92 (72%) patients.
The coronary artery was evaluated within 3 months by CAG in 89 (70%) patients and by CCTA in 59 (46%). There was no disagreement in diagnosis in any patient who underwent both examinations. In total, 35 patients were determined to be positive for ischemia, of which 34 diagnoses were determined based on stenosis and 1 on FFR. The results of CAG were considered true if the CCTA was unassessable.
Patient groups were also compared according to whether or not imaging was performed in the prone position (Table 1). Patients who underwent imaging in the prone position were more likely to be male and have higher PTP scores and previous myocardial infarction compared with those that did not.
Comparison of diagnostic performance between the conventional method and MUS method
Ischemia was defined as negative when stenosis was ≤ 50% in the RCA and LCX and as positive when there was ≥ 90% stenosis or when FFR was ≤ 0.8 in the RCA. According to these definitions, the sensitivity and specificity of the FBP and OSEM methods were 45.7% (95% confidence interval [CI]: 41.6–50.4) vs 54.3% (45.6–63.0) and 75.8% (68.5–83.5) vs 76.9% (64.1–89.7), respectively. Sensitivity using the OSEM method (54%) was comparable to that using the FBP method (46%) (p = 1.00), and specificity using the OSEM method (79%) was comparable to that using the FBP method (76%) (p = 0.763). The AUC of the OSEM method was 0.61 (95%CI 0.52–0.70), which was comparable to that of the FBP method (p = 0.873). Using the MUS method, sensitivity was 51.4% (95% CI: 42.3–59.7) and specificity was 86.8% (81.1–92.9) (Table 2).
Comparing diagnostic performance between the MUS and OSEM methods, sensitivity was comparable between the methods (51% vs 54%, p = 0.366), whereas specificity was significantly higher using the MUS method (87% vs 77%, p < 0.05). Figure 5 shows ROC curves for the OSEM and MUS methods. AUC using the MUS method was 0.69 (95%CI: 0.59–0.77), which was higher than that using the OSEM method, 0.61 (95%CI: 0.52–0.70), but the difference was not significant (p = 0.138). Use of the MUS method resulted in a change in diagnosis from false positive to negative in 14 patients (11%) in the ischemia-negative group (Fig. 6).
After excluding the 39 patients who received additional prone imaging, evaluation of the remaining 87 patients showed sensitivity and specificity for the FBP and OSEM methods of 43.8% (95% CI: 35.3–52.7) vs 43.8% (35.3–52.7) and 74.6% (67.4–82.6) vs 76.9% (68.0–85.8), respectively. Sensitivity using the OSEM method (44%) was comparable to that using the FBP method (44%) (p = 1.00), and specificity using the OSEM method (77%) was also comparable to that using the FBP method (75%) (p = 0.763). The AUC of the OSEM method was 0.60 (95%CI 0.46–0.72), which was comparable to that of the FBP method (p = 0.891).
Comparing diagnostic performance between the OSEM and MUS methods, sensitivity using the MUS method was 43.8% (95% CI: 35.3–52.7), which was comparable to that using the OSEM method (44%) (p = 1.000). Specificity was 90.0% (84.8–95.2) using the MUS method, which was significantly higher than that using the OSEM method (77%) (p < 0.05) (Table 3). Figure 7 shows ROC curves for the OSEM and MUS methods. AUC using the MUS method was 0.67 (95%CI: 0.59–0.77), which was higher than that using the OSEM method (0.60; 95%CI: 0.46–0.72), but the difference was not significant (p = 0.185).
Evaluation of the 39 patients who received additional prone imaging showed sensitivity and specificity for the FBP and OSEM methods of 47.4% (95% CI: 31.7–63.1) vs 47.4% (31.7–63.1) and 80.0% (67.5–92.6) vs 80.0% (67.5–92.6), respectively. Sensitivity using the OSEM method (47%) was comparable to that using the FBP method (47%) (p = 1.000), and specificity using the OSEM method (80%) was also comparable to that using the FBP method (80%) (p = 1.000). The AUC of the OSEM method was 0.64 (95%CI 0.48–0.77), which was comparable to that using the FBP method (p = 1.000).
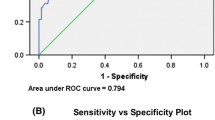
Comparing diagnostic performance between the OSEM and MUS methods, sensitivity using the MUS method was 73.7% (95% CI: 59.9–87.5), which was higher than that using the OSEM method (47%) but the difference was not significantly different (p = 0.480). Specificity using the MUS method was 85.0% (73.8–96.2), which was comparable to that using the OSEM method (80%) (p = 0.564) (Table 4). Figure 8 shows ROC curves for the MUS and OSEM methods. AUC using the MUS method was 0.79 (95%CI: 0.64–0.89), which was significantly higher than that using the OSEM method (0.64; 95%CI: 0.48–0.77) (p = 0.045).
Reproducibility analysis
Regarding the degree of coincidence of the diagnosis, the κ coefficient was satisfactory using both the conventional (0.81) and MUS (0.82) methods.
Discussion
Artifact due to extracardiac accumulation in stress MPS is one of the major causes of decline in diagnostic performance, particularly in the inferior wall region [16, 17]. The recently developed MUS method mitigates such artifact. Although based on our experience with only a small number of cases, the present study is the first to compare diagnostic accuracy between the conventional and MUS methods. Compared with the conventional method, use of the MUS method improved specificity with no change in sensitivity, and this tendency was more notable in patients who did not undergo prone imaging. In patients who did undergo prone imaging, diagnostic performance was significantly improved using the MUS method compared with the conventional method.
In stress MPS using a Tc radiopharmaceutical, artifact due to extracardiac accumulation was observed in approximately 70% of the total patients. The effects of extracardiac accumulation include (1) an apparent relative decrease in the count of the entire myocardium due to a higher count of extracardiac accumulation than the myocardial count, (2) a decrease in the count of the inferior myocardial wall due to extracardiac accumulation caused by the ramp filter in FBP, and (3) an increase in the count in part of the inferior wall and a decrease in the count in the surrounding areas of the myocardium due to PVE caused by the effect of extracardiac accumulation. To reduce these artifacts, measures such as changing the display conditions and masking out areas other than the myocardium concerning effect (1) and adoption of a statistical image reconstruction method (OSEM) concerning effect (2) have been tentatively used [18]. However, in effect (3), PVE is enhanced by the smoothing process and is made more prominent by AC, which increases the relative count of extracardiac accumulation. Currently, the addition of prone imaging is reported as a useful method for minimizing this effect and improving the diagnostic accuracy [8, 9, 16, 17], but it has the drawbacks of increased patient burden and the limited number of patients for whom it can be applied.
The improvement in specificity using the MUS method that was observed in the present study can be ascribed largely to the suppression of the effect of PVE and artifact associated with FBP among the effects of extracardiac accumulation mentioned above, and a consequent decrease in false positives. The MUS method can be implemented simply by changing the image processing method and the order of processes from the conventional method, by extracting the myocardial area first and performing image smoothing next. Applying the smoothing process (which was originally performed to reduce high-frequency noise) also to extracardiac accumulation affects the myocardial count and causes artifact; however, by physically cutting extracardiac accumulation before this process, its effect can be reduced. This is considered to have prevented decreases in count occurring around the near myocardium due to the PVE from extracardiac accumulation, and decreased the number of false positives accordingly. Furthermore, in this study, only OSEM was used as the image reconstruction method in the MUS method group. The OSEM method has advantages including (1) less effect of radial artifact caused by extracardiac accumulation, (2) good signal/noise ratio (S/N) in the low-count region, and (3) the possibility of incorporation of absorption correction and scattered ray correction. However, the OSEM method requires longer processing time compared with FBP and depending on the conditions, the contrast ratio may decrease and noise may increase. For these reasons, some facilities, such as ours, have not adopted the OSEM method [19]. In the MUS method, careful isolation of the myocardium in reconstruction in the first step of the flow enables the most effective reduction of artifact in the subsequent smoothing process. Therefore, we consider that the initial myocardial isolation can be achieved better by reconstruction using the OSEM method, which is less susceptible to the effect of artifact than FBP, resulting in a greater improvement in diagnostic performance. In this study, the change to the OSEM method alone did not improve diagnostic performance. Although the effect of artifacts was suppressed compared to FBP, the effect of PVE due to extracardiac accumulation was not suppressed, and thus the change in the order of processing flow by the MUS method was considered to be of importance.
In this study, no difference in sensitivity was observed between the conventional and MUS methods. We consider that sensitivity did not improve for the reason that both the MUS and OSEM methods contributed to reducing the count due to extracardiac accumulation. Sensitivity was low compared with specificity in the present study, possibly for the reason that ischemia may not be inducible at stenosis of ≥ 90%, depending on the site and morphology of the stenosis.
Moreover, the improvement in specificity in the MUS group compared with the conventional method group was more notable in the patients who did not undergo prone imaging than in the overall patients. Several studies have reported that the accuracy of detection of coronary artery disease (CAD) was improved by prone imaging in stress myocardial perfusion scintigraphy [8, 9], and have reported improvement in specificity from 67 to 84% by prone imaging compared with supine imaging, particularly in the area supplied by the RCA [5]. In our study, specificity in the MUS group was 86.8% in all patients and 90.0% in patients who did not undergo prone imaging. Tolerance of prone imaging is limited by the patient’s condition because additional imaging inevitably increases patient burden and prolongs the examination time. However, the MUS method requires no additional imaging and is expected to offer improved diagnostic performance in all patients compared with the conventional method by shortening the examination time and improving the efficiency of the workflow without increasing patient burden.
Lastly, when comparison with the conventional method was made in the 39 patients who underwent prone imaging, diagnostic performance was significantly improved using the MUS method. As masking is performed first in the image processing procedure of the MUS method, masking is considered to be more accurate when the myocardium is isolated from extracardiac accumulation, which contributes to improved diagnostic performance. Prone imaging has the advantages of inferior displacement of the diaphragm and abdominal organs, compression of the precordial soft tissues including breast tissues, anterior displacement of the heart, and a decrease in the patient’s body movements [8], which are considered to more clearly isolate the myocardium from extracardiac accumulation and significantly improve the diagnostic performance. Concerning the improvement in sensitivity, prone imaging in particular may have caused an additional decrease in accumulation due to the closeness of the heart to the detector and the increase in the distance of the heart from the diaphragm, but its concomitant use with the MUS method may have contributed to the observed decrease in accumulation by eliminating effects other than artifacts. Although we did not select patients for this study based on whether or not imaging could be performed in the prone position, patient selection on this basis could have better proven the usefulness of the MUS method. The present findings suggest that diagnostic performance can be most improved by combining the MUS method with prone imaging.
Limitations
This study has several limitations. First, it was a single-center retrospective study, and careful interpretation of the results is necessary because of the small number of subjects. Second, as the judgment of whether prone imaging should be added was made by a cardiologist and a nuclear medicine technologist after checking the stress scintigrams, there may have been bias in the selection of patients. Third, the presence or absence of ischemia was judged according to the evaluation of stenosis of the epicardial coronary artery alone, which may have resulted in underestimation of the presence of microvascular CAD. Fourth, ischemia was determined as “Percent diameter stenosis ≥ 90% and FFR positive,” which is not always consistent with ischemia and may underestimate or overestimate sensitivity.
Conclusion
Using the MUS method, the specificity of the diagnosis in the inferior wall region could be improved while maintaining sensitivity compared with the conventional method, and diagnostic accuracy could be improved to that obtainable by prone imaging in patients in whom prone imaging was difficult to perform. In addition, diagnostic accuracy was maximized by the addition of the MUS method to prone imaging.
Data availability
All data that support the findings of this study are available from the corresponding authors.
References
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77.
Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, et al. AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain. J Am Coll Cardiol. 2021. https://doi.org/10.1016/j.jacc.2021.07.053.
Hesse B, Tagil K, Cuocolo A, Anagnostopoulos C, Bardies M, Bax J, et al. EANM/ESC procedural guidelines for myocardial perfusion imaging in nuclear cardiology. Eur J Nucl Med Mol Imaging. 2005;32:855–97.
Mirshahvalad SA, Chavoshi M, Hekmat S. Diagnostic performance of prone-only myocardial perfusion imaging versus coronary angiography in the detection of coronary artery disease: A systematic review and meta-analysis. J Nucl Cardiol. 2020. https://doi.org/10.1007/s12350-020-02376-x.
Singh B, Bateman TM, Case JA, Heller G. Attenuation artifact, attenuation correction, and the future of myocardial perfusion SPECT. J Nucl Cardiol. 2007;14:153–64.
Segall GM, Davis MJ. Prone versus supine thallium myocardial SPECT: a method to decrease artifactual inferior wall defects. J Nucl Med. 1989;30:548–55.
Zhang D, Yang BH, Wu NY, Mok GSP. Respiratory average CT for attenuation correction in myocardial perfusion SPECT/CT. Ann Nucl Med. 2017;31:172–80.
Arsanjani R, Hayes SW, Fish M, Shalev A, Nakanishi R, Thomson LE, et al. Two-position supine/prone myocardial perfusion SPECT (MPS) imaging improves visual inter-observer correlation and agreement. J Nucl Cardiol. 2014;21:703–11.
Taasan V, Wokhlu A, Taasan MV, Dusaj RS, Mehta RS, Kraft S, et al. Comparative accuracy of supine-only and combined supine-prone myocardial perfusion imaging in men. J Nucl Cardiol. 2016;23:1470–6.
Komuro A, Teraoka S, Ishikawa Y, Tsuboko M, Tanno M, Ishimori K, et al. Devising a method to suppress the effect of subdiaphragmatic high activity in 99mTc myocardial perfusion SPECT and examining its usefulness lncluding lmage reconstruction method. Kakuigakugijutsu. 2021;41:431–41.
Komuro A, Teraoka S, Ishikawa Y, Tsuboko M, Tanno M, Ishimori K, et al. A novel method to suppress the effect of subdiaphragmatic activity in 99mTc myocardial perfusion spect and evaluation of its usefulness using a myocardial phantom. Ann Nucl Cardiol. 2022;8:30–5.
Tilkemeier PL, Bourque J, Doukky R, Sanghani R, Weinberg RL. ASNC imaging guidelines for nuclear cardiology procedures. J Nucl Cardiol. 2017. https://doi.org/10.1007/s12350-017-1057-y.
Agresti A. Categorical data analysis. New York: John Wiley & Sons; 1990. p. 350–4.
Feuer EJ, Kessler LG. Test statistic and sample size for a two-sample McNemar test. Biometrics. 1989;45:629–36.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
DePuey EG. How to detect and avoid myocardial perfusion SPECT artifacts. J Nucl Med. 1994;35:699–702.
Nishina H, Slomka PJ, Abidov A, Yoda S, Akincioglu C, Kang X, et al. Combined supine and prone quantitative myocardial perfusion SPECT: method development and clinical validation in patients with no known coronary artery disease. J Nucl Med. 2006;47:51–8.
Juan Ramon A, Yang Y, Pretorius PH, Johnson KL, King MA, Wernick MN. Personalized models for injected activity levels in spect myocardial perfusion imaging. IEEE Trans Med Imaging. 2019;38:1466–76.
Bai J, Hashimoto J, Suzuki T, Nakahara T, Kubo A, Iwanaga S, et al. Comparison of image reconstruction algorithms in myocardial perfusion scintigraphy. Ann Nucl Med. 2001;15:79–83.
Acknowledgements
We appreciate the nuclear radiographers who assisted with the imaging and image composition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no financial conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kudo, A., Fujimoto, S., Aoshima, C. et al. First validation of stress myocardial perfusion scintigraphy using a novel reconstruction process. Ann Nucl Med 37, 390–399 (2023). https://doi.org/10.1007/s12149-023-01837-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-023-01837-w