Abstract
Objectives
225Ac-PSMA-617 therapy has shown good response in many recent studies. We report our experience of targeted alpha therapy with 225Ac-PSMA-617 in mCRPC patients who have failed therapy with taxanes.
Materials and methods
Thirty-eight patients with CRPC with progressive disease following at least one taxane-based chemotherapy received 225Ac-PSMA-617 between July 2017 and Nov 2019. Primary end point was a composite 50% PSA and radiological response. Secondary endpoints were PFS, OS, and changes in QOL. The differences in outcomes between patients with skeletal and lymph-node metastases versus those with visceral metastases were also studied.
Results
A composite response by predetermined criteria was observed in 25 (66%) of 38 patients. The median PFS was 8 months (95% CI 5.3–10.6 months). Median overall survival was 12 months (95% CI 9.1–14.9) with 16 patients alive at the time of censorship. There was no difference in response rates or survival statistics between patients with visceral metastases versus those with only bone and lymph-node metastases (Chi-square 1.51, df 1, Sig 0.218). The most common adverse effect was xerostomia. On the QOL Symptom score, Pain, Fatigue Insomnia, and constipation showed a significant improvement as compared to baseline.
Conclusions
225Ac-PSMA-617 is a safe and tolerable treatment option for mCRPC that demonstrates marked anti-tumour activity with improvement in quality of life even in patients of metastatic CRPC who have been previously treated with taxane-based chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Men with metastatic castration-resistant prostate cancer (mCRPC) who have failed treatments with agents like Abiraterone and Enzalutamide and taxane-based chemotherapy have limited options of therapy. Patients with soft-tissue metastases other than lymph nodes have a particularly poor prognosis [1]. Targeted alpha therapy (TAT) with 225Ac-PSMA-617 therapy has in recent studies shown durable responses in most patients [2]. We report our experience with 225Ac-PSMA-617 therapy in 38 patients where it was offered as a salvage therapy. We tried to analyse the therapeutic efficacy, toxicity profile, quality-of-life (QOL) changes, and the differential response in patients with bone only versus visceral metastases.
Materials and methods
Patients and study design
This is a single-centre, non-randomised retrospective analysis of the safety and efficacy of 225Ac-PSMA-617 therapy in 38 consecutive patients with mCRPC who underwent 225Ac-PSMA-617 treatments as a salvage therapy between July 2017 and Nov 2019 at the Fortis Memorial Research Institute, Gurugram, India. Eligible patients were men, aged 18 years or older, with histologically confirmed prostate adenocarcinoma. Patients with tumours showing neuroendocrine differentiation or small cell subtypes were not eligible. Patients were required to have previously received at least one but not more than two taxane-based chemotherapy regimens, regardless of prior exposure to novel hormonal drugs. Other inclusion criteria included: documented prostate cancer progression at study entry defined by either rising serum prostate-specific antigen (PSA) levels or radiological progression [3, 4]; a castrate testosterone concentration of less than 50 ng/dL; an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less; and adequate organ function (including haemoglobin ≥ 9 g/dL, platelet counts > 100,000 thou/ml3, eGFR > 60 ml/min and albumin > 2.5 g/dl). All patients underwent a 68 Ga-PSMA-11 scan prior to the therapy to confirm the overexpression of PSMA receptors. Only patients who demonstrated 68 Ga-PSMA-11 uptake more than or equal to the 68 Ga-PSMA-11 uptake in the parotid glands, in most of the metastatic sites, were considered eligible for the 225Ac-PSMA-617 therapy [5, 6]. The patient selection and tailoring process was based on visual imaging interpretation of the 68 Ga-PSMA-11 scans as there was no objective scoring of PSMA expression at the time of recruitment of the patients. Patients were informed about the experimental nature of this therapy. Patients provided written informed consent at enrolment and prior to administration of each dose of 225Ac-PSMA-617 therapy. The study was approved by the Institutional Ethics Committee.
Treatment protocol
All patients received 225Ac-PSMA-617 at a dose of 100 kBq/kg body weight at intervals of 8 weeks for 3 doses or until evidence of radiographic progression, unacceptable toxicity, or the patient’s decision to discontinue [7]. Discontinuation based solely on rising PSA in the absence of radiological or clinical progression was discouraged. Patients with limited radiological progression but with continuing clinical benefit were offered the option of further 225Ac-PSMA-617 doses till evidence of unequivocal clinical progression, provided that the patient did not have any limiting toxicities. Since 225Ac-PSMA-617 therapy was offered to patients after they had exhausted most standard clinical options, nearly all patients received only metronomic chemotherapy or best supportive care after they became ineligible for any further 225Ac-PSMA-617 treatments.
Radiopharmaceutical administration
225Ac-PSMA-617 labelling was performed using protocol described [8]. The radio peptide was injected intravenously through a venous cannula using a syringe pump over 5 min. An average of 7.04 ± 1.6 MBq of 225Ac-PSMA-617 was administered per dose. During the course of administration, none of the patients developed any immediate side effects. The patients were isolated as inpatients for 24 h, covering urinary clearance of non-tumour-bound radioactivity. Patients underwent intravenous hydration using 1000 ml of Ringer Lactate/Normal Saline infusion. Patients were also advised oral hydration of 2L/m2 BSA in the ensuing week post-therapy.
Toxicity and response evaluation
Clinical assessments, review of adverse events, and routine haematology and biochemical tests were carried out at intervals of 2 weeks during the therapy cycles and subsequently every 8 weeks. Assessments of adverse events were recorded and reported as per the National Cancer Institute Common Terminology Criteria for Adverse Events [CTCAE] version 5 [9]. PSA concentrations were measured every month 68 Ga-PSMA-11 PET CT scans were performed at 8 weeks following 2 doses of 225Ac-PSMA-617 and thereafter if there was evidence of PSA progression or onset of a new symptom. In patients with definite evidence of PSA progression, the 68 Ga-PSMA-11 PET CT scan was performed earlier than scheduled to confirm progression. The 68 Ga-PSMA-11 PET CT scans were done with diagnostic CT with intravenous contrast administration unless contraindicated due to deranged renal function or contrast allergy. The CT part of the PET CT scan images was used for assessing response by RECIST 1.1 criteria.
QOL outcomes using the patient reported EORTC CLC30 questionnaire were measured at baseline and then at 8 weeks following each dose of 225Ac therapy till the date of censure/progression or death. Domain scores were calculated according to the established scoring manual provided by the EORTC QOL group [10, 11]. Unidimensional assessment of pain was done using the standard numerical pain scale (NPS) score ranging from 0 to 10 (where 0 meant no pain and 10 meant worst possible pain). Multidimensional assessment of pain was done with Brief Pain Inventory Questionnaire (BPI) [12]. The patients were assessed for pain prior to start of therapy and then reassessed using both the NPS and BPI on each visit.
Definition of response
The primary endpoint was confirmed response; defined as a composite of a decline of 50% from baseline PSA concentration (PSA50) and objective response by RECIST 1.1 criteria in patients with measurable lesions [13]. A composite of 50% decline in PSA level with the absence of less than 2 new lesions was considered as a measure of response in patients with skeletal only metastases.
Secondary endpoints were: progression-free survival, defined as the time from the first dose of 225Ac-PSMA-617 to first evidence of progression or death or the end of the study period; overall survival, defined as the time from administration of first dose of 225Ac-PSMA-617 to death from any cause. The changes in QOL, safety, and tolerability of 225Ac therapy were also studied. A comparison was also made of the response rates and survival of patients with lymph node and skeletal metastases versus those with visceral metastases at study entry.
Progression was again defined as a composite of PSA progression and radiological progression. PSA progression was defined as an increase of 25% from baseline value. Radiological progression in cases with measurable lesions was considered according to RECIST Criteria. Radiological progression in skeletal only metastatic lesions was based on visualization of at least 2 new skeletal lesions on the 68 Ga-PSMA-11 PET CT scans. Isolated PSA progression without radiological progression was not considered as progressive disease.
Analysis of the primary endpoint was triggered when all patients had completed at least 8 weeks since last treatment. Toxicity was analysed in all patients and the worst grades of adverse events that occurred during treatment were reported. Patients who discontinued treatment prior to completion of two doses of 225Ac-PSMA-617 therapy due to progression or toxicity and had no follow-up assessments for the primary endpoint were considered non-responders. For progression-free survival, patients who did not progress were censored at the last scheduled disease assessment during the study or date of death, whichever, occurred first. Patients alive at the end of the study were censored at the date of last scheduled assessment for the analysis of overall survival.
Statistical analysis
Response is presented along with exact two-sided 95% CIs. Percentage changes from baseline in PSA concentration and the sum of target lesions (RECIST 1.1) are represented in waterfall plots. Time to event endpoints are summarised by Kaplan–Meier curves, and median times estimated with 95% CIs. Descriptive statistics were used for the baseline demographic and clinical characteristics. The duration of follow-up was calculated by the reverse Kaplan–Meier approach. Mean (with standard deviation) of each scale of the EORTC QLQ-C30 at each time point was calculated. Paired t test was used to compare the scores at baseline and 8 weeks after the last dose of 225Ac-PSMA-617 therapy with a p < 0.05 considered statistically significant.
The trial was not powered for head to head direct comparisons of the two cohorts of patients with lymph node and skeletal metastases versus those with visceral metastases at study entry, and so, tests to compare them were considered hypothesis generating (i.e., χ2 test to compare the proportion of patients with a response and log-rank test to compare Kaplan–Meier curves). Statistical analyses were done with the SPSS software (version 23).
Results
Patient characteristics
Thirty-eight patients underwent 225Ac-PSMA-617 therapy between July 2017 and Nov 2019. The median age of the patients was 68 (range 53–84). None of the patients had any history of any other malignancy other than prostate cancer. All patients had radiological evidence of progressive disease at entry. Radiological progression was determined as per the RECIST 1.1 Criteria in patients with measurable lesions and/or appearance of more than two new sites of skeletal metastases on a serial bone scan or serial 68 Ga-PSMA scans in patients with bone only metastases. In 35 patients, there was also an increase in serum PSA levels of either greater than 25% and > 2 ng/ml above nadir, confirmed on at least two timepoints at least 3 weeks apart.
Prior therapies
12 (31.5%) patients had organ confined disease at initial diagnosis, while the rest were metastatic at initial diagnosis. The details of prior therapies are mentioned in Table 1. All patients had achieved castrate resistant status prior to study entry. The median duration of achieving castrate resistance was 28 months from initial diagnosis. 24 (63%) of the 38 patients received Abiraterone therapy in the course of their treatment. The median duration of response on Abiraterone was 11.16 months. 13 (34%) of the 38 patients also received Enzalutamide therapy and the median duration of clinical benefit on Enzalutamide was 5.1 months. All patients had received at least one taxane-based chemotherapy, having received a median of 6 cycles of therapy. The median duration between the diagnosis of prostate cancer and the first dose of 225Ac-PSMA-617 therapy was 68 months. The details of prior therapies are mentioned in Table 1. The durations of response of prior therapies and their relative contribution to the treatment chronology are shown in a Swimmers plot in Fig. 1a and Fig. 1b.
Pattern of metastatic disease at entry
All the patients had progressive disease prior to 225Ac-PSMA-617 therapy. All patients had a high burden of metastatic disease having four or more sites of skeletal metastases with at least one lesion outside the axial skeleton and/or visceral metastasis. Nine (24%) patients had visceral metastases in addition to skeletal and nodal metastases. The sites of metastases at study entry are mentioned in Table 1.
Treatment efficacy
Between July 2017 and Nov 2019, thirty-eight patients of mCRPC received 225Ac-PSMA-617 therapy. The median number of therapies was 2 (Range 2–5). While our initial protocol included administering at least 3 doses of 225Ac-PSMA-617 therapy to all patients, 3 patients could receive only a single dose and 28 patients could receive only 2 doses of 225Ac-PSMA-617 therapy due to disease progression while on therapy. At the time of the data snapshot, four patients remained on 225Ac-PSMA-617 treatment. The median duration of follow-up was 14 months (CI 95% 8.3–19.6).
17 (45%) of the 38 patients had measurable lesions and were evaluable by the RECIST 1.1 criteria. Radiological response by RECIST 1.1 criteria was seen in 10 (59%) of the 17 patients (Fig. 2a). 16 patients had PR by RECIST 1.1 criteria, while 1 patient had CR. No other criteria of response were included in the analysis. All patients were evaluable for a PSA response. PSA50 response was observed in 25 (66%) of 38 patients (Fig. 2b); 20 (52.6%) patients achieved 50% PSA reduction after the 1st dose of 225Ac-PSMA-617, 3 (8.5%) patients achieved it after 2 doses of 225Ac-PSMA-617, while 2 (6%) patients achieved 50% PSA response only in the follow-up PSA concentrations after 3 doses of 225Ac-PSMA-617 therapy. A composite response by predetermined criteria was observed in 25 of the 38 patients (66%) patients (Figs. 3, 4, 5).
68Ga-PSMA-11 image done in a patient with extensive bone and lung metastases shows a near complete resolution of the lung metastases with a partial resolution of the skeletal metastases, 6 weeks post 225Ac-PSMA-617 therapy. The patient went on to receive two more doses of 225Ac-PSMA-617 and remained in response till the end of the study
a 68 Ga-PSMA-11 PET CT scan at study entry showed multiple skeletal metastases overexpressing PSMA receptors. b Post 3 doses of 225Ac-PSMA-617 patient had > 90% reduction in PSA levels with a complete disappearance of all PSMA avid lesions. c He developed a PSA relapse with reappearance of some of the PSMA avid lesions after about 4 months. d He was treated with 2 doses more of 225Ac-PSMA-617 with again a good PSA and radiological response
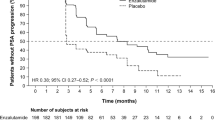
At the time of analysis, 26(68%) of the 38 patients either had progression or had died. The median progression-free survival was 8 months (95% CI 5.3–10.6 months) Fig. 6. 20 of the 38 patients had died of disease-related cause, while 2 had died of non-disease-related causes. Median overall survival was 12 months (95% CI 9.10–14.9) (Fig. 7a). The longest surviving patient without radiological progression was alive at 29 months from the 1st dose of 225Ac-PSMA-617 therapy at the last date of disease assessment.
a Kaplan–Meier survival curve showing overall survival in patients treated with 225Ac-PSMA-617 therapy. b Kaplan–Meier survival curve showing progression-free survival between the patients with visceral metastases versus those with only bone and lymph-node metastases in patients treated with 225Ac-PSMA-617 therapy
7 (18%) of the 38 patients had visceral metastases at trial entry. No statistically significant difference was found in the radiological progression-free survival between the patients with visceral metastases versus those with only bone and lymph-node metastases (Chi-square 1.51, df 1, Sig 0.218) (Fig. 7b). No significant difference in overall survival was found between the cohorts showing 50% fall in PSA levels and those who showed less than 50% fall in PSA levels from baseline (Chi-square 0.925, df 1, Sig 0.336).
Quality of life
The QOL measurements were analysed for 35 of the 38 patients. The QOL scores were missing for the 3 patients who progressed following a single dose of 225Ac-PSMA-617 therapy. EORTC QOL-C30 scores of the patients at baseline and 8 weeks following 2nd dose of 225Ac-PSMA-617 therapy are shown in Table 2. The Global QOL assessed by the EORTC QLQ-C30 showed a significant improvement over time. On the QOL Symptom score, Pain, Fatigue Insomnia, and constipation showed a significant improvement as compared to baseline. The physical, role, cognitive, and emotional functioning also improved as compared to baseline. There was no significant change in the social functioning.
Pain assessment
The unidimensional mean Non-Parametric Score (NPS) at the beginning of the study was 5. At 8 weeks following the first dose of 225Ac-PSMA-617, the NPS reduced to 3 which further reduced to 1 at the end of 8 weeks from 2nd dose of alpha therapy (Fig. 8a).Footnote 1 On the BPI score, there was a statistically significant fall in pain severity with an improvement in the metrics of worst pain, least pain, average pain, and pain right now. There was a reduction in the analgesic use with an improved response to analgesics. A statistically significant improvement from baseline was observed for BPI measurement of pain interference in general activity, sleep, and mood. Improvement in BPI measure of pain interference in walking ability was statistically significant only after 2 doses of 225Ac-PSMA-617. Values of other indicators also showed no significant difference (Table 3).
Adverse effects
The safety population included all 38 patients treated (Table 4). The tolerability profile was in line with what has been previously reported for 225Ac-PSMA-617 therapy [2, 14, 15] The common adverse events were dryness of mouth [37/38, (97%)] anaemia [29/38 (76%)], weight loss [15/38 (38%)], fatigue [18/38 (47%)] platelet count decreased [6/38 (16%)], and hearing loss [2/38 (5%)].
Dryness of mouth
The most common adverse effect was dryness of mouth with 37 (97%) of the 38 patients reporting varying degrees of dryness of mouth during the course of the treatments. 17 (45%) of the 38 patients had some dryness of mouth prior to the study entry. The dryness of mouth worsened in all these patients following 225Ac-PSMA-617 therapy. Of the 38 patients, 5 patients reported Gr III dryness of mouth according to the CTCAE Version 5, and 3 patients reported Grade II dryness of mouth, while 29 reported Gr I dryness. None of the patients, however, dropped out of the treatment schedules due to xerostomia. All 5 patients who developed CTCAE Gr III dryness of mouth had pre-existing xerostomia prior to start of 225Ac-PSMA-617 therapy. 5 of the 8 patients who survived beyond 1 year following 225Ac-PSMA-617 therapy reported partial reversal of the dryness of mouth with an improvement in the perception of taste with time.
Haematotoxicity
18 (47%) of the 38 patients had Grade I anaemia prior to the start of therapy, while 3 (8%) had grade III anaemia and thrombocytopenia requiring periodic blood/platelet transfusions. The bone marrow aspiration and biopsy of these three patients showed diffuse marrow infiltration by metastatic cells. Two of these three patients showed a marked radiological and biochemical response coupled with a progressive reduction in the need for blood transfusions, likely indicating a repopulation of their marrow by haematopoietic cells. 10 (55.5%) of the 18 other patients who had Gr I anaemia at baseline showed a worsening to Gr III anaemia. In all these patients, the worsening of anaemia was likely attributable to the progression of prostate cancer rather than the 225Ac-PSMA-617 treatment. There was no worsening of the anaemia in the rest of the 8 patients. There was appearance of Gr I anaemia in 11(61%) of the remaining 20 patients which, however, did not deteriorate with subsequent doses or with time. There was no Gr II or febrile neutropenia or thrombocytopenia noted in any of the patients in the duration of the study.
Weight loss
21 (55%) of the 38 patients demonstrated weight loss following 225Ac-PSMA-617 therapy with 5 reporting a loss of more than 10% of their body weight from baseline. There was a mean loss of 3.5% of body weight as compared to baseline following 225Ac-PSMA-617 therapy (Fig. 8b).
Hearing loss
Two patients reported severe acute sensorineural deafness post 2nd dose of 225Ac-PSMA-617 therapy. Neither of the patients had any skeletal metastases in the mastoid region nor in the base of the skull. Both these patients were treated with high-dose oral corticosteroids and they improved. Both, however, had some residual hearing loss. There was no other evidence of peripheral neuropathy in any of the patients.
Organ toxicity
There was no worsening of renal or hepatic function tests seen following 225Ac-PSMA-617 therapy. The details of the baseline and post-therapy kidney and liver function test values are shown in Table 5. Even patients with extensive hepatic metastases did not demonstrate deterioration of liver function following 225Ac-PSMA-617 therapy.
Discussion
Docetaxel-based chemotherapy has been the mainstay of treatment for metastatic CRPC for several years [16, 17]. It is only recently that the novel molecules like Abiraterone, Enzalutamide, Cabazitaxel, and the alpha-emitter Ra-223 have been introduced in the schedule of therapies for patients, refractory to the conventional ADT [18,19,20,21,22,23]. While numerous clinical trials to investigate the optimal positioning and sequence of these therapies are underway, the overall survival for patients with CRPC remains relatively short. A meta-analysis studying 13,909 patients in 23 Phase 3 trials of novel therapies demonstrates a median survival of 19 months [3].
Targeted alpha therapy (TAT) of prostate cancer uses alpha emitters conjugated to highly specific immune conjugates, which selectively targets tumour cells. Alpha emitters work by inducing clusters of DNA damage such as double-stranded DNA breaks and base chemical modifications that evoke large number of cellular responses and pathways that include apoptosis, autophagy, and necrosis and cell-cycle arrest. This damage is difficult to repair by the cell. Alpha emitter range is in micrometres in tissue, so surrounding normal tissue is unaffected. Targeted alpha therapy has potential to eradicate cancer cells with minimal damage to normal tissue.
In this single-arm single institutional retrospective study, we found that 225Ac-PSMA-617 targeted alpha therapy produced significant clinical, radiological, and PSA response in patients who have developed disease progression following ADT and following at least one taxane-based chemotherapy.
The Prostate Cancer Clinical Trials Working Group (PCWG3) recommends an independent reporting of PSA, imaging, and clinical measures while designing the end points of clinical trials in patients with advanced progressive prostate cancer [4]. In our study, 66% of patients achieved a 50% PSA response, while the median radiological progression-free survival was 8 months. These response rates compare favourably to the outcomes of the AFFIRM study, one of the pivotal studies which led to the approval of Enzalutamide in the post-Docetaxel setting, in which 54% of patients reportedly had a 50% PSA response [24].
Contrary to the recommendations of the PCWG3, we used the 68Ga-PSMA PET CT scans instead of a combination of 99mTc-MDP Bone scan and a CT abdomen and pelvis to assess radiological response. The advantages of using the 68Ga-PSMA-11 PET CT scan for response evaluation are twofold; (1) the CT component of the scan provides accurate size measurements for calculating responses in measurable lesions by RECIST Criteria; (2) the sensitivity of a 68Ga-PSMA-11 PET CT scan for detecting small new skeletal lesions is higher than a 99mTc-MDP bone scan which is important in excluding progression in patients with skeletal only metastases. In India, where the patient pays for his therapies and diagnostics, the cost of the combination of a 99mTc-MDP Bone scan and a CT abdomen and pelvis versus a 68Ga-PSMA-11 PET CT scan is not vastly different, the cost being approximately 200USD. This cost of each dose of 225Ac-PSMA-617 therapy (approximately 5000USD) is on the contrary much more expensive. Using a more accurate technique for ascertaining response prior to administering further doses of this often prohibitively expensive 225Ac-PSMA-617 therapy thus is a preferred practice. In this study, we have not used the reduction in PSMA expression in existing sites of metastatic disease as an indicator of response as the criteria for using changes in PSMA uptake in response to PSMA-based radio ligand therapy is still not validated.
225Ac-PSMA-617 response rates were found to be equivalent to prior reported response rates with 177Lu-PSMA-617 therapy [14, 25]. In our cohort, we had 23.6% patients (n = 9) who had earlier received 177Lu-PSMA-617 therapy and subsequently progressed on it. The number of doses given and the duration of response between 177Lu-PSMA therapy and 225Ac-PSMA therapy is shown in Table 6. The number of patients who received 177Lu PSMA therapy prior to 225Ac-PSMA therapy was very small with significant heterogeneity in the total activity received, number of doses of 177Lu-PSMA therapy received, the interval between the doses, and the intervening therapies received between the 177Lu-PSMA and 225Ac-PSMA therapies. All the patients received 225Ac-PSMA therapy much later in their disease chronology than 177Lu-PSMA. As a consequence, their duration of response to 225Ac-PSMA was more modest as compared to 177Lu-PSMA possibly due to the increasing aggressiveness of the tumour following each kind of therapy. Enduring responses demonstrated with 225Ac-PSMA-617 therapy likely proves alpha emitting Ac-225′s ability to overcome the radio-resistance to beta emitters. We did not find any significant difference in adverse effects in patients who had prior exposure to 177Lu-PSMA therapy except a somewhat increased incidence and severity of xerostomia.
Remarkable therapeutic efficacy of 225Ac-PSMA-617 has been shown in advanced metastatic chemotherapy-naïve prostate cancer patients with ≥ 90% decline in serum PSA in 82% of patients who remained in remission 12 months after therapy [15]. These results in chemotherapy naïve group of patients are better than our results, and thus, sequencing 225Ac-PSMA-617 therapy prior to chemotherapy might be a more promising approach in mCRPC treatment.
In our patient groups, there was no significant difference found in the survival statistics of the patients presenting with visceral versus lymph node and bone only metastases. While our study was underpowered to truly assess the impact of 225Ac-PSMA-617 on patients with visceral metastases, the relatively good objective response rates and survival in response to 225Ac-PSMA-617 in the small cohort of patients with visceral metastases are is definitely encouraging.
Potential delayed nephrotoxicity of the rebound daughters is an apprehension with alpha therapies. However, none of the patients in our study developed any reduction in eGFR during the course of their therapies. None of the patients including patients with extensive hepatic metastases did not demonstrate any deterioration of liver function post 225Ac-PSMA-617 therapy.
Xerostomia was a common adverse effect. Unlike the South African study, we did not empirically de-escalate the dose in subsequent therapies. Yet, unlike similar studies in the past, none of the patients discontinued therapy due to intolerable xerostomia [15, 26].
While none of the patients had any Grade 3 or 4 treatment emergent haematological toxicity, most patients demonstrated Gr 1/2 anaemia. In patients showing inadequate response to 225Ac-PSMA-617 therapy, the distinction between treatment emergent anaemia and anaemia due to disease progression, or a combination of the two conditions was not always possible. However, even patients showing good durable biochemical, radiological, and clinical response showed evidence of persistent anaemia. Reason for persistent anaemia could be a combination of myelosuppression, nutritional deficiency, and castrate level testosterone levels. All studied patients were heavily pre-treated and had poor haematological reserve. Logically, introduction of 225Ac-PSMA-617 therapy earlier in disease trajectory of metastatic prostate cancer may avoid potential challenges with hematologic toxicity.
Recent research has emphasised balancing prolonged life expectancy against men’s quality of life in end-stage metastatic prostate cancer. To our knowledge, however, none of the other studies on 225Ac-PSMA-617 therapy have evaluated the impact on quality of life in detail.
In our study, there was an almost uniform improvement in the physical, emotional, and cognitive functioning of patients in response to 225Ac-PSMA-617 therapy. The most remarkable improvement was seen in pain relief. Poorly controlled pain in terminal metastatic prostate cancer patients produces debilitating physical and psychological effects. The relief in pain brought on by the 225Ac-PSMA-617 therapy was thus very encouraging. There was a loss of body weight seen in nearly half of the patients which is similar to the weight loss reported in earlier studies. The weight loss may have been related to a decrease in food intake, consequent to dryness of mouth brought on by 225Ac-PSMA-617 therapy.
Among patients who progressed following 225Ac-PSMA-617 therapy, practically all had a very rapidly deteriorating course with emergence of multiple visceral metastases. While pathological and immunohistochemistry assessment of all the progressive lesions was beyond the scope of this study, the aggressive behaviour of the disease was suspicious for a cellular trans-differentiation from an epithelial to neuroendocrine phenotype (Fig. 9). Impact of 225Ac-PSMA-617 therapy and neuroendocrine differentiation of castration-resistant disease may be a potential field of further study. Also in patients with neuroendocrine tumours with a relatively low proliferation index, there may be a potential to investigate the somatostatin receptor expression status by a68Ga DOTANOC scan and subsequent treatment with 177Lu or 225Ac PRRT [27].
a Baseline 68Ga-PSMA-11 PET CT scan showing good PSMA uptake prior to 225Ac-PSMA-617 therapy in a patient with biopsy proven acinar adeno carcinoma prostate. b Disappearance of PSMA uptake despite rise in PSA levels and clinical deterioration following 2 doses of 225Ac-PSMA-617 therapy with appearance of multiple hepatic metastases. c, d HE stains in 20× and 40× from the core biopsy of liver metastases shows infiltration by tumour with appearances favouring small cell neuroendocrine carcinoma. e, f Immunohistochemical staining positive for Chromogranin and CD56 suggesting a cellular trans-differentiation to a neuroendocrine tumour
The principal limitation of the study was the small number of patients with relative heterogeneity in terms of their initial disease characteristics, the sites of metastases, and response to prior therapies. Comparing with historical controls in such a setting is often inaccurate. The duration of follow-up is also short. Further investigation in a clinical trial setting with strict cohorting and a larger number of patients with a longer follow-up may be worthwhile.
In our study, we found minimal myelotoxicity with 225Ac-PSMA-617 therapy despite potentially compromised bone marrow reserve due to the either disease or multiple prior therapies. It stands to reason thus that we can safely administer 225Ac-PSMA-617 therapy in patients with extensive bone or bone marrow disease without fear of life threatening bone marrow suppression, possibly an advantage over 177Lu-PSMA-617 therapy where marrow toxicity is known in patients with extensive bone metastases. Also, since the 225Ac-PSMA-617 therapy causes a significant and rapid improvement in the quality-of-life parameters, especially in pain relief, 225Ac-PSMA-617 therapy may be a better alternative over 177Lu-PSMA-617 in patients with severe pain. However, comparative strictly cohorted trials comparing 225Ac-PSMA-617 and 177Lu-PSMA-617 may be worthwhile to assess the true benefits of 225Ac-PSMA-617 over 177Lu-PSMA-617 therapy and to determine whether incorporating 225Ac-PSMA-617 therapy earlier in the course of the disease has an impact on overall outcomes.
Conclusions
225Ac-PSMA-617 is a safe and tolerable treatment option for mCRPC. The therapy demonstrates marked anti-tumour activity with improvement in quality of life even in patients of metastatic CRPC who have been previously been treated with taxane-based chemotherapy. Significant response rates and improvements in the quality of life could be achieved even in patients with visceral disease who have otherwise very limited therapeutic options. It is hoped that with greater availability and use of targeted alpha PSMA therapy in the future, a significant change may be made in the outcomes of these patients.
Notes
Data could not be received for patient 11.
References
Cui PF, Cong XF, Gao F, Yin JX, Niu ZR, Zhao SC, et al. Prognostic factors for overall survival in prostate cancer patients with different site-specific visceral metastases: a study of 1358 patients. World J Clin Cases. 2020;8(1):54–67. https://doi.org/10.12998/wjcc.v8.i1.54.
Van der Doelen MJ, Mehra N, Smits M, van Oort IM, Janssen MJR, et al. Clinical experience with PSMA-Actinium-225 (Ac-225) radioligand therapy (RLT) in end-stage metastatic castration-resistant prostate cancer (mCRPC) patients. J Clin Oncol. 2018;36(6_suppl):344. https://doi.org/10.1200/JCO.2018.36.6_suppl.
West TA, Kiely BE, Stockler MR. Estimating scenarios for survival time in men starting systemic therapies for castration-resistant prostate cancer: a systematic review of randomised trials. Eur J Cancer. 2014;50:1916–24. https://doi.org/10.1016/j.ejca.2014.04.004.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working Group 3. J Clin Oncol. 2016;34(12):1402–18. https://doi.org/10.1200/JCO.2015.64.2702.
Scher HI, Morris MJ, Basch E, Heller G. End points and outcomes in castration-resistant prostate cancer: from clinical trials to clinical practice. J Clin Oncol. 2011;29(27):3695–704. https://doi.org/10.1200/JCO.2011.35.8648.
Eiber M, Herrmann K, Calais J, Hadaschihk B, Giesel F, Hartenbach M, et al. Prostate cancer Molecular Imaging Standardized Evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59(3):469–78. https://doi.org/10.2967/jnumed.117.198119.
Kratochwil C, Bruchertseifer F, Rathke H, Bronzel M, Apostolidis C, Weichert W. Targeted alpha therapy of mCRPC with 225Actinium-PSMA-617: Dosimetry estimate and empirical dose finding. J Nucl Med. 2017;58(10):1624–31. https://doi.org/10.2967/jnumed.117.191395.
Shukurov R, Veliyev M, Dadashov Z, İsayev J, Novruzov F. Labeling process and quality control results of 225Ac-PSMA-617 for targeted alpha particle therapy for metastatic prostate cancer. J Nucl Med. 2019;60(supplement 1):1611.
Common Terminology Criteria for Adverse Events (CTCAE) (2017) Version 5.0 Published: November 27. U.S. Department of Health and Human Services. National Institutes of Health, National Cancer Institute
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76.
Van Andel G, Bottomley A, Fossa SD, Efficace F, Coens C, Guerif S, et al. An international field study of the EORTC QLQ-PR25: a questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur J Cancer. 2008;44:2418–24.
Cleeland CS, Ryan KM. Pain assesment: global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23:129–38.
Schwartz LH, Litière S, Elisabeth de Vries, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1–Update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–7. https://doi.org/10.1016/j.ejca.2016.03.081.
Fuerecker B, Knorr K, Beheshti A, Seidl C, D’Alessandrai C, et al. Safety and efficacy of Ac-225-PSMA-617 in mCRPC after failure of Lu-177-PSMA. J Med Imaging Radiat Sci. 2019;50(1):S20–1. https://doi.org/10.1016/j.jmir.2019.03.066.
Sathekge M, Bruchertseifer F, Knoesen O, Reyneke F, Lawal I, et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2019;46(1):129–38. https://doi.org/10.1007/s00259-018-4167-0.
Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. https://doi.org/10.1056/NEJMoa041318.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. https://doi.org/10.1056/NEJMoa040720.
De Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. https://doi.org/10.1016/S0140-6736(10)61389-X.
De Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. https://doi.org/10.1056/NEJMoa1014618.
Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–60. https://doi.org/10.1016/S1470-2045(14)71205-7.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. https://doi.org/10.1056/NEJMoa1207506.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. https://doi.org/10.1056/NEJMoa1405095.
Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. https://doi.org/10.1056/NEJMoa1213755.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. for the AFFIRM investigators, increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. https://doi.org/10.1056/NEJMoa1207506.
Hofman M, Violet JA, Hicks RJ, Ferdinandus J, Thang SP, Iravani A, et al. Results of a 50 patient single-center phase II prospective trial of Lutetium-177 PSMA-617 theranostics in metastatic castrate-resistant prostate cancer. J Clin Oncol. 2019;37(7_suppl):228. https://doi.org/10.1200/JCO.2019.37.7_suppl.228.
Kratochwil C, Bruchertseifer F, Rathke H, Hohenfellner M, Frederik L Giesel FL, et al. Targeted Alpha Therapy of mCRPC with 225Actinium-PSMA-617: Swimmer-Plot analysis suggests efficacy regarding duration of tumor-control. J Nucl Med. 2018;59(5):795–802. https://doi.org/10.2967/jnumed.117.203539.
Gofrit ON, Frank S, Meirovitz A, Nechushtan H, Orevi M. PET/CT With 68Ga-DOTA-TATE for diagnosis of neuroendocrine: differentiation in patients with castrate-resistant prostate cancer. Clin Nucl Med. 2017;42(1):1–6. https://doi.org/10.1097/RLU.0000000000001424.
Acknowledgements
The authors have no conflicts of interest to declare that are relevant to the content of this article. No funds, grants, or other support was received.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sen, I., Thakral, P., Tiwari, P. et al. Therapeutic efficacy of 225Ac-PSMA-617 targeted alpha therapy in patients of metastatic castrate resistant prostate cancer after taxane-based chemotherapy. Ann Nucl Med 35, 794–810 (2021). https://doi.org/10.1007/s12149-021-01617-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-021-01617-4