Abstract
Objective
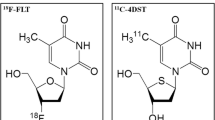
A novel radiopharmaceutical, 4′-[methyl-11C]thiothymidine (11C-4DST), has been developed as an in vivo cell proliferation marker based on the DNA incorporation method. The purpose of this study was to evaluate 11C-4DST uptake in patients with newly diagnosed glioma and to correlate the results with proliferative activity and tumor grade, in comparison with l-[methyl-11C]-methionine (11C-MET).
Methods
Investigations of 11C-4DST and 11C-MET PET/CT were performed retrospectively in 23 patients with newly diagnosed glioma. The maximum standardized uptake value (SUVmax) for tumor (T) and the mean SUV for normal contralateral hemisphere (N) were calculated, and the tumor-to-normal (T/N) ratio was determined. Metabolic tumor volume (MTV) was defined as the volume with a threshold of 40 % of the SUVmax. Proliferative activity as indicated by the Ki-67 index was estimated in tissue specimens.
Results
Of 23 gliomas examined, 11C-4DST PET/CT and 11C-MET PET/CT detected 20 and 22, respectively. Linear regression analysis between 11C-4DST and 11C-MET indicated a weak correlation for SUVmax (r = 0.54, P < 0.008), for T/N ratio (r = 0.56, P < 0.006), and for MTV (r = 0.60, P < 0.003). Linear regression analysis indicated a weak correlation between 11C-4DST and Ki-67 index for SUVmax (r = 0.46, P < 0.03), for T/N ratio (r = 0.43, P < 0.05), and for MTV (r = 0.68, P < 0.001) and between 11C-MET MTV and Ki-67 index (r = 0.43, P < 0.04). Using 11C-4DST, there was a significant difference in SUVmax between grades II and IV (P < 0.03) and in MTV between grades II and IV (P < 0.009) and grades III and IV (P < 0.02). Using 11C-MET, there was a significant difference in SUVmax (P < 0.009) and T/N ratio (P < 0.02) between grades II and IV and in MTV between grades II and IV (P < 0.03) and grades III and IV (P < 0.02).
Conclusion
11C-4DST PET/CT is feasible for imaging of brain gliomas, as well as 11C-MET PET/CT. Especially, it showed the highest correlation coefficient between 11C-4DST MTV and Ki-67 index in newly diagnosed gliomas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Markers of proliferative activity and accurate tumor grading are essential for individualized patient therapy and management of glioma [1, 2]. Tissue sampling is often performed by stereotactic biopsy, has a limit on size, and is thus sometimes not representative of the entire tumor. Therefore, the sample may not be an accurate representation of the true malignant potential and may lead to a misclassification of the true tumor grade [3]. Thus, noninvasive imaging-based technology for assessment of malignant progression is required to select the best possible treatment planning and assess treatment response.
Positron emission tomography (PET) is now an indispensable modality for assessment of various tumors. l-[methyl-11C]-methionine (11C-MET), an amino acid, is the most widely used radiopharmaceutical that can be used for brain tumor imaging [4–11]. Its uptake reflects increased amino acid transport and protein synthesis and is related to cellular proliferative activity [4]. High-contrast images can be acquired due to high amino acid uptake in both low- and high-grade gliomas and low uptake in normal brain tissue [1].
The radiotracer 3′-deoxy-3′-18F-fluorothymidine (18F-FLT) has been investigated as a promising PET tracer for evaluating tumor proliferative activity in several types of malignant tumor including brain tumor [5–7]. In brain gliomas, 18F-FLT has been validated for evaluation of cellular proliferation and tumor grade in newly diagnosed gliomas [5, 7]. A theoretic limitation of 18F-FLT as a radiotracer for the salvage pathway of DNA synthesis is that it is not incorporated into DNA because of the lack of a 3′-hydroxyl [12].
Toyohara et al. [13, 14] developed 4′-thiothymidine labeled with 11C at the methyl group (4′-[methyl-11C]-thiothymidine [11C-4DST]), as a new candidate for cell proliferation imaging that is resistant to degradation by thymidine phosphorylase and is incorporated into DNA. 11C-4DST has shown significantly higher uptake into proliferating tissues than [methyl-3H]FLT, in an animal study [14]. A 11C-4DST PET pilot study of six patients with various brain tumors showed that 11C-4DST PET is feasible for brain tumor imaging and can be performed with acceptable dosimetry and pharmacologic safety at a suitable dose for adequate imaging [15]. In a mixed population of patients with newly diagnosed and recurrent gliomas, Toyota et al. [16] have recently demonstrated that 11C-4DST PET is feasible for evaluating cell proliferation. These results indicate that 11C-4DST has great potential for imaging cell proliferation. However, there is still only limited experience of use of 11C-4DST in patients with brain tumors [15, 16].
The purpose of the present study was to retrospectively evaluate 11C-4DST uptake in patients with newly diagnosed glioma and to correlate the results with proliferative activity as indicated by the Ki-67 index and tumor grade, in comparison with 11C-MET.
Materials and methods
Patients
A total of 23 patients (12 men, 11 women; mean age, 53.6 years; age range 13–84 years) with newly diagnosed gliomas who underwent 11C-4DST and 11C-MET PET/CT studies between October 2011 and April 2015 were retrospectively selected. Some of the data from 9 of these patients were used in a previous study [16]. The median interval between 11C-4DST and 11C-MET PET/CT scans was 5 days (range 1–29 days). All patients underwent routine magnetic resonance imaging (MRI) examination including contrast-enhanced T1-weighted MRI. Pathologic diagnosis was obtained by stereotactic biopsy or open surgery. The tumor was graded according to the World Health Organization classification for neuroepithelial tumors [17]. The tumor numbers, grades and types were as follows: 1 grade I glioma, 5 grade II astrocytomas, 1 grade II pilomyxoid astrocytoma, 6 grade III anaplastic astrocytomas, 2 grade III anaplastic oligoastrocytomas, and 8 grade IV glioblastomas.
This study was approved by our institutional ethical review board, and written informed consent was obtained from all patients.
Radiotracer synthesis and PET/CT
The radiotracers 11C-4DST and 11C-MET were produced using an automated synthesis system with HM-18 cyclotron (QUPID; Sumitomo Heavy Industries Ltd, Tokyo, Japan). The 11C-4DST was synthesized using the method described by Toyohara et al. [15]. The 11C-MET was synthesized using a modified method of Ishiwata et al. [18].
All acquisitions were performed using a Biograph mCT 64-slice PET/CT scanner (Siemens Medical Solutions USA Inc., Knoxville, TN, USA). Patients were instructed to fast for at least 4 h before PET/CT imaging. Data acquisition began with CT at the following settings: no contrast agent, 120 kV, 192 mA, 1.0-s tube rotation time, 3-mm slice thickness, 3-mm increments, and pitch 0.55. PET emission scanning of the head region with a 15-min acquisition of one bed position was performed 15 min after intravenous injection of 11C-4DST or 11C-MET (6 MBq/kg). The PET data were acquired in three-dimensional mode and were reconstructed with the baseline ordered-subsets expectation maximization (OSEM) bases, incorporating correction with point spread function and time-of-flight model (five iterations, 21 subsets). A Gaussian filter with a full-width at half-maximum of 3 mm was used as a post-smoothing filter.
Data analysis
The images were visually assessed independently by two experienced nuclear physicians. Any difference of opinion was resolved by consensus. Tumor lesions were identified as areas of focally increased uptake, exceeding that of normal brain background.
Semiquantitative analysis using the standardized uptake value (SUV) was performed by an experienced nuclear physician using a syngo.via (Siemens Healthcare, Erlangen, Germany). The region of interest (ROI) was placed over the tumor including the area of biopsy or surgery using the transverse PET image. For lesions not visible on each PET scan, a ROI was drawn on one or both PET images in the area corresponding to the area of the lesion on MRI. For the reference tissue, a circular region of interest of 10 × 10 mm was manually placed on the uninvolved contralateral hemisphere in the plane that showed maximum tumor uptake. Radioactivity concentrations measured in the ROI were normalized to the injected dose per patient’s body weight by calculation of SUV. The maximal SUV (SUVmax) for tumor and the mean SUV (SUVmean) for reference tissue were calculated. The tumor-to-contralateral normal brain tissue (T/N) ratio was determined by dividing the tumor SUVmax by the SUVmean of the contralateral hemisphere. The volumetric ROI around the outline of the tumor was placed on the transverse PET images and adjusted manually with 3-D reconstructions to avoid overlap with adjacent non-tumor structures (Fig. 1). A threshold was selected by visual inspection of the lesion’s outline to best fit the tumor and to determine metabolic tumor volume (MTV). MTV was determined as the volume with a threshold of 40 % of the SUVmax. If the percentage threshold was not appropriate, further adjustment was performed until a satisfactory outcome was achieved.
Ki-67 immunohistochemistry
Formalin-fixed, paraffin-embedded sections of resected specimens from brain tumors were prepared for immunohistochemical staining. The Ki-67 index was estimated as the percentage of Ki-67-positive cell nuclei per 500–1000 cells in the region of the tumor with the greatest staining density.
Statistical analysis
The detection rates of gliomas using 11C-4DST and 11C-MET were compared using Fisher’s exact test. All semiquantitative data were expressed as mean ± SD. The 11C-4DST and 11C-MET uptake were compared using paired t test. Correlations between 11C-4DST and 11C-MET uptake; 11C-4DST uptake and Ki-67 index; and 11C-MET uptake and Ki-67 index were assessed using linear regression analysis. Differences in 11C-4DST and 11C-MET uptake among glioma grades were compared using analysis of variance (ANOVA). The significance of individual differences was evaluated using the Tukey honestly significant difference test. Differences were considered statistically significant at a P value of less than 0.05.
Results
11C-4DST and 11C-MET uptake
On visual analysis, all but 3 grade II gliomas showed increased uptake on 11C-4DST PET/CT. On 11C-MET PET/CT, all but one grade II glioma showed increased uptake. Three grade II gliomas that were not visualized with 11C-4DST had no enhancement on contrast-enhanced MRI. Two of them were visualized with 11C-MET. Although 11C-4DST PET/CT showed a slightly lower detection rate than that for 11C-MET PET/CT for detection of glioma (87 vs. 96 %), the difference was not statistically significant. Figures 2, 3, and 4 show typical cases of grade II, III, and IV gliomas, respectively.
Radiologic images of a 14-year-old girl with newly diagnosed World Health Organization grade II pilomyxoid astrocytoma. Transverse T1-weighted MR image with contrast enhancement (a) shows enhanced mass in pineal region. Transverse 11C-4DST PET image (b) shows inhomogeneous faint uptake (SUVmax = 1.57, T/N ratio = 4.91, MTV = 10.49 cm3) in tumor. Transverse 11C-MET PET image (c) shows mild uptake (SUVmax = 2.92, T/N ratio = 2.16, MTV = 18.02 cm3) in tumor
Radiologic images of a 73-year-old man with newly diagnosed World Health Organization grade III anaplastic astrocytoma. Transverse fluid-attenuated inversion recovery MR image (a) shows high signal intensity mass in right frontal lobe. Transverse T1-weighted MR image with contrast enhancement (b) shows small enhanced areas in tumor. Transverse 11C-4DST PET image (c) shows increased uptake (SUVmax = 2.35, T/N ratio = 6.18, MTV = 2.92 cm3) in tumor. Transverse 11C-MET PET image (d) also shows increased uptake (SUVmax = 9.13, T/N ratio = 5.67, MTV = 5.89 cm3) in tumor
Radiologic images of an 81-year-old man with newly diagnosed World Health Organization grade IV glioblastoma. Transverse T1-weighted MR image with contrast enhancement (a) shows inhomogeneous enhanced mass in left temporal lobe. Transverse 11C-4DST PET image (b) shows inhomogeneous uptake (SUVmax = 2.49, T/N ratio = 6.55, MTV = 46.79 cm3) in tumor. Transverse 11C-MET PET image (c) also shows inhomogeneous uptake (SUVmax = 6.49, T/N ratio = 5.85, MTV = 50.83 cm3) in tumor
Table 1 shows the results of semiquantitative analysis using 11C-4DST and 11C-MET. The mean (±SD) value of SUVmean for reference tissue using 11C-4DST was significantly lower than that using 11C-MET (P < 0.001). The SUVmax using 11C-MET was significantly higher than that using 11C-4DST (P < 0.001). There were no significant differences in T/N ratio or MTV between 11C-4DST and 11C-MET.
Linear regression analysis between 11C-4DST and 11C-MET indicated a weak correlation for SUVmax (r = 0.54, P < 0.008; Fig. 5a), for T/N ratio (r = 0.56, P < 0.006; Fig. 5b), and for MTV (r = 0.60, P < 0.003; Fig. 5c).
11C-4DST and 11C-MET uptake and Ki-67 immunohistochemistry
Linear regression analysis between 11C-4DST and the Ki-67 index indicated a weak correlation for SUVmax (r = 0.46, P < 0.03; Fig. 6a), for T/N ratio (r = 0.43, P < 0.05; Fig. 6b), and for MTV (r = 0.68, P < 0.001; Fig. 6c). Linear regression analysis indicated a weak correlation between 11C-MET MTV and the Ki-67 index (r = 0.43, P < 0.04; Fig. 6d). There was no significant correlation between 11C-MET and the Ki-67 index for SUVmax (r = 0.19, P = 0.39) or for T/N ratio (r = 0.23, P = 0.30).
Linear regression analysis demonstrates a weak correlation between 11C-4DST and the Ki-67 index for SUVmax (r = 0.46, P < 0.03) (a), for T/N ratio (r = 0.43, P < 0.05) (b), and for MTV (r = 0.68, P < 0.001) (c) and between 11C-MET MTV and the Ki-67 index (r = 0.43, P < 0.04) (d), in 23 patients with newly diagnosed gliomas
11C-4DST and 11C-MET uptake and glioma grade
Table 2 shows the results of semiquantitative analysis according to the glioma grade using 11C-4DST and 11C-MET. Using 11C-4DST, there was a significant difference in SUVmax (P < 0.03) and MTV (P < 0.009) between gliomas of grades II and IV. Using 11C-4DST, a significant difference in MTV was also observed between gliomas of grades III and IV (P < 0.02). No significant difference in T/N ratio using 11C-4DST was noted among glioma grades.
Using 11C-MET, significant differences were noted in SUVmax (P < 0.009), T/N ratio (P < 0.02) and MTV (P < 0.03) between gliomas of grades II and IV. Using 11C-MET, a significant difference in MTV was also observed between gliomas of grades III and IV (P < 0.02).
Discussion
In the present study, we evaluated 11C-4DST uptake in patients with newly diagnosed gliomas, in comparison with 11C-MET. On visual assessment, 11C-4DST PET/CT showed a slightly lower overall detection rate of glioma than did 11C-MET PET/CT (87 vs. 96 %), especially for grade II glioma, although this difference was not statistically significant. In semiquantitative analysis, tumor SUVmax of 11C-4DST was significantly lower than that of 11C-MET. However, there was no significant difference in T/N ratio between these two tracers. The background 11C-4DST level in brain parenchyma was low compared with 11C-MET. Lesion detection is influenced not only by absolute tumor uptake but also by the tumor-to-non-tumor contrast. This advantage of 11C-4DST may be of benefit in the localization of tumors. These findings are similar to those from a previous study using 18F-FLT and 11C-MET [6]. The T/N ratio of 18F-FLT was significantly higher than that of 11C-MET, although SUVmax of 18F-FLT was significantly lower than that of 11C-MET [6].
Increased cell proliferation and DNA replication is a characteristic of malignant transformation [1]. The assessment of cellular proliferation rate by means of PET is useful as a noninvasive clinical approach. In the present study, 11C-4DST PET/CT was found to be useful in the assessment of tumor proliferation in newly diagnosed gliomas. In a previous study, an initial clinical trial in only six patients with brain tumor indicated that 11C-4DST PET was feasible for imaging brain tumors [15]. Toyota et al. [16] evaluated 11C-4DST uptake and proliferative activity in 20 patients with gliomas, including 11 recurrent gliomas. They showed a weak correlation between 11C-4DST SUVmax and T/N ratio and Ki-67 index. The present study also showed a weak correlation between 11C-4DST SUVmax and T/N ratio and Ki-67 index but in gliomas that were all newly diagnosed. Moreover, a correlation was also observed between 11C-4DST MTV and Ki-67 index. The correlation coefficient in MTV was higher than that in SUVmax and T/N ratio. Although a weak correlation was also noted between 11C-MET MTV and Ki-67 index, the correlation coefficient between 11C-4DST MTV and Ki-67 index was the highest. Minamimoto et al. [19] evaluated 11C-4DST uptake and Ki-67 index in patients with non-small cell lung cancer. They showed a significant correlation between 11C-4DST SUVmax and Ki-67 index [19]. However, Ito et al. [20] found no significant correlation between 11C-4DST SUVmax and Ki-67 index in head and neck squamous cell carcinoma. One possible reason for Ito et al’s findings may be that the Ki-67 index was obtained mainly from biopsy specimens, and not from resected specimens.
In the present study, according to the glioma grade, on 11C-4DST PET/CT, there was a significant difference in SUVmax and MTV between grades II and IV and in MTV between grades III and IV. However, Toyota et al. [16] observed no significant differences in 11C-4DST T/N ratio among different glioma grades. Their study analyzed the data of newly diagnosed and recurrent gliomas together but in the present study, data for only newly diagnosed gliomas were analyzed. To date, only the study of Toyota et al. and the present study have published results using 11C-4DST according to the glioma grade. Further research with larger numbers of patients in a variety of tumor types is needed to evaluate the reliability of 11C-4DST as a proliferation imaging tracer.
No significant differences in 11C-4DST T/N ratio were observed among different glioma grades in the present study. The mean (±SD) value of T/N ratio in grade II glioma using 11C-4DST was high compared with that using 11C-MET. Three grade II gliomas that were visualized with 11C-4DST had enhancement on contrast-enhanced MRI. In addition to increased proliferation, breakdown of the blood–brain barrier may contribute to the degree of 11C-4DST uptake. We did not compare 11C-4DST PET images with contrast-enhanced MRI. Further studies are needed to evaluate the relationship between 11C-4DST uptake and enhancement on contrast-enhanced MRI in patients with gliomas.
The present study evaluated three parameters (SUVmax, T/N ratio and MTV) of tracer uptake in the tumor. Of them, MTV, which is a volume-based parameter, showed the best results in the assessment of tumor proliferation and tumor grade using 11C-4DST and 11C-MET. Galldiks et al. [11] compared the prognostic value of pretreatment T/N ratio and MTV using 11C-MET in patients with malignant gliomas and suggested that MTV but not T/N ratio is a useful biologic prognostic marker. MTV may be an important imaging marker for tumor biology in patients with glioma. Further studies including volumetric analysis are needed to determine the clinical usefulness of 11C-4DST PET.
Although 11C-4DST PET is feasible for brain glioma imaging, its mechanism of uptake and biological significance in gliomas are not yet understood. Limitations of the present study were the small sample size and retrospective design. The short physical half-life of 11C places a significant limitation on counting statistics and on commercial convenience for widespread routine clinical use. However, it has benefits with regard to lower radiation burden, and therefore, diagnosis using multiple tracers to determine tumor characteristics, and repeated scans during the course of therapy are possible. If an alternative thymidine analogue labeled with a longer half-life radioisotope that could be incorporated into DNA synthesis was available, it could provide additional information for clinical use.
Because the most actively proliferating part of a tumor is mainly responsible for its progression, 11C-4DST analysis enables a more precise estimation of the malignancy. More important indication is the possibility that the cell proliferation imaging could be used for early evaluation of treatment effects. In the report of Toyohara et al. [15], although 11C-MET PET detected all the contrast-enhanced lesions visualized with MRI, a clinically stable tumor with contrast enhancement was not detected with 11C-4DST. The role of 11C-4DST in therapy monitoring has not been evaluated so far. Further prospective studies involving a larger number of patients with a variety of tumor types are required to determine the clinical usefulness of 11C-4DST PET for early evaluation of treatment response.
Conclusion
Based on the preliminary results of the present study in a small patient population, 11C-4DST PET/CT is feasible for assessment of tumor proliferation and tumor grade in newly diagnosed gliomas, as well as 11C-MET PET/CT. Especially, the correlation coefficient between 11C-4DST MTV and Ki-67 index in newly diagnosed gliomas was the highest.
References
la Fougère C, Suchorska B, Bartenstein P, Kreth FW, Tonn JC. Molecular imaging of gliomas with PET: opportunities and limitations. Neuro Oncol. 2011;13:806–19.
DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–23.
Ceyssens S, Van Laere K, de Groot T, Goffin J, Bormans G, Mortelmans L. [11C]methionine PET, histopathology, and survival in primary brain tumors and recurrence. Am J Neuroradiol. 2006;27:1432–7.
Hatazawa J, Ishiwata K, Itoh M, Kameyama M, Kubota K, Ido T, et al. Quantitative evaluation of l-[methyl-C-11]methionine uptake in tumor using positron emission tomography. J Nucl Med. 1989;30:1809–13.
Hatakeyama T, Kawai N, Nishiyama Y, Yamamoto Y, Sasakawa Y, Ichikawa T, et al. 11C-methionine (MET) and 18F-fluorothymidine (FLT) PET in patients with newly diagnosed glioma. Eur J Nucl Med Mol Imaging. 2008;35:2009–17.
Jacobs AH, Thomas A, Kracht LW, Li H, Dittmar C, Garlip G, et al. 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med. 2005;46:1948–58.
Ullrich R, Backes H, Li H, Kracht L, Miletic H, Kesper K, et al. Glioma proliferation as assessed by 3′-fluoro-3′-deoxy-l-thymidine positron emission tomography in patients with newly diagnosed high-grade glioma. Clin Cancer Res. 2008;14:2049–55.
Kaschten B, Stevenaert A, Sadzot B, Deprez M, Degueldre C, Del Fiore G, et al. Preoperative evaluation of 54 gliomas by PET with fluorine-18-fluorodeoxyglucose and/or carbon-11-methionine. J Nucl Med. 1998;39:778–85.
Kim S, Chung JK, Im SH, Jeong JM, Lee DS, Kim DG, et al. 11C-methionine PET as a prognostic marker in patients with glioma: comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2005;32:52–9.
Sasaki M, Kuwabara Y, Yoshida T, Nakagawa M, Fukumura T, Mihara F, et al. A comparative study of thallium-201 SPET, carbon-11 methionine PET and fluorine-18 fluorodeoxyglucose PET for the differentiation of astrocytic tumours. Eur J Nucl Med. 1998;25:1261–9.
Galldiks N, Dunkl V, Kracht LW, Vollmar S, Jacobs AH, Fink GR, et al. Volumetry of [11C]-methionine positron emission tomographic uptake as a prognostic marker before treatment of patients with malignant glioma. Mol Imaging. 2012;11:516–27.
Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwartz JL. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med. 2002;43:1210–7.
Toyohara J, Kumata K, Fukushi K, Irie T, Suzuki K. Evaluation of [methyl-14C]4′-thiothymidine for in vivo DNA synthesis imaging. J Nucl Med. 2006;47:1717–22.
Toyohara J, Okada M, Toramatsu C, Suzuki K, Irie T. Feasibility studies of 4′-[methyl-11C]thiothymidine as a tumor proliferation imaging agent in mice. Nucl Med Biol. 2008;35:67–74.
Toyohara J, Nariai T, Sakata M, Oda K, Ishii K, Kawabe T, et al. Whole-body distribution and brain tumor imaging with 11C-4DST: a pilot study. J Nucl Med. 2011;52:1322–8.
Toyota Y, Miyake K, Kawai N, Hatakeyama T, Yamamoto Y, Toyohara J, et al. Comparison of 4′-[methyl-11C]thiothymidine (11C-4DST) and 3′-deoxy-3′-[18F]fluorothymidine (18F-FLT) PET/CT in human brain glioma imaging. EJNMMI Res. 2015;5:7.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109.
Ishiwata K, Ido T, Vaalburg W. Increased amounts of d-enantiomer dependent on alkaline concentration in the synthesis of l-[methyl-11C]methionine. Appl Radiat Isot. 1988;39:311–4.
Minamimoto R, Toyohara J, Seike A, Ito H, Endo H, Morooka M, et al. 4′-[Methyl-11C]-thiothymidine PET/CT for proliferation imaging in non-small cell lung cancer. J Nucl Med. 2012;53:199–206.
Ito K, Yokoyama J, Miyata Y, Toyohara J, Okasaki M, Minamimoto R, et al. Volumetric comparison of positron emission tomography/computed tomography using 4′-[methyl-11C]-thiothymidine with 2-deoxy-2-18F-fluoro-d-glucose in patients with advanced head and neck squamous cell carcinoma. Nucl Med Commun. 2015;36:219–25.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any potential conflicts of interest relevant to this article to report.
Rights and permissions
About this article
Cite this article
Tanaka, K., Yamamoto, Y., Maeda, Y. et al. Correlation of 4′-[methyl-11C]-thiothymidine uptake with Ki-67 immunohistochemistry and tumor grade in patients with newly diagnosed gliomas in comparison with 11C-methionine uptake. Ann Nucl Med 30, 89–96 (2016). https://doi.org/10.1007/s12149-015-1035-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-015-1035-x