Abstract
Objective
Optimized production and quality control of gallium-68 labeled ethylenediamine tetramethylene phosphonate (68Ga-EDTMP) as an efficient PET radiotracer for bone scans have been presented.
Methods
Efforts have been made to present a fast, efficient, cost-effective and facile protocol for 68Ga-EDTMP productions for clinical trials. 68Ga-EDTMP was prepared using generator-based 68GaCl3 and EDTMP at optimized conditions for time, temperature, ligand amount, gallium content followed by proper formulation. The biodistribution of the tracer in rats was studied using tissue counting and PET/CT imaging up to 155 min.
Results
68Ga-EDTMP was prepared at optimized conditions in 5–10 min at 50–60 °C (radiochemical purity ≈99 ± 0.88 % ITLC, >99 % HPLC, specific activity: 15–18 GBq/mM). The biodistribution of the tracer demonstrated high bone uptake of the tracer in 10–20 min while yielding the best images in 2 h.
Conclusion
The whole production and quality control of 68Ga-EDTMP including labeling, purification, HPLC analysis, sterilization and LAL test took 18–20 min with significant specific activity for administration to limited number of patients in a PET center.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the increasing rate of morality and mortality of malignant diseases and low access to radionuclide sources, developing generator-based PET radiopharmaceuticals are of great importance. Thus, the high costs of installation and running a cyclotron in every nuclear medicine center are of less concern especially for large countries. Many Ga-68 generators have been developed by the industries based on organic and inorganic solid phases [1–3].
Bone metastases are common in the progression of various tumors such as prostate, breast, and lung carcinoma; often entail an occurrence of progressive pain [4] and occur in many patients with solid malignant tumors [5].
The longer half-life and intensive radiation dose to the patients from F-18 sodium fluoride has led to develop Ga-68-based bone radiopharmaceuticals including 68Ga-EDTMP [6, 7].
Interesting studies have been performed for the development of new phosphonate-based Ga-68 tracers for PET bone imaging [6] based on aliphatic and cyclic molecules. Recently, novel radiogallium-labeled bone imaging agents using oligo-aspartic moieties have been presented due to their high affinity for hydroxyapatite [7].
Another interesting research project has been initiated using 68Ga-BPAMD presenting a possible PET/CT imaging agents based on Ga-68 generator [8] leading to few human studies [9].
Ethylene diamine tetra-methylene phosphonic acid (EDTMP) has been envisaged as an ideal carrier moiety, for the development of therapeutic/diagnostics bone avid radiopharmaceuticals leading to a high probability that 68Ga-EDTMP would enter possible human studies. The FDA has already approved Sm-153 EDTMP [10] and recent clinically used Lu-177 EDTMP ligands [11], demonstrating high bone uptake and fast urinary clearance of activity and these ligands have paved the way for developing EDTMP-based radiopharmaceuticals.
Recently, the production, ex vivo and in vivo evaluations of 68Ga-EDTMP have been reported using commercial Ga-68 generators [12]; however in this work, an attempt has been made to use available SnO2-based generator for developing customized production and quality control 68Ga-EDTMP, for clinical imaging.
Materials and methods
The 68Ge/68Ga generator (30 mCi/day activity) was obtained from Pars Isotope Co. Karaj, Iran. Chemicals were purchased from the Aldrich Chemical Co. (Germany). Reverse-phase liquid chromatography (RP-HPLC) was performed for radiolabeling and specific activity analyzed of the final product using a KNAUER-D-14163 system, Berlin, Germany using a gradient mobile phase of A: Ultrapure water–TFA 1 % (V/V); B: Acetonitrile HPLC Grade using gradient-elution: 0–3 min, A: 100 %, B: 0 %; 3–10 min, A: 50 %, B: 50 %; 10–15 min, A: 0 %, B: 100 %; Flow rate: 1.5 mL/min, Injection volume: 20 µL. The used was, MZ-Analysentechnik, ODS-H 5 µm (100 × 4.0 mm), Gamma detector: Ray test, Gabi gamma ray detector. Thin-layer chromatography (TLC) for DOTATATE quality control was performed on polymer-backed silica gel, F 1500/LS 254, 20 × 20 cm, TLC Ready Foil, Schleicher and Schuell®, Germany. Normal saline and sodium acetate used for radiolabeling were of high purity and had been filtered through 0.22-μm Cativex filters. Instant thin-layer chromatography (ITLC) was performed by counting Whatman No. 2 papers using a thin-layer chromatography scanner, Bioscan AR2000, Bioscan Europe Ltd., France. Biodistribution data were acquired by counting normal saline-washed tissues after weighting on a Canberra™ high-purity germanium (HPGe) detector (model GC1020-7500SL). Radionuclidic purity was checked with the same detector. For activity measurement of the samples a CRC Capintech Radiometer (NJ, USA) was used. Animal studies were performed in accordance with the United Kingdom Biological Council’s Guidelines on the Use of Living Animals in Scientific Investigations, 2nd edition.
68Ge/68Ga generator characterization and quality control
A prototype 30 mCi68Ge/68Ga generator developed at Pars Isotope Co. Iran was used in this study [2]. The generator is SnO2-based loaded by germanium production at a 30-MeV IBA cyclotron irradiating metallic gallium powder. The characterization of the generator for clinical studies has been reported previously [13].
Synthesis of EDTMP
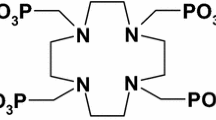
EDTMP was synthesized from phosphorous acid, ethylenediamine and formaldehyde in the presence of HCl by a modified Mannich-type reaction [14] using phosphorous acid, conc. HCl, ethylenediamine and aq. formaldehyde and recrystallization from water/methanol, m.p. 214–215 °C. IR (KBr, ν cm−1): 3308, 2633, 2311, 1668, 1436, 1356. 1H-NMR (D2O, δ ppm): 3.53 (d, J = 12.3 Hz, 8H, –N–CH2–P=O), 3.85 (s, 4H, –N–CH2–). 13C NMR (D2O, δ ppm): 51.63, 52.73. 31P NMR (D2O, δ ppm): 10.52.
Preparation of [68Ga]EDTMP
A 7-month-old locally available generator was used in the radiolabeling procedure. The acidic solution of [68Ga]GaCl3 with highest activity from the 3 first 0.5-mL elution of the generator (1500 µL, 15 ± 0.2 mCi, in 0.6 M HCl) was transferred to a 10-ml borosilicate Reacti-vial containing solid HEPES (352 mg), acetate buffer 200 µl (0.1 M), and EDTMP solution (12.5, 6.25, 3.6, 1.8 μg in DDH2O) and sealed vial was heated at 50–60 °C for 5–10 min. The mixture put in an ice bath for 2 min followed by the addition of 0.8 ml of normal saline. The reaction mixture was then injected into a 0.22-micron filter (Waters). The pH of the active solution with acceptable radiochemical purity was adjusted to 5.5.
Quality control of [68Ga]EDTMP
Radio thin-layer chromatography
A 5-μl sample of the final fraction was spotted on Whatman no. 2 paper, and SG-TLC stationary phases and methanol:saline mixture (5:1) as well as normal saline were used as mobile phases.
High-performance liquid chromatography
HPLC was performed with a flow rate of 1.5 ml/min, pressure: 130 psi for 15 min. HPLC was performed on the final preparation using a gradient of water:acetonitrile (0–100:100–0 % added: 1 % trifluoroacetic acid) as the eluent by means of reversed-phase column chromatography as explained in the experimental section.
Biodistribution in wild-type rats
The distribution of the radiolabeled complex and free Ga-68 cation among tissues was determined in rats. The animals were killed by CO2 asphyxiation at selected times after injection (n = 5 for each time interval); the tissues were weighed and rinsed with normal saline and their specific activities were determined with a HPGe detector equipped with a sample holder device as percent of injected dose per gram of tissues.
Imaging studies
PET/CT imaging was performed with a PET/CT scanner (Biograph 6 TrueX; Siemens Medical Solutions). The rats were placed in a supine position. Static PET images were acquired for 10 min with 3 sets of emission images starting 60 and 155 min after 68Ga-EDTMP injection for the rats. In addition, PET emission scans were preceded by CT scans performed for anatomical reference and attenuation correction (spatial resolution 1.25 mm, 80 kV, 150 mAs) with a total CT scanning time of 20 s. Reconstruction was performed using the iterative algorithm with attenuation correction. The reconstruction settings were 4 iterations and 21 subsets to a 256 × 256 matrix, with a post-filtering of 2 mm. Transmission data were reconstructed into a matrix of equal size by means of filtered back-projection, yielding a co-registered image set. The reconstructed emission images were reformatted into coronal, sagittal and maximum intensity projection (MIP) image sets.
Results and discussion
Radiolabeling
Many considerations must be taken into account for 68Ga-radiolabeling using a generator: some influence the radiochemical purity of the complex and some would change the quality of the formulation for human applications [15]. The interaction of all factors is usually crucial for obtaining suitable radiopharmaceutical compounds (Fig. 1).
Elution
The elution portfolio of the generator was measured using a suitable concentration of HCl (0.6–0.7 M) leading to the elution of most of daily generator activity in the first 0.5–1.5 ml of elution (Fig. 2).
Acidity
It has been shown that the pH of radiolabeling for most of Ga-68 labeling reactions is in the range of 4–5; thus, Ga activity elutions using 0.6–0.7 M HCl usually possess low pHs at the range of 1–2 which does not allow the direct use of the elution in the radiolabeling process. Due to the limited physical half-life of the radionuclide, the addition of calculated amount of solid HEPES to the elution was applied and checked by ITLC (Fig. 3).
Time
All considerations are taken into account for minimizing the reaction/purification/formulation process times. Evaporation of the eluent was replaced by the addition of the appropriate base (HEPES) as described above; the fact that heating the mixture would shorten the reaction time also should be carefully investigated.
Ligand amount
EDTMP is a rather low-toxic ligand at milligram scales, usually used in 20–50 mg range for the preparation of therapeutic agents. In case of carrier-free Ga-68, the ligand amount was reduced using optimization reactions (3–5 mg of ligand used) (Fig. 4).
The optimizations were controlled using ITLC method using normal saline as eluent on Whatman paper, the radiolabeled compound migrates to higher \( R_{{{\text{f}}_{\text{s}} }} \) (0.9) while the cation retains at the base (R f 0.1) (Fig. 5).
In HPLC experiments using a gradient of water:acetonitrile (0–100:100–0 % added: 1 % trifluoroacetic acid) would let to the fast removal of any free cation from the column with retention time of 0.9–1 min starting from water, while with increasing the amount of acetonitrile content in the mobile phase mixture the radiolabeled complex is washed out (9–10 min).
The reason for the close retention times is the ionic nature of 68Ga-EDTMP complex. Usually for better reproducibility, the column is washed for 20 min followed by washing with water again (Fig. 6).
Biodistribution
The % ID/g data for free Ga3+ are summarized in Fig. 7 which is excreted mostly from gastrointestinal tract (GIT) with high blood, colon, bone and stomach activity. 68Ga-EDTMP on the other hand is mostly excreted from the circulation almost instantly into the bone tissues with constant accumulation up to 120 min (Fig. 8).
PET/CT Imaging of 68Ga-EDTMP in rats
As shown in Figs. 9 and 10, the only visible organs were the vertebrae, limb long bones and bladder. There was a significant uptake of 68Ga-EDTMP in kidneys with low levels of accumulation in other organs. For quantitative uptake behavior, maximum standard uptake value (SUVmax) ratios were calculated for liver to kidney in all imaging studies.
To visualize three-dimensional image of the injected animal, Volume Rendering Technique (VRT) was applied to use a maximum intensity projection (MIP) leading to a two-dimensional projection pixel. The brightest pixel along each line of sight going through the three-dimensional object in a fashion starting from the (virtual) viewer was used. Figure 11 demonstrates the VRT MIP image of 68Ga-EDTMP-injected rat 155-min post-injection.
Conclusion
68Ga-EDTMP complex was prepared in high radiochemical purity (≈99 ± 0.88 % ITLC, >99 % HPLC) as well as significant specific activity of 15–18 GBq/mM. The time, temperatures and buffer content factors were all optimized for developing a clinical batch under sterile conditions. A 15- to 18-mCi eluted Ga activity from the generator was radiolabeled at 50–60 °C using 3–12 mg of the starting synthesized ligand in 6 min followed by formulation of the tracer for injection. A 5- to 8-min quality control time for simultaneous RTLC and HPLC was also needed. The biodistribution of the tracer demonstrated high bone uptake and also kidney uptake all in accordance with the reported bone-avid radiopharmaceuticals in mammals. This study can be used as an optimized protocol for production and quality control of 68Ga-EDTMP and possibly other radiolabeled Ga-68 tracers for clinical studies in nuclear medicine centers far from cyclotrons capable of using a Ga-68 generator, considering 7–10 millicurie human dose for injection.
References
Sudbrock F, Fischer T, Zimmermann B, Guliyev M, Dietlein M, Drezezga A, et al. Characterization of SnO2-based 68Ge/68Ga generators and 68Ga-DOTATATE preparations: radionuclide purity, radiochemical yield and long-term constancy. EJNMMI Res. 2014;4:36.
Fazaeli Y, Jalilian AR, Amini MM, Ardaneh K, Rahiminejad A, Bolourinovin F, et al. Development of 68Ga-fluorinated porphyrin complex as a possible PET imaging agent. Nucl Med Mol Imaging. 2012;46:20–6.
Velikyan I. Prospective of 68Ga-radiopharmaceutical development. Theranostics. 2014;4:47–80.
Serafini AN. Therapy of metastatic bone pain. J Nucl Med. 2001;42:895–906.
Bayouth JE, Macey DJ, Kasi LP, Fosselia FV. Dosimetry and toxicity of samarium-153-EDTMP administered for bone pain due to skeletal metastases. J Nucl Med. 1994;35:63–9.
Fellner M, Riss P, Loktionova N, Zhernosekov K, Thews O, Geraldes CFGC, et al. Comparison of different phosphorus-containing ligands complexing 68Ga for PET-imaging of bone metabolism. Radiochem Acta. 2011;99:43–51.
Ogawa K, Ishizaki A, Takai K, Kitamura Y, Kiwada T, Shiba K, et al. Development of novel radiogallium-labeled bone imaging agents using oligo-aspartic acid peptides as carriers. PLoS One. 2013;8:e84335.
Fellner M, Biesalski B, Bausbacher N, Kubicek V, Herman P, Rösch F, et al. 68Ga-BPAMD: PET-imaging of bone metastases with generator based positron emitter. Nucl Med Biol. 2012;39:993–9.
Fellner M, Baum RP, Kubicek V, Hermann P, Lukes I, Prasad V, et al. PET/CT imaging of osteoblastic bone metastases with 68Ga-bisphosphonates: first human study. Eur J Nucl Med Mol Imaging. 2010;37:834.
Ayati N, Aryana K, Jalilian AR, Hoseinnejad T, Samani AB, Ayati Z, et al. Treatment efficacy of 153Sm-EDTMP for painful bone metastasis. Asia Oceania J Nucl Med Biol. 2013;1:27–31.
Bahrami-Samani A, Anvari A, Jalilian AR, Shirvani-Arani S, Yousefnia H, Aghamiri MR, et al. Production, quality control and pharmacokinetic studies of 177Lu-EDTMP for human bone pain palliation therapy trials. Iran J Pharm Res. 2012;11:137–44.
Mitterhauser M, Toegei S, Wadsak W, Lanzenberger RR, Mien LK, Kunter C, et al. Pre vivo, ex vivo and in vivo evaluations of [68Ga]-EDTMP. Nucl Med Biol. 2007;34:391–7.
Mirzaei A, Jalilian AR, Aghanejad A, Mazidi M, Yousefnia H, Shabani G, et al. Preparation and evaluation of 68Ga-ECC as a PET renal imaging agent. Nucl Med Mol Imag. 2015. doi:10.1007/s13139-015-0323-7.
Goeckeler WF, Troutner DE, Volkert WA, Edwards B, Simon J, Wilson D. 153Sm radiotherapeutic bone agents. Int J Rad Appl Instrum B. 1986;13:479–82.
Breeman WA, de Jong M, de Blois E, Bernard BF, Konijnenberg M, Krenning EP. Radiolabeling DOTA-peptides with 68Ga. Eur J Nucl Med Mol Imaging. 2005;32:478–85.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mirzaei, A., Jalilian, A.R., Badbarin, A. et al. Optimized production and quality control of 68Ga-EDTMP for small clinical trials. Ann Nucl Med 29, 506–511 (2015). https://doi.org/10.1007/s12149-015-0971-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-015-0971-9