Abstract
177Lu-maltolate (177Lu-MAL) was successfully developed which can be widely used in bone palliation therapy. At optimized conditions a radiochemical purity of about >99 % was obtained for 177Lu-MAL shown by ITLC (specific activity, 970–1,000 MBq/mmole). Biodistribution studies of 177Lu chloride and 177Lu-MAL were carried out in wild-type rats comparing the critical organ uptakes. Compartmental analysis was used to determine temporal biodistribution model of 177Lu-MAL in different organs. 177Lu-MAL is a possible therapeutic agent in human malignancies for the bone palliation therapy so the efficacy of the compound should be tested in various animal models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many beta emitters such as 153Sm, 166Ho and 177Lu can be produced in reasonable amounts using (n, gamma) reactions [1–3]. Due to the half-life limitations in the application of mentioned radionuclides the emerging need for a long half-life beta emitter such as 177Lu is obvious [1].
Owing to 177Lu suitable decay characteristics [T1/2 = 6.73 d, Eβmax = 497 keV, Eγ = 112 keV (6.4 %), 208 keV (11 %) [4] as well as the feasibility of large-scale production in adequate specific activity and radionuclidic purity using a moderate flux reactor, 177Lu has been considered as a promising radionuclide for developing therapeutic radiopharmaceuticals [5]. The mean range of 177Lu beta particles is 0.67 mm, which makes it ideal for treating micro-metastatic disease [6].
Maltol (3-Hydroxy-2-methyl-4-pyron) is commonly formed when sugars are heated. Maltol loses its hydroxyl proton at neutral into basic pH levels, forming the maltolate anion; this anionic molecule forms a strong bidentate/tridentate chelates with gallium, iron, zinc, aluminum, vanadium and many other metals [7].
Most of metal-maltolato complexes are reported as biologically active compounds. Gallium-maltolate is a superior antitumor oral drug in clinical trial phases in contrast to gallium nitrate as well as many other metal based antitumoral compounds [8]. Ga-maltolate is an effective anti-lymphoma compound with activity against Ga nitrate-resistant lymphomas [9] and urothelial malignancies [10]. A more recent clinical trial confirmed the efficacy of gallium nitrate in patients with non-Hodgkin’s lymphoma whose disease had relapsed following treatment with conventional chemotherapeutic agents [11]. The chemistry and pharmacokinetics of Ga-maltolate have been extensively reported before starting the clinical phase [12] moreover, the pharmacokinetic has been shown in animals after oral administration [13]. On the other hand, aluminum-maltolate complex has demonstrated in vitro apoptotic cell death pathway in man [14] as well as anti-microbial effects [15]. Mixed copper-maltolate complexes have also been reported for high cytotoxicity against HeLa (cervical) cancer cell lines demonstrating a synergistic effect between the metal and ligand in the cell death [16].
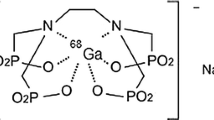
Due to the interesting pharmacological properties of maltolato complexes such as solubility in serum, rapid wash-out, tumor avidity and feasible complexation with various metals [17], the idea of developing a possible bone pain palliation agent through incorporating 177Lu into a suitable anionic ligand, i.e. Tris (maltolato) 177Lu(III) was investigated (Fig. 1).
In this investigation, radiolabeling, partition coefficient determination, quality control and biodistribution studies of 177Lu-MAL complex in wild-type rats was reported. In addition, time dependant biodistribution model of 177Lu-MAL was procured by using compartmental analysis with respect to anatomical data [18] from ICRP Report 89 (2002).
Experimental
Maltol was purchased from Aldrich Co., Germany, without further purification. Chromatography paper (Whatman No. 2) was obtained from Whatman (Maidstone, UK). Radio-chromatography was performed using a bioscan AR-2000 radio TLC scanner (Bioscan, Paris, France). A high purity germanium (HPGe) detector coupled with a Canberra™ (model GC1020-7500SL) multichannel analyzer and a dose calibrator ISOMED 1010 (Dresden, Germany) were used for counting distributed activities in rat organs. All other chemical reagents were purchased from Merck (Darmstadt, Germany). Calculations were based on the 112 keV peak for 177Lu. All values were expressed as mean ± standard deviation (Mean ± SD) and the data were compared using Student’s t test. Statistical significance was defined as P < 0.05. Animal studies were carried out in accordance with The United Kingdom Biological Council’s Guidelines on the use of living animals in scientific investigations, 2nd ed. Male healthy rats were purchased from Pasteur Institute, Tehran, Iran.
Production and quality control of 177LuCl3 solution
177Lu was produced by irradiation of natural Lu2O3 target (1 mg) at a thermal neutron flux of approximately 5 × 1013 n/cm2.s for 5 days at Tehran Research Reactor (TRR) according to the reported procedures [19]. The irradiated target was dissolved in 200 µl of 1.0 mol/L HCl, to prepare 177LuCl3 and diluted to the appropriate volume with ultra pure water, for producing a stock solution of final volume of 5 ml. The mixture was filtered through a 0.22 µm biological filter and sent for use in the radiolableing step. Radionuclide purity of the solution was checked for the presence of other radionuclides using beta spectroscopy and HPGe spectroscopy based on two major photons of 177Lu (6.4 % of 112 keV and 11 % of 208 keV) to detect various interfering beta and gamma emitting radionuclides. The radiochemical purity of the 177LuCl3 was checked using 2 solvent systems for ITLC [A: 10 mM DTPA pH.4 and B: ammonium acetate 10 %: methanol (1:1)] for parallel determination of colloids as well as other ionic species.
Labeling of maltolate with 177LuCl3
Most of the papers have reported the maltolate metal complex synthesis in aqueous phase [12]. However, we made the synthesis in ethanolic media. Briefly, 177LuCl3 (111 MBq, 0.1 ml) was added to a borosilicate vial and dried by heating (50 °C) under a nitrogen flow for about 15 min. Then, maltol (31 mg, 0.25 mmol) dissolved in absolute ethanol (1 ml) was added to the dried residue and the mixture was agitated and incubated at 60 °C for 2 h. The radiochemical purity of free Lutetium and 177Lu-MAL was determined by counting Whatman No. 2 sheets as stationary phase using various mobile phases (A: ammonia: water: methanol (2:40:20), B: 1 mM DTPA aqueous solution, C: 10 % ammonium acetate: methanol system, 1:1). After obtaining the desired radiochemical purity, the ethanolic solution was concentrated by warming 40–50 °C to 0.05 ml and then diluted to a 5 % solution by adding 1 ml of normal saline.
Stability studies
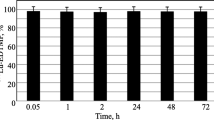
The stability of the 177Lu-MAL in final preparation stored at room temperature (25 °C ambient) and in presence of freshly prepared human serum (37 °C, 300 µl) was studied at different intervals of time by determining the radiochemical purity of the complex by ITLC analysis using ammonia: water: methanol (2:40:20) mobile phase (n = 3).
Determination of partition coefficient
The partition coefficient (log P) [20] of the 177Lu-MAL was measured following 1 min of vigorous vortex mixing of 1 ml of 1-octanol and 1 ml of isotonic acetate-buffered saline (pH = 7) with approximately 3.7 MBq (100 μCi) of the radiolabeled complex at 37 °C. Following further incubation for 5 min, the octanol and aqueous phases were sampled and counted in an automatic well counter. A 500 µl sample of the octanol phase from this partitioning was repartitioned two or three times with fresh buffer to ensure that traces of hydrophilic 177Lu impurities did not alter the calculated P values. The reported log P values are the averages of the second and third extractions from three to four independent measurements, log P values represent the mean (standard deviation) of five measurements.
Biodistribution of 177Lu-MAL and 177LuCl3 in normal rats
To determine comparative biodistribution, 177Lu-MAL and 177LuCl3 were administered to the normal rats in separate groups (n = 3). Each of the experimental animals was injected with 100–120 μL of the final radio labeled solution corresponding to 4.75 ± 0.2 MBq (130 ± 5 μCi) of activity through lateral caudal vein (vena caudalis lateralis). The animals were sacrificed by cardiac puncture post-anesthesia at the exact time intervals (2, 4, 24 h and 7 days) post-injection (p.i.). Various organs and tissues were excised following sacrifice, washed with normal saline, dried and radioactivity associated with each organ and tissue was determined using a flat type Nal(Tl) counter. Percent injected dose (% ID) accumulated in various organs were calculated from the data acquired by counting the radioactivity. The activity excreted by each animal was inferred from the difference between total injected dose and the % ID accounted for in all the organs and tissues.
Biodistribution modeling of 177Lu-MAL
The first approach of the modeling studies included the knowledge of chemical kinetics and mimetism of the lutetium and the possible targets of the diagnosis/therapy for choosing the possible models to apply over the sampling standard methods used in experimental works [21, 22].
Biodistribution modeling consists of two steps. At first stage a model with only one physical compartment (whole body) and one chemical compartment (177Lu-MAL) generated with the compartmental analysis. The values used in this work were residence time from three different kinds of study with free 177Lu: whole body, average excretion and maximum excretion as a chemical compartment. Activity concentration values as time function in measurements of total whole body and activity measurement in samples of blood with projection to total circulating blood volume with 177Lu-MAL. Considering the two sources of data in the same modeling a better consistence was obtained.
The second step was the statistic treatment of biodistribution and dosimetry in rats considering three chemical fractions of the designed radiopharmaceutical: 177Lu-MAL, free 177Lu, and total radiopharmaceutical (free 177Lu + (177Lu-MAL) (Fig. 2). Using a mamillar models with six compartments and Human anatomic data from ICRP Report 89, these studies were also performed in rats. The selected parameters were very critical, considering the blood flux in each body region and tissue.
Results
Production and quality control of 177Lu
The radionuclide was prepared in a research reactor according to the regular methods within the range of specific activity 2.6–3 GBq/mg for radiolabeling use. The obtained radionuclidic purity was 99.98 % (Fig. 3).
The radioisotope was dissolved in acidic media as a starting sample and was further diluted and evaporated for obtaining the desired pH and volume followed by sterile filtering. The radiochemical purity of the 177Lu solution was checked in two solvent systems on Whatman No. 2 papers in 10 mM DTPA. Free Lu3+ cation was chelated to the more lipophilic Lu-DTPA form and migrated to higher Rf, while small radioactive fraction remained at the origin which could be related to other Lu ionic species, not forming Lu-DTPA complex, such as LuCl4 −, etc. and/or colloids (4 %).
On the other hand, 10 % ammonium acetate: methanol mixture was also used for the determination of radiochemical purity. In this solvent system, the fast eluting species were possibly 177Lu cations, other than Lu3+ (2 %) and the remaining fraction at Rf.0 was a possible mixture of Lu3+ and/or colloids. The difference in values of impurity in two solvent systems was possibly due to the presence of colloidal impurity in the sample (2 %).
Preparation of 177Lu-MAL
In order to obtain the highest specific activity in the shortest possible time, a quantitative study was designed using different amounts of MAL and various time intervals for a specific amount of radioactivity (2 mCi of LuCl3 for instance) while 50 °C was considered a suitable temperature. A satisfactory labeling yield of higher than 99 % was obtained at this temperature using 18–22 mg of MAL within 2 h. Because of relative lipophilic 177Lu-MAL complex and participation of several polar functional groups in its structure, 177Lu-MAL migrated to the solvent frontline in ITLC (Rf.08) while 177Lu cation was retained at the origin (Rf. 0.05) in a mixture of ammonia: water: methanol (Fig. 4).
As shown in Fig. 1, 177Lu-MAL is majorly prepared in a 3:1 ligand: cation ratio 2 components with reported ratio mixture, considering the molar ratio, a molecular weight of 552 can be calculated for 177Lu(MAL)3, resulting in a specific activity of 970–1,000 MBq/mmol under optimized radiolabeling conditions. The labeling step took about 2 h. In all radiolabeling procedures (n = 5), the labeling yield was over 99 %.
The partition coefficient for the labeled compound was calculated (log P. 1.869) demonstrating a rather lipophilic complex as it could be observed from the chromatographic behavior.
The final radiolabeled complex diluted in normal saline was then passed through a 0.22 µm (Millipore) filter for sterilization. Incubation of 177Lu-MAL in freshly prepared human serum for 24 h at 37 °C showed no loss of 177Lu from the complex (less than 0.1 %).
Biodistribution studies for free 177Lu cation in rats
The animals were sacrificed by CO2 asphyxiation at selected times after injection. Dissection began by drawing blood from the aorta followed by removing the heart, spleen, muscle, bone, kidneys, liver, intestine, stomach, lungs and skin samples. The tissue uptakes were calculated as the percent of area under the curve of the related photo peak per gram of tissue (ID/g %) (Fig. 5).
The liver uptake of the cation is comparable with many other radio-lanthanides mimicking calcium cation accumulation; about 0.58 ± 0.04 % of the activity accumulates in the liver after 48 h. The metal-transferrin complex uptake and the final liver delivery seem the possible route of accumulation.
The blood content is low at all intervals which show the rapid removal of activity in the circulation. The lung, muscle and also skin do not demonstrate significant uptake which is in accordance with other cation accumulation. A 4 % bone uptake is observed for the cation which remains almost constant after 48 h (data not shown). The spleen also has significant uptake possibly related to reticuloendothelial uptake. The kidney plays an important role in 177Lu cation excretion especially after 24 h.
Biodistribution studies for 177Lu-MAL in rats
The accumulation of 177Lu-MAL is demonstrated in Fig. 6. The bone, liver and kidney were the major accumulated sites of the radiolabeled compound. A major route of excretion for the tracer was the urinary tract. Comparison of vital organ uptake for 177LuCl3 and 177Lu-MAL demonstrates kinetic pattern difference for both species. 177Lu cation is accumulated in the liver in the first 24 h (p.i.), and it can be assumed that later on, the activity is excreted from the liver via the biliary tract, while 177Lu-MAL is excreted through the kidneys with an exponential rate in 5 days.
As a result of the comparison of Figs. 5, 6, 24 h after injection free 177Lu accumulates in the bone (2.2 ± 0.3 %), while in case of 177Lu-MAL, the uptake is significantly higher (5.5 ± 0.6 %) thus at least 70 % more uptake is observed for the labeled compound. On the other hand, according to many published and unpublished data on the most important 177Lu bone pain palliation complex, 177Lu-EDTMP [23–26] accumulates in this tissue up to the maximum of 2.5–3 % which shows even less uptake compared with that of 177Lu-MAL. In case of the kidney, free 177Lu is excreted through the kidney in a linear manner and this uptake is rapidly decreased after 24 h, while in case of 177Lu-MAL, the uptake increases after 24 h. 4 h after injecting the two spices the biodistribution showed a different pattern while in 2–24 h the labeled compound is not significantly accumulated in the liver (0.6 ± 0.02–0.8 ± 0.01 %), whereas the cation itself is drastically accumulated in the liver up to 1.8 %. However, compared to analogous Ga-maltolate, the biological evaluation of 177Lu-MAL upon tumor models can be carried out.
Time-dependant model of 177Lu-MAL
The activity concentration in each organ was measured with the use of detectors at specified time after injection. The results show variation with time. The compartmental model was used to produce a mathematical description of these variations. The following equations were obtained for each organ. In each case, t = 0 corresponds to the time of injection.
-
1.
Blood
$$ \begin{gathered} {\text{f}}_{ 1} = 1. 7 9 1 3 4 2 {\text{ e}}^{{ - 0. 4 5 {\text{t}}}} \quad+ \left( { 2. 40 8 {\text{E}} - 4} \right){\text{ e}}^{{ - 0.0 6 {\text{t}}}}\quad + \left( { 6. 6 5 {\text{E}} - 2} \right){\text{ e}}^{{{-}( 9. 9 {\text{E}} - 4){\text{t}}}}\quad + \left( { 1. 3 7 2 {\text{E}} - 4} \right){\text{ e}}^{{ - 0.00 1 {\text{t}}}} \quad+ \left( { 1. 1 9 {\text{E}} - 1} \right){\text{ e}}^{{ - 0.0 8 7 {\text{t}}}} - \hfill \\ \left( { 1. 2 9 5 {\text{E}} - 5} \right){\text{ e}}^{{ - 5.0 4 {\text{t}}}} \, - \left( { 5. 8 8 {\text{E}} - 8} \right){\text{ e}}^{{ - 0. 1 4 {\text{t}}}} \hfill \\ \end{gathered} $$ -
2.
Bone
$$ \begin{gathered} {\text{f}}_{ 2} = 3. 5 7 1 3 3 {\text{ e}}^{{{-}( 9. 9 {\text{E}} - 4){\text{t}}}}\quad + 1. 7 5 2 8 {\text{ e}}^{{ - 0. 9 7 7 {\text{t}}}} - { 2}. 5 9 6 3 3 {\text{ e}}^{{ - 3.0 4 {\text{t}}}} - { 2}. 9 4 4 70 4 {\text{ e}}^{{ - 0. 1 6 {\text{t}}}} - \, \hfill \\ \left( { 6. 2 2 2 4 4 {\text{E}} - 1} \right){\text{ e}}^{{ - 8. 1 4 {\text{t}}}} - \left( { 4. 2 9 4 3 6 {\text{E}} - 3} \right){\text{ e}}^{{ - 0.0 1 {\text{t}}}} \hfill \\ \end{gathered} $$ -
3.
Heart
$$ \begin{gathered} {\text{f}}_{ 3} = \left( { 7. 8 5 3 8 5 {\text{E}} - 2} \right){\text{ e}}^{{ - 0. 3 8 {\text{t}}}}\quad + \left( { 7. 7 4 {\text{E}} - 5} \right){\text{ e}}^{{ - 0.0 7 {\text{t}}}}\quad + \left( { 1.0 1 2 5 {\text{E}} - 3} \right){\text{ e}}^{{ - ( 9. 9 {\text{E}} - 4){\text{t}}}} \quad+ \left( { 4. 4 1 {\text{E}} - 4} \right){\text{ e}}^{{ - 0.00 1 {\text{t}}}} \quad+ \left( { 1. 9 3 5 {\text{E}} - 2} \right){\text{ e}}^{{ - 0.0 7 7 {\text{t}}}} - \hfill \\ \left( { 4. 1 6 2 5 {\text{E}} - 6} \right){\text{ e}}^{{ - 5. 1 4 {\text{t}}}} - \, \left( { 6. 3 9 {\text{E}} - 9} \right){\text{ e}}^{{ - 0. 2 4 {\text{t}}}} \hfill \\ \end{gathered} $$ -
4.
Kidneys
$$ \begin{gathered} {\text{f}}_{ 4} = 1. 1 7 8 7 2 3 {\text{ e}}^{{ - 0. 4 8 {\text{t}}}} \quad+ \left( { 1. 5 6 5 2 {\text{E}} - 2} \right){\text{ e}}^{{ - 1. 7 {\text{t}}}} \quad+ \left( { 2. 9 5 7 5 {\text{E}} - 1} \right){\text{ e}}^{{ - ( 9. 9 {\text{E}} - 4){\text{t}}}} \quad+ \left( { 8. 9 1 8 {\text{E}} - 3} \right){\text{ e}}^{{ - 0.00 1 {\text{t}}}} \quad+ \hfill \\ \left( { 4. 3 6 8 {\text{E}} - 1} \right){\text{ e}}^{{ - 0.00 7 7 {\text{t}}}} - \left( { 8. 4 1 7 5 {\text{E}} - 3} \right){\text{ e}}^{{ - 4. 1 4 {\text{t}}}} - \left( { 1. 2 9 2 2 {\text{E}} - 4} \right){\text{ e}}^{{ - 1. 2 4 {\text{t}}}} \hfill \\ \end{gathered} $$ -
5.
Large Intestine
$$ \begin{gathered} {\text{f}}_{ 5} = \left( { 4. 8 1 6 {\text{E}} - 2} \right){\text{ e}}^{{ - 0.0 7 {\text{t}}}}\quad + \left( { 2. 8 {\text{E}} - 2} \right){\text{ e}}^{{ - ( 9. 9 {\text{E}} - 4){\text{t}}}} \quad+ \left( { 2. 7 4 4 {\text{E}} - 2} \right){\text{ e}}^{{ - 0.00 1 1 {\text{t}}}} \quad+ \left( { 3. 9 2 {\text{E}} - 1} \right){\text{ e}}^{{ - 0.0 8 4 2 {\text{t}}}} - \, \hfill \\ \left( { 3. 8 9 9 5 4 1 1 {\text{E}} - 1} \right){\text{ e}}^{{ - 0. 5 9 {\text{t}}}} - \left( { 2. 5 9 {\text{E}} - 2} \right){\text{ e}}^{{ - 0. 8 7 9 {\text{t}}}} - \, \left( { 3. 9 7 6 {\text{E}} - 2} \right){\text{ e}}^{{ - 0. 1 3 8 {\text{t}}}} - \left( { 8.0 9 9 5 4 1 1 {\text{E}} - 1} \right){\text{ e}}^{{ - 1.0 6 {\text{t}}}} \hfill \\ \end{gathered} $$ -
6.
Liver
$$ \begin{gathered} {\text{f}}_{ 6} = \left( { 5. 8 8 4 3 {\text{E}} - 1} \right){\text{ e}}^{{ - ( 9. 9 {\text{E}} - 4){\text{t}}}} \quad+ \left( { 2. 8 8 8 {\text{E}} - 1} \right){\text{ e}}^{{ - 0. 7 7 {\text{t}}}} - \, \left( { 1. 3 8 9 8 5 {\text{E}} - 1} \right){\text{ e}}^{{ - 1.0 4 {\text{t}}}} - \, \hfill \\ \left( { 1.0 2 5 2 4 {\text{E}} - 1} \right){\text{ e}}^{{ - 1. 1 4 {\text{t}}}} - \, \left( { 1. 2 4 1 8 4 {\text{E}} - 1} \right){\text{ e}}^{{ - 0. 6 6 {\text{t}}}} - \left( { 7.0 7 5 6 {\text{E}} - 3} \right){\text{ e}}^{{ - 0. 1 {\text{t}}}} - \, \left( { 1. 8 7 7 2 {\text{E}} - 8} \right){\text{ e}}^{{0. 1 {\text{t}}}} \hfill \\ \end{gathered} $$ -
7.
Lung
$$ \begin{gathered} {\text{f}}_{ 7} = \left( { 6. 9 4 4 {\text{E}} - 1} \right){\text{ e}}^{{ - 0.0 8 7 {\text{t}}}}\quad + \left( { 8. 5 3 1 2 {\text{E}} - 2} \right){\text{ e}}^{{ - 0.0 6 {\text{t}}}} \quad+ \left( { 4. 9 6 {\text{E}} - 2} \right){\text{ e}}^{{ - ( 9. 9 {\text{E}} - 4){\text{t}}}} \quad+ \left( { 4. 8 60 8 {\text{E}} - 2} \right){\text{ e}}^{{ - 0.00 1 {\text{t}}}} - \hfill \\ { 1}. 4 3 4 7 7 5 8 5 2 {\text{ e}}^{{ - 1.0{\text{t}}}} - \left( { 6. 90 7 7 5 8 5 2 {\text{E}} - 1} \right){\text{ e}}^{{ - 0. 6 2 {\text{t}}}} - \, \left( { 4. 5 8 8 {\text{E}} - 2} \right){\text{ e}}^{{ - 0. 9 7 3 {\text{t}}}} - \, \left( { 7.0 4 3 2 {\text{E}} - 1} \right){\text{ e}}^{{ - 0. 1 4 {\text{t}}}} \hfill \\ \end{gathered} $$ -
8.
Muscle
$$ \begin{gathered} {\text{f}}_{ 8} = \left( { 1. 1 7 8 7 2 3 {\text{E}} - 1} \right){\text{ e}}^{{ - 0. 3 9 {\text{t}}}} \quad + \left( { 1. 5 6 5 2 {\text{E}} - 3} \right){\text{ e}}^{{ - 1. 7 9 {\text{t}}}}\quad + \left( { 3. 4 1 2 5 {\text{E}} - 2} \right){\text{ e}}^{{ - ( 9. 9 {\text{E}} - 3){\text{t}}}} \quad+ \left( { 8. 9 1 8 {\text{E}} - 4} \right){\text{ e}}^{{ - 0.0 3 1 {\text{t}}}} + \hfill \\ \left( { 4. 3 6 8 {\text{E}} - 2} \right){\text{ e}}^{{ - 0.00 7 3 {\text{t}}}} - \left( { 8. 4 1 7 5 {\text{E}} - 4} \right){\text{ e}}^{{ - 2. 4 8 4 {\text{t}}}} - \, \left( { 1. 2 9 2 2 {\text{E}} - 5} \right){\text{ e}}^{{ - 1. 6 7 4 {\text{t}}}} \hfill \\ \end{gathered} $$ -
9.
Small Intestine
$$ \begin{gathered} {\text{f}}_{ 9} = \left( { 1. 2 6 6 8 3 7 {\text{E}} - 1} \right){\text{ e}}^{{ - 0. 1 1 {\text{t}}}} \quad + \left( { 7. 3 7 8 8 {\text{E}} - 4} \right){\text{ e}}^{{ - 0. 1 7 {\text{t}}}} \quad + \left( { 9. 6 5 2 5 {\text{E}} - 2} \right){\text{ e}}^{{ - ( 9. 9 {\text{E}} - 3){\text{t}}}}\quad + \hfill \\ \left( { 4.0 7 5 5 {\text{E}} - 2} \right){\text{ e}}^{{ - 0.00 1 {\text{t}}}} \quad+ \left( { 2.0 5 9 2 {\text{E}} - 1} \right){\text{ e}}^{{ - 0.0 7 7 {\text{t}}}} - \left( { 3. 9 6 8 2 5 {\text{E}} - 5} \right){\text{ e}}^{{ - 3. 1 4 {\text{t}}}} - \, \left( { 6.0 9 1 8 {\text{E}} - 6} \right){\text{ e}}^{{ - 0. 3 6 {\text{t}}}} \hfill \\ \end{gathered} $$ -
10.
Spleen
$$ \begin{gathered} {\text{f}}_{ 10} = \left( { 1. 3 7 6 {\text{E}} - 1} \right){\text{ e}}^{{ - 0.0 6 {\text{t}}}} + \left( { 4.0{\text{E}} - 2} \right){\text{ e}}^{{ - ( 9. 9 {\text{E}} - 4){\text{t}}}} + \left( { 7.8 4 {\text{E}} - 2} \right){\text{ e}}^{{ - 0.00 1 {\text{t}}}} + \left( { 7.0{\text{E}} - 1} \right){\text{ e}}^{{ - 0.0 8 7 {\text{t}}}} - \hfill \\ 2. 3 1 4 1 5 4 6 {\text{ e}}^{{ - 0.5 8 {\text{t}}}} - 1.5141546 {\text{ e}}^{{ - 1.6 {\text{t}}}} - \, \left( { 4.7 4 {\text{E}} - 1} \right){\text{ e}}^{{ - 3. 9 7 3 {\text{t}}}} - \, \left( { 1.1 3 6 {\text{E}} - 1} \right){\text{ e}}^{{ - 10. 1 4 {\text{t}}}} \hfill \\ \end{gathered} $$ -
11.
Sternum
$$ \begin{gathered} {\text{f}}_{ 1 1} = 1. 1 7 6 {\text{ e}}^{{ - 0.0 7 7 {\text{t}}}} + \left( { 1.4 4 4 8 {\text{E}} - 1} \right){\text{ e}}^{{ - 0.0 6 {\text{t}}}} + \left( { 8. 4 {\text{E}} - 2} \right){\text{ e}}^{{ - ( 9. 9 {\text{E}} - 4){\text{t}}}} + \left( { 2.60 2 3 2 {\text{E}} - 2} \right){\text{ e}}^{{ - 0.00 1 {\text{t}}}} + \left( { 2.1 8 4 {\text{E}} - 2} \right){\text{ e}}^{{0.0 1 9 {\text{t}}}} - \hfill \\ 2. 42986233 {\text{ e}}^{{ - 0. 6 3 {\text{t}}}} - { 6}. 6 2 9 8 2 6 {\text{ e}}^{{ - 2. 1 7 {\text{t}}}} - \, \left( { 7. 7 7 {\text{E}} - 2} \right){\text{ e}}^{{ - 0. 9 7 3 {\text{t}}}} - \, \left( { 1.1928 {\text{E}} - 1} \right){\text{ e}}^{{ - 0.14 {\text{t}}}} \hfill \\ \end{gathered} $$ -
12.
Stomach
$$ \begin{gathered} {\text{f}}_{ 1 2} = \left( { 9. 8 3 8 4 {\text{E}} - 1} \right){\text{ e}}^{{ - 2. 5 6 1 {\text{t}}}} + \left( { 1. 1 {\text{E}} - 3} \right){\text{ e}}^{{ - 0. 1 5 {\text{t}}}} + \left( { 2.0 3 5 {\text{E}} - 2} \right){\text{ e}}^{{ - 0. 9 9 {\text{t}}}} + \left( { 9. 9 {\text{E}} - 3} \right){\text{ e}}^{{ - ( 1. 1 {\text{E}} - 5){\text{t}}}} + \left( { 3.0 8 {\text{E}} - 1} \right){\text{ e}}^{{ - 0.0 3 4 1 {\text{t}}}} - \hfill \\ \left( { 6. 3 6 3 9 2 5 1 5 {\text{E}} - 1} \right){\text{ e}}^{{ - 0. 9 4 9 8 {\text{t}}}} - \, \left( { 1.0 8 3 5 {\text{E}} - 2} \right){\text{ e}}^{{ - 1.0 8 3 5 {\text{t}}}} - \, \left( { 8. 6 3 9 2 5 1 5 {\text{E}} - 2} \right){\text{ e}}^{{ - 0. 1 8 {\text{t}}}} \hfill \\ \end{gathered} $$
The deviation of 177Lu-MAL concentration in all organs and tissues is described with summation of six to nine exponential terms. Comparison with animal data showed that our experimental data with precision better than 1 %. It should be noted that the concentration of activity had been in a good statistics rang of measurement.
Discussion
The worthiness of an ideal bone-avid agent, especially with respect to therapeutic applications, contingent on the accumulation of the complex in the bone during the delayed time intervals compared with its accumulation in critical organ at risks such as the liver and kidneys is important for developing a therapeutic agent. Therefore, clearance from blood, accumulation in bone, and the ratio of accumulated activity in bone to that in critical organs is parameters that should be considered for bone-avid radiopharmaceuticals.
As shown in Fig. 6, significant bone uptake of 177Lu-MAL (5.52 ± 0.6 %/g) was observed 24 h after injection. Bone uptake decreases to 48 h p.i. (3.4 ± 0.5 %/g) and no significant uptake was observed in critical organs. The target/nontarget ratios for this complex are given in Table 1.
There is no known mechanism and behavior of lanthanide maltolate complexes for bone accumulation. In the case of Ga-maltolate, 2 % bone uptake has been observed [8]. This amount is higher than free gallium uptake in bones (possibly due to bone marrow accumulation of Ga mimicking Ferric ion). In this study, 177Lu-MAL showed a significant bone uptake. In comparison with free cation, there are less liver and kidney uptake.
Although the distribution mode and biological behavior of some metal maltol complexes, including Zinc ethyl maltol [27] and vanadyl maltol [28] seems to be very similar to free cation, they showed some increased biopharmaceutical behavior and less unwanted accumulation sites including kidney and liver. These studies suggest that the maltol complex is increasing biological distribution and passing through the membranes such as vessels and cell lines. While these complexes reaching the same tissue uptake percentage in bone compared to free lutetium, decreases the liver uptake.
Nowadays, various bone-seeking radiopharmaceuticals such as 166Ho-DOTMP [29], 186Re-HEDP [30, 31], 153Sm-EDTMP [32], and 177Lu-EDTMP [33] are being used clinically for bone pain palliation. 153Sm-EDTMP is the most widely used radiopharmaceutical in the USA for this purpose [34, 35].
The most important point to be considered while developing radiopharmaceuticals as bone pain palliative agents is the dose delivered to the bone marrow. Although the skeletal lesions should receive adequate dose, the dose to the bone marrow should be kept as low as possible [36, 37]. Because of the importance of the dose delivered to the bone marrow, radiopharmaceuticals with lower beta energy are preferred for bone pain palliation. As seen in Table 2, the maximum and average beta energy varies considerably with each radionuclide. The highest average energy beta particle is seen with 90Y and the lowest with 177Lu. From this point of view, 177Lu seems to be a better candidate for bone pain palliation compared with 153Sm, 186Re, and 166Ho. In addition, the longer half-life of 177Lu compared with that of the mentioned radionuclides can be an advantage for transportation to distant centers from the research reactor.
177Lu-MAL demonstrated a higher bone uptake compared with the only clinically used 177Lu bone pain palliative therapeutic agent 177Lu-EDTMP. Further, both 177Lu-MAL and 177Lu-EDTMP complexes cleared from the blood rapidly, and after 24 h no radioactivity existed in the blood.
Conclusion
The 177Lu-MAL complex was prepared with high radiochemical yield (>99 %). Under optimized conditions, total labeling and formulation took about 2 h. The prepared complex was stable in the final solution at room temperature and the presence of human serum at 37 °C and can be used even 48 h after preparation. No significant amount of other radioactive species was detected by ITLC. IV injection of 177Lu-MAL complex to male wild-type rats demonstrated activity distribution among rat tissues. Biodistribution studies showed different accumulation from free 177Lu cation. Most of the 177Lu-MAL was accumulated in the bone while liver and spleen are major dose-limiting tissues. 177Lu-MAL can be a potential candidate for bone pain palliation therapy in skeletal metastases, although further biological studies in other mammals and also exact oral administration upon appropriate animal model are still needed.
References
Troyer G, Schenter R (2009) Medical isotope development and supply opportunities in the 21st century. J Radioanal Nucl Chem 282:243–246. doi:10.1007/s10967-009-0267-4
Yousefnia H, Jalilian AR, Zolghadri S, Bahrami-Samani A, Shirvani-Arani S, Ghannadi-Maragheh M (2010) Preparation and quality control of lutetium-177 bleomycin as a possible therapeutic agent. Nukleonika 55:285–291
Naseri Z, Hakimi A, Jalilian A, Nemati Kharat A, Shirvani-Arani S, Bahrami-Samani A, Ghannadi-Maragheh M (2012) Synthesis, quality control and biological evaluation of tris [(1, 10-phenanthroline)[153Sm] samarium(III)] trithiocyanate complex as a therapeutic agent. Radiochim Acta Int J Chem Asp Nucl Sci Technol 100:267–271. doi:10.1524/ract.2012.1910
Firestone RB, Shirley VS, Baglin CM, Zipkin J (1996) In: Table of isotopes. Wiley, New York, p 1447
Chakraborty S, Das T, Sarma HD, Venkatesh M, Banerjee S (2008) Comparative studies of <sup> 177 </sup> Lu–EDTMP and <sup> 177 </sup> Lu–DOTMP as potential agents for palliative radiotherapy of bone metastasis. Appl Radiat Isot 66:1196–1205. doi:10.1016/j.apradiso.2008.02.061
Ishfaq M, Hussain N, Jehangir M (2007) DOTA-Tyr3-Octreotate: labeling with β-emitting radionuclides for the preparation of potential therapeutic radiopharmaceuticals. J Radioanal Nucl Chem 273:689–694. doi:10.1007/s10967-007-0932-4
Naseri Z, Hakimi A, Jalilian AR, Kharat AN, Bahrami-Samani A, Ghannadi-Maragheh M (2011) Preparation and quality control of the [153Sm]-Samarium maltolate complex as a lanthanide mobilization product in rats. Sci Pharm 79:265. doi:10.3797/scipharm.1011-08
Fazaeli Y, Jalilian AR, Amini MM, Majdabadi A, Rahiminejad A, Bolourinovin F, Pouladi M (2012) Development of Ga-67 maltolate complex as an imaging agent. Iran J Pharm Res: IJPR 11:755 PMCID: PMC3813137
Chitambar CR, Purpi DP, Woodliff J, Yang M, Wereley JP (2007) Development of gallium compounds for treatment of lymphoma: gallium maltolate, a novel hydroxypyrone gallium compound, induces apoptosis and circumvents lymphoma cell resistance to gallium nitrate. J Pharmacol Exp Ther 322:1228–1236. doi:10.1124/jpet.107.126342
Chitambar CR (2004) Gallium nitrate for the treatment of non-Hodgkin’s lymphoma. Expert Opin on Investig drugs 13:531–541. doi:10.1517/13543784.13.5.531
Pro B, Bociek R, Chitambar CR, Gregory SA, Leonard JP, Smith S, Novick S. (2004) Phase 2 multicenter trial of gallium nitrate in patients with advanced non-hodgkin’s lymphoma (NHL). In: ASH annual meeting abstracts. Vol. 104, pp 2487
Bernstein LR, Tanner T, Godfrey C, Noll B (2000) Chemistry and pharmacokinetics of gallium maltolate, a compound with high oral gallium bioavailability. Met Base Drugs 7:33. doi:10.1155/MBD.2000.33
Martens RJ, Mealey K, Cohen ND, Harrington JR, Chaffin MK, Taylor RJ, Bernstein LR (2007) Pharmacokinetics of gallium maltolate after intragastric administration in neonatal foals. Am J Vet Res 68:1041–1044. doi:10.2460/ajvr.68.10.1041
Satoh E, Yasuda I, Yamada T, Suzuki Y, Ohyashiki T (2007) Involvement of NO generation in aluminum-induced cell death. Biol Pharm Bull 30:1390–1394. doi:10.1248/bpb.30.1390
DeLeon K, Balldin F, Watters C, Hamood A, Griswold J, Sreedharan S, Rumbaugh KP (2009) Gallium maltolate treatment eradicates Pseudomonas aeruginosa infection in thermally injured mice. Antimicrob Agents Ch 53:1331–1337. doi:10.1128/AAC.01330-08
Barve A, Kumbhar A, Bhat M, Joshi B, Butcher R, Sonawane U, Joshi R (2009) Mixed-ligand copper(II) maltolate complexes: synthesis, characterization, DNA binding and cleavage, and cytotoxicity. Inorg Chem 48:9120–9132. doi:10.1021/ic9004642
Chitambar CR, Purpi DP (2010) A novel gallium compound synergistically enhances bortezomib-induced apoptosis in mantle cell lymphoma cells. Leuk Res 34:950–953. doi:10.1016/j.leukres.2010.02.034
Valentin J (2002) Basic anatomical and physiological data for use in radiological protection: reference values: ICRP Publication 89. Ann ICRP 32:1–277. doi:10.1016/S0146-6453(03)00002-2
IAEA (2003) Manual for reactor produced radioisotopes. IAEA-TECDOC-1340, Vienna
Jalilian A, Hakimi A, Garousi J, Bolourinovin F, Kamali-Dehghan M, Aslani G (2008) Development of [201Tl](III) oxinate complex for in vitro cell labeling. Iran J Radiat Res 6:145–150
Sardari D, Hakimi A (2012) Modeling the time dependent distribution of a new <sup> 153 </sup> Sm complex for targeted radiotherapy purpose. Rep Pract Oncol Radiother 17:358–362. doi:10.1016/j.rpor.2012.07.001
Abbasian P, Foroghy M, Jalilian AR, Hakimi A, Shirvani-Arani S (2014) Modeling the time dependent biodistribution of Samarium-153 ethylenediamine tetramethylene phosphonate using compartmental analysis. Rep Pract Oncol Radiother 19:214–220. doi:10.1016/j.rpor.2013.12.002
Máthé D, Balogh L, Polyák A, Király R, Márián T, Pawlak D, Zaknun JJ, Pillai MR, Jánoki GA (2010) Multispecies animal investigation on biodistribution, pharmacokinetics and toxicity of <sup> 177 </sup> Lu-EDTMP, a potential bone pain palliation agent. Nucl Med Biol 37:215–226. doi:10.1016/j.nucmedbio.2009.09.004
Garnuszek P, Pawlak D, Licińska I, Kamińska A (2003) Evaluation of a freeze-dried kit for EDTMP-based bone-seeking radiopharmaceuticals. Appl Radiat Isot 58:481–488. doi:10.1016/S0969-8043(03)00057-5
Bahrami-Samani A, Anvari A, Jalilian AR, Shirvani-Arani S, Yousefnia H, Aghamiri MR, Ghannadi-Maragheh M (2012) Production, quality control and pharmacokinetic studies of 177Lu-EDTMP for human bone pain palliation therapy trials. Iran J Pharm Res 11:137–144
Abbasi A (2011) Studies on 177Lu-labeled methylene diphosphonate as potential bone-seeking radiopharmaceutical for bone pain palliation. Nucl Med Biol 38:8. doi:10.1016/j.nucmedbio.2012.02.001
Akbar B, Niloufar N, Abolfazl M, Lofollah S, Ali KQ, Soheyla V. (2013) Evaluation and comparison of zinc absorption level from 2-Alkyle 3-Hydroxy pyranon-zinc complexes and zinc sulfate in rat in vivo. Adv Biom Res, 2 DOI: 10.4103/2277-9175.116432
Barrio D, Braziunas M, Etcheverry S, Cortizo A (1997) Maltol complexes of vanadium(IV) and (V) regulate in vitro alkaline phosphatase activity and osteoblast-like cell growth. J Trace Elem Med Bio 11:110–115. doi:10.1016/S0946-672X(97)80035-1
Bayouth JE, Macey DJ, Kasi LP, Garlich JR, McMillan K, Dimopoulos MA, Champlin RE (1995) Pharmacokinetics, dosimetry and toxicity of holmium-166-DOTMP for bone marrow ablation in multiple myeloma. J Nucl Med: Off Publ, Soc Nucl Med 36:730–737
Brenner W, Kampen WU, von Forstner C, Brümmer C, Zuhayra M, Muhle C, Czech N, Henze E (2001) High-dose treatment with 186Re-HEDP or 153Sm-EDTMP combined with amifostine in a rabbit model. J Nucl Med 42:1545–1550
Brenner W, Kampen WU, Brümmer C, von Forstner C, Zuhayra M, Czech N, Muhle C, Henze E (2003) Bone uptake studies in rabbits before and after high-dose treatment with 153Sm-EDTMP or 186Re-HEDP. J Nucl Med 44:247–251
Bayouth JE, Macey DJ, Kasi LP, Fossella FV (1994) Dosimetry and toxicity of samarium-153-EDTMP administered for bone pain due to skeletal metastases. J Nucl Med: Off Publ, Soc Nucl Med 35:63–69
Yuan J, Liu C, Liu X, Wang Y, Kuai D, Zhang G, Zaknun JJ (2013) Efficacy and safety of 177Lu-EDTMP in bone metastatic pain palliation in breast cancer and hormone refractory prostate cancer: a phase II study. Clin Nucl Med 38:88–92. doi:10.1097/RLU.0b013e318279bf4d
Pandit-Taskar N, Batraki M, Divgi CR (2004) Radiopharmaceutical therapy for palliation of bone pain from osseous metastases. J Nucl Med 45:1358–1365
Yousefnia H, Jalilian AR, Zolghadri S, Ghannadi-Maragheh M (2014) Production, quality control, biodistribution assessment and preliminary dose evaluation of 177Lu-PDTMP as a possible bone palliative agent. Nucl Med Commun 35:99–107. doi:10.1097/MNM.0000000000000018
Hosain F, Spencer RP (1992) Radiopharmaceuticals for palliation of metastatic osseous lesions: biologic and physical background. Semin Nucl Med 22:11–16. doi:10.1016/S0001-2998(05)80152-7
Volkert WA, Hoffman TJ (1999) Therapeutic radiopharmaceuticals. Chem Rev 99:2269–2292
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hakimi, A., Jalilian, A.R., Shirvani-Arani, S. et al. Production, quality control, biological evaluation and biodistribution modeling of Lutetium-177 maltolate as a viable bone pain palliative in skeletal metastasis. J Radioanal Nucl Chem 303, 1–10 (2015). https://doi.org/10.1007/s10967-014-3603-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3603-2