Abstract
The COVID-19 pandemic has exacerbated anxiety and depression disorders. We used network analysis to identify core symptoms and comorbid pathways between the two disorders and reveal temporal changes in symptoms that traditional assessment tools fail to capture. Data was collected from 533 clinically comorbid patients (Mean age = 43.4; female = 363) who completed the Hamilton Anxiety Scale and Hamilton Depression Scale before and after treatment (T1→ T2). Node and bridge strengths were calculated, and analyses included a network comparison test (NCT) and cross-lagged network analyses (CLPN) to examine the interconnectedness and changing features of anxiety and depression comorbidity. Results indicate that (a) In contemporaneous networks before treatment, the most central nodes were tension, somatization, anxiety (somatic), depressed mood, psychomotor retardation, somatic symptoms (GI), autonomic symptoms, and hopelessness. Additionally, depressed mood, psychomotor retardation, somatic symptoms (GI), and hopelessness were also bridge nodes associated with psychological and physical symptoms of anxiety. (b) Although the total score of the assessment tools decreased during treatment and NCT confirmed overall network strength remained stable during treatment (p = 0.38), specific symptoms changed significantly (p < 0.05), underscoring the importance of nuanced evaluations beyond total scores. Specifically, nodes such as fear, insomnia, intellectual, suicide, psychomotor agitation, anxiety (psychic), loss of weight, and helplessness exhibited a significant reduction (p < 0.05). In contrast, nodes including genitourinary symptoms, insomnia (late), work and interests, and somatic symptoms (general) demonstrated a significant enhancement (p < 0.05). (c) Genital symptoms, hypochondriasis, and paranoid symptoms positively predicted other symptoms, while insight negatively predicted tension in longitudinal networks. In summary, our results contribute additional perspectives on the mechanisms underlying comorbid symptomatology and its dynamics throughout the treatment process, identifying meaningful targets for intervention. Thus, recommending the integration of network analysis into current diagnostic, treatment, and follow-up procedures promotes individualized interventions and improves patient recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The COVID-19 pandemic has posed substantial threats to personal health and life (Zhang et al., 2023), and researchers suggested that the subsequent disasters might endure for some time and mental health disorders probably would peak later than the actual pandemic (Xiao et al., 2023; Zhang et al., 2023), such as psychological distress like anxiety disorders and depressive disorders (anxiety and depression hereafter). Anxiety is an adaptive response involving physical and cognitive symptomatology to react to a state of unease and agitation from real or imagined danger; depression is broadly defined as an affective disorder associated with reduced vitality and engagement in activities that previously generated pleasure, along with extreme burnout, discouragement, and loss of meaning in life (van Borkulo et al., 2015; Wolk et al., 2016; Zhang et al., 2023). Both are assumed to be reactions to stressful events producing biological and psychological alterations in the organism (Bao et al., 2017). They usually occur together at high rates, with symptoms often superimposing. Possible explanations for the comorbidity were that anxiety might cause depression or vice versa (Cummings et al., 2014), alternatively that some core symptom dimensions of psychopathology including depression, anxiety, and psychosis-like symptoms were transdiagnostic with a combined presentation of both potentially structuring a unique diagnosis (Angold et al., 1999). However, the co-occurrence could lead to more severe chronic psychopathology (Schoevers et al., 2005), poorer treatment outcomes (Furukawa et al., 2018), and poorer prognoses (Ding et al., 2023). In a recent study of 1,840 comorbidity patients randomly selected for follow-up in a national psychiatric hospital, up to 451 patients reported no improvement or recurrence after treatment (Ding et al., 2023). Therefore, the prevalence of comorbidity underscores a pervasive and substantial concern, warranting expeditious inquiry into the intricate mechanisms that underlie this complex condition. Presently, the prevailing method employed for exploring mental disorders centers on discerning common causes, wherein a conjectural pathological entity dictates a spectrum of symptoms (McNally, 2021). Within this framework, mental disorders are construed as a basis for symptom clusters, and the association between two disorders is frequently conceptualized as comorbidity (Angold et al., 1999; Cramer et al., 2010; Kaiser et al., 2021).
Nevertheless, the conventional causal model might obfuscate the genuine nature of comorbidity. Initially, investigations tend to concentrate on the diagnosis of disorders (Cramer et al., 2010), potentially neglecting the intricate interplay among symptoms. For instance, as per the ICD-10, diminished interest and reduced activity are indicative of a depressive episode. In the common cause model, the robust positive correlation between these symptoms is exclusively ascribed to the shared impact of depression. Yet, one may question the existence of a direct causal link between symptoms, such as whether decreased interest precedes reduced activity. Meanwhile, the scrutiny of psychopathological data heavily leans on the latent variable model (Borsboom & Cramer, 2013). In these models, observed variables (e.g., symptoms or scale items) are construed as measurements of underlying variables (e.g., disorders). Symptoms are amalgamated into a total score to portray the disorder’s severity (Borsboom, 2022). However, this unweighted total score implies equal consideration for all symptoms (Cramer et al., 2010), potentially obscuring authentic changes in the condition. For instance, two patients with identical total scores may manifest different symptoms; one may exhibit a higher score for depressive mood but a lower score for somatic symptoms, while the other’s situation could be the reverse. Notwithstanding, it is evident that the former may have a more severe condition. Despite diagnostic criteria (e.g., DSM-5) emphasizing the significance of core symptoms, the common cause model in comorbidity research falls short of addressing individual symptoms or symptom clusters (Cramer et al., 2010). Consequently, to better understand associations between anxiety and depression symptoms, it is required to examine the symptoms as a holistic dynamic system. Applying network analysis to observe these relationships contemporaneously and longitudinally, with a more microscopic understanding of symptom interactions from a “small world” perspective (Bringmann et al., 2022; Epskamp et al., 2012), may provide novel insights into addressing these psychological health problems (Beard et al., 2016).
Network analysis has received increasing attention (Bringmann et al., 2022; McNally, 2021), and the theory assumes that mental disorders are derived from the overall interconnectedness of their symptoms (Borsboom & Cramer, 2013; Epskamp et al., 2018). Specifically, the appearance of one symptom is considered to increase the probability of the emergence of interrelated symptoms, in turn, which could lead to episodes of illness. It differed from the latent variable model, which supposed unobservable latent variables resulting in observable symptoms (Borsboom et al., 2021; Bringmann et al., 2022). In a network, nodes represent symptoms, edges signify the relationship between symptoms (Jones et al., 2021), and centralities indicate node importance, referring to the connectivity of a node and its contribution to sustaining the disorder (McNally, 2021). Namely, centralities serve to evaluate the degree of interconnectedness relative to other nodes (Kalisch et al., 2019), and centrally activated symptoms might cause other symptoms to develop (Borsboom & Cramer, 2013). Thus, it would be useful to use sophisticated interactive systems to examine the dynamics between anxiety and depression in more depth and to develop targeted interventions. For example, shared symptoms, such as insomnia and fatigue, act as connecting nodes in comorbidity (Cramer et al., 2010); core symptoms, including depressed and anxious moods, play a pivotal role in the initiation and perpetuation of mental disorders (Beard et al., 2016). The interconnections between depression and anxiety serve as conduits for information transmission, contributing to their elevated comorbidity rate (Ding et al., 2023). In another study, Jones et al. (Jones et al., 2021) expanded upon bridge centrality indices to identify pivotal symptoms that facilitate the propagation of comorbidity. Meanwhile, networks could be compared across groups or time intervals (van Borkulo et al., 2022), offering insight into the dynamics of symptom strength before and after treatment. Furthermore, the dynamic interaction of symptoms between two or more temporal structures can be detected by cross-lagged panel network analysis (CLPN), that is, it can indicate and distinguish symptoms that are more predictable in networks depending on given time points, and understand transdiagnostic processes by identifying symptoms that play a predictive or influential role across-construct (Bringmann et al., 2022; McNally, 2021; Wysocki et al., 2022).
Therefore, in the current study, we aimed to explore the network features of depression and anxiety comorbidity through before (T1) and after treatment (T2) networks, identifying core symptoms and pathways, and exploring symptom changes that would not be captured by existing assessment tools. Specifically, (a) to construct network structures for anxiety and depression at T1 and T2, and explore the core symptoms of both structures and identify the bridge nodes of different syndromes at T1. (b) Compare T1 and T2 network structures and recognize strength variations in symptom centralities. (c) Utilize CLPN to confirm dynamic interactions within symptoms and identify significant predictive and outcome symptoms from the T1→ T2 symptom network.
Methods
Participants and procedure
Participants (N = 533) seeking routine care outpatient psychotherapy were referred to the outpatient clinic of a department of psychiatry in western China. It is a specialist center for early psychosis, anxiety, and depression disorders. The diagnosis of the referred patient was confirmed by a joint examination of two trained diagnosticians in a talk-and-see format. It was primarily based on the Comprehensive International Diagnostic Interview (M-CIDI) and allowed for the estimation of mental disorders with a DSM-IV or DSM-IV-TR diagnosis using operationalized criteria for the DSM-IV diagnosis. Two diagnosticians, one scoring independently and the other validating, jointly evaluated the severity of the medical condition and the treatment effect.
Inclusion criteria were primarily (a) a diagnosis of anxiety (F40 or F41) and depression (ICD codes F32 or F33) according to the structured clinical interview and excluded participants with other mental disorders, (b) the ability to provide informed consent and fluency in Chinese, and (c) hospitalized and discharged cured (i.e., not taking psychiatric medications and scores of anxiety and depression below clinical criteria) from January 1, 2021 to December 31, 2022. The final valid sample was 533 (female = 363; Mean age = 43.4, SD age = 17.9), with a mean interval between hospitalization and discharge of 14 days (Mean = 14.1, SD = 1.4, range day 12–16). To enhance the rigor of our study design, we collected baseline data (T1) right before the first session of treatment and follow-up data (T2) right after the last session. All participants received conventional psychotropic medications during hospitalization, with 10.1% also undergoing psychotherapy. Considering potential differences in the samples, we conducted t-tests to examine the impact of psychological intervention on prognostic depression and anxiety. The results indicated no significant differences in depression (t = 0.30, p = 0.768, Cohen’s d = 0.04) and anxiety (t = 0.27, p = 0.787, Cohen’s d = 0.04) based on whether participants received psychological intervention or not. Table 1 provides detailed demographics of the sample.
Measures
Hamilton Anxiety Scale (HAMA) and Hamilton Depression Scale (HAMD) have been broadly adopted to assess the development of anxiety and depression. HAMA contains 14 items, and HAMD used in this study contains 24. Most items have 5 measurements and a scale of 0 (never), 1 (mild), 2 (moderate), 3 (severe), or 4 (extremely severe), with a few questions having 3 or 4 measurements (see Supplementary Table A for details). Overall, according to precious studies (Lu et al., 2020; Yang et al., 2019), a HAMA total score is operationally categorized as no anxiety (score 0–6), mild and moderate (score 7–13), and severe (score ≥ 14); the total HAMD score can be graded as normal (score 0–7), mild (score 8–19), moderate (score 20–34), and severe depression (score ≥ 35). Previous studies demonstrated that the epidemiology of anxiety and depression was strongly affected by sociocultural and economic contexts, and both scales have been validated in China and are widely used (Lu et al., 2020; Yang et al., 2019).
Statistical analysis
Descriptive statistics and missing data handling
SPSS 25.0 was used for descriptive statistics. With a low rate of missing data in our study (∼ 5%), it is likely that these missing values have little impact on the findings (Schlechter et al., 2022). Yet CLPN modeling required a full-case analysis (Wysocki et al., 2022), and the mice package was used in predicted mean matching R to estimate the data (van Borkulo et al., 2015). The package utilizes multivariate imputation of chained equations, which offers unbiased effect estimates and valid inferences after implementation (Schlechter et al., 2022; van Borkulo et al., 2015). After these steps, the final sample included 533 participants, and their data were used in the subsequent network analysis. Additionally, the data and R script have been uploaded to OSF (https://osf.io/mt9gx/).
Contemporaneous networks
First, we constructed network structures for the sample using the qgraph R package at T1 and T2, respectively (Epskamp et al., 2018). Due to the massive estimated parameters in the network (i.e., 38 nodes need to estimate 741 parameters: 38 threshold parameters and 38*37/2 = 703 pairwise correlation parameters), it might lead to some false positive edges. Therefore, all edges in the network were reduced by the graphical lasso (glasso) algorithm (Epskamp et al., 2018; Friedman et al., 2008), and the minor edges were set precisely to zero, which maximized the fit and explained the covariances across nodes with the fewest possible edges. Further, the hyperparameter γ, controlling the weighing between false positive edges (i.e. specificity) and removed true ones (i.e. sensitivity), was set to 0.5 (Beard et al., 2016), along with the extended Bayesian Information Criterion model selected to obtain a parsimoniously accurate structure. Second, for graphical visualization, the edge thickness marks the degree of association between nodes, solid lines indicate positive associations and dashed lines indicate negative ones. We used the averageLayout function for network averaging, which presents a coherent layout using the average position of different network structures (Epskamp et al., 2012). That is, the same nodes in different structures were fixed in the same position, and we also added partial correlation coefficients to the network graph. Third, in contemporaneous networks, centralities include node strength, betweenness, and closeness (Epskamp et al., 2018). Previous studies have considered betweenness and closeness as unstable, and strength is primarily used in research (Bringmann et al., 2022). Finally, we utilized the networktools package to identify critical nodes for cluster connectivity by computing the bridge strength based on edge weights from a given node to other clusters (Heeren et al., 2018; Jones et al., 2021). Nodes exhibiting a higher bridge strength are deemed to play a more significant role in activating nodes from the opposite clusters. To avoid confirmation bias in interpreting centrality statistics, we employed a stringent approach that involved blind 80th percentile cutoffs on both node bridge strength values when determining the centrality and bridge nodes (Jones et al., 2021).
Network comparison
In comparing the global connectivity and local differences between the networks, we employed the R package Network Comparison Test (NCT; van Borkulo et al., 2022). This analysis encompassed the examination of invariant global strength and invariant edge weights through permutation tests involving 1000 iterations. Participants were randomly allocated to distinct groups (i.e., T1 and T2), with subsequent construction, estimation, and comparison of networks using a bootstrap resampling method iterated 1000 times to derive the null distribution of network differences under the null hypothesis. A significance level of 0.05 was set. Global strength, defined as the weighted sum of all edges, served as the metric for assessing overall strength differences (Opsahl et al., 2010). To address potential issues of multiple testing, the NCT utilized the Holm-Bonferroni method for post hoc corrections. Furthermore, local network properties were assessed by examining strength invariance, reflecting variations in node strength (van Borkulo et al., 2022).
CLPN network analysis
The network modeling approach in psychology revolves around the concept that constructs are dynamic systems. Cross-sectional data have limited utility in assessing network dynamics because, while they can generate dynamic hypotheses, they cannot to test them. We used the glmnet package to calculate a directed CLPN from T1 to T2 (Tay et al., 2023; Wysocki et al., 2022), CLPN can model a directed network for two-time points, offering a novel opportunity compared to established panel data methods, which necessitate intensive longitudinal data, typically a minimum of three-time points (Epskamp, 2020). The glmnet package used for network estimation incorporates various link functions suitable for non-normally distributed data, including binary, multinomial, and Poisson. It comprises autoregressive paths of nodes and examines cross-lagged effects between nodes by regularizing regression estimates (Funkhouser et al., 2021). Given the numerous potential causal paths to estimate, regularization contributes to shrinking many near-zero paths precisely to zero, which leads to the effective estimation of cross-time network relations, particularly when dealing with limited longitudinal data. In the network analysis, nodes denote symptoms and directed edges signify autoregressive and cross-lagged effects. In the autoregressive pathway, a baseline symptom predicted itself at follow-up after adjustment for all others at the first time point. In the cross-lagged pathway, baselines predicted differential symptoms at follow-up after adjustment for all others at the first time point. Specifically, as with the contemporaneous network, a LASSO penalty was introduced on the estimated regression coefficients to increase interpretability by reducing small regression paths to zero and expanding others (Wysocki et al., 2022). Meanwhile, we computed centrality indices: the cross-construct “in” expected impact (iEI) and “out” expected impact (oEI). iEI quantifies the extent to which other symptoms predict each symptom (i.e., the sum of the incoming edge weights), as compared to oEI, describing the extent to which other symptoms are predicted by each symptom (i.e., the sum of the outgoing edge weights). High oEI is particularly highlighted in clinical practice because it predicts a wide range of other symptoms, namely, its activation might subsequently trigger other symptoms across the network (McNally, 2016; Wysocki et al., 2022).
Accuracy and stability test
We used the bootnet package bootstrapping method to evaluate the estimation accuracy and robustness of contemporaneous networks at T1 and T2 (Epskamp et al., 2018). First, 2000 nonparametric bootstraps were performed to draw 95% confidence intervals (CIs) from each edge weight to test the accuracy estimation. Higher CIs of overlapping edge weights indicate lower accuracy of the graphical depiction. Second, the correlation stability (CS) coefficients of 2000 case-dropping subsets bootstrap examined the centrality stability. Specifically, it shows how the centrality index changes as the proportion of the sample subset decreases (e.g., comparing the total sample with only 50% of them). The more rapidly the centrality shifts with decreasing sample proportions, the less stable it is. The CS coefficient shows the maximum acceptable degree of sample reduction, with strong stability above 0.50 and a minimum not less than 0.25. Finally, for CLPN, we performed stability checks on the accuracy of its edge weights, the difference, and the stability of iEI and oEI.
Results
Descriptive statistics
Table 2 presents the mean and standard deviation of all items assessed at T1 and T2, a higher score indicated a stronger propensity for the trait (Schlechter et al., 2022). From T1 to T2, mean symptom levels decreased and total HAMA scores significantly lower, t (532) = -34.32, p < 0.001; Hedges’ g = -1.77 (95% CI [-1.908, -1.624]). Similarly, HAMD decreased significantly in mean symptoms, t (532) = -34.73, p < 0.001; Hedges’ g = -1.75 (95% CI [-1.890, -1.607]).
Contemporaneous networks
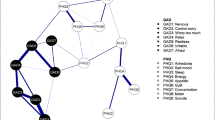
Figure 1 shows the connectivity between the symptoms (i.e., nodes) of samples before T1 treatment and after T2 treatment. To visualize which symptoms are more connected, we set “minimum value = 0.16, cut value = 0.05,” meaning that edges with values less than 0.16 are not displayed. Overall, symptom connectivity changed before and after treatment, whereas sleep-related entries HA4, HD4, HD5, and HD6 did not change markedly in their clustering. Within the network at T1, the strongest edges were HA11 (gastrointestinal symptoms) and HD12 (somatic symptoms, GI); HA2 (tension) and HA3 (fear); HA4 (insomnia) and HD4 (insomnia, early). Meanwhile, the most central nodes were HA2 (tension), HA7 (somatization), HA8 (anxiety, somatic), HD1 (depressed mood), HD8 (psychomotor retardation), HD12 (somatic symptoms, GI), HD13 (autonomic symptoms), and HD23 (hopelessness), which indicated these items were the more cored nodes. Based on the values of bridge strength presented in Table 3; Fig. 1 illustrate the symptoms that function as bridge nodes. These nodes, which scored in the top 20% for bridge strength, included HD1 (depressed mood), HD7 (work and interests), HD8 (psychomotor retardation), HD12 (somatic symptoms, GI), HD13 (somatic symptoms, general), HD14 (genital symptoms), HD15 (hypochondriasis), and HD18 (diurnal variation). Edge investigation showed that the connections between anxiety and depression symptoms primarily occurred via these bridge nodes. Notably, most of these bridge nodes corresponded to the retardation and anxiety/ somatization symptom domains of the HAMD, and the only exception was diurnal variation.
Network comparisons
The global strength values of the T1 and T2 networks were 18.19 and 18.69, respectively. Comparing both networks, no significant differences were found in terms of global strength (p = 0.38), yet the centrality test indicated significant differences (p < 0.05). A more detailed examination of local strength invariance unveiled notable variations. Specifically, nodes such as HA3 (fear), HA4 (insomnia), HA5 (intellectual), HD3 (suicide), HD9 (psychomotor agitation), HD10 (anxiety, psychic), HD16 (loss of weight), and HD22 (helplessness) exhibited a significant reduction (p < 0.05). In contrast, nodes including HA12 (genitourinary symptoms), HD6 (insomnia, late), HD7 (work and interests), and HD13 (somatic symptoms, general) demonstrated a significant enhancement (p < 0.05), as detailed in Table 3.
CLPN networks
Figure 2 indicates the complex cross-lagged effects. First, many nodes revealed substantial autoregressive effects, “HD21: obsessive, compulsive, β = 0.15”; “HA6: depressed mood, HA8: anxiety, somatic, HD17: insight; all β = 0.14”; “HA5: intellectual, HD24: worthlessness; both β = 0.13”. Second, one of the most predictive nodes in the CLPN was “HD14: genital symptoms”, HD14→ “HA7: somatization, β = 0.10”, HD14→ “HA11: gastrointestinal symptoms, β = 0.05”, HD14→ “HD11: anxiety, somatic, β = 0.05”, HD14→ “HD18: diurnal variation, β = 0.05”. Following is “HD15: hypochondriasis”, which predicted respectively “HD10: anxiety, psychic, β = 0.07”, “HA14: behavioral changes, β = 0.06”, and “HA3: fear, β = 0.05”. Additionally, “HD20: paranoid symptoms”→ “HA2: tension, β = 0.07”, “HD20: paranoid symptoms”→ “HA5: intellectual, β = 0.06”, and “HD20: paranoid symptoms”→ “HA2: feelings of guilt, β = 0.06”. Finally, the strongest negative edges were “HD17: insight”→ “HA2: tension, β = -0.05”.
(a) A cross-lagged panel network with auto-regressive paths, green edges represent positive associations while red edges represent negative associations with arrows indicating a directed association between nodes (T1→ T2). (b) Cross-construct in-expected influence and out-expected influence. Higher z-scores indicate greater centrality. Out-expected should not be interpreted due to the low stability. See Table 2 for symptom descriptions and names
Accuracy of the networks
The relatively narrow 95% CIs of the bootstrap indicate that the edges of the contemporaneous and CLPN network would be judged accurately (see Supplementary Figures S1, S2, and S9), and we performed bootstrap difference tests for edge weights and centralities separately (see Supplementary Figures S3 to S8). In the contemporaneous networks, the CS coefficients for edges, strength, and bridge strength at T1 (0.67/ 0.67/ 0.60) and T2 (0.60/ 0.44/ 0.52) were > 0.25, which meet the cutoff score suggested by Epskamp et al. (2018). Additionally, stability was low for iEI but strong for oEI (CS coefficient of 0.13 and 0.75, respectively), which indicated weak iEI interpretability (see Supplementary Figures S10 and S11 for details).
Discussion
Our study is based on clinicians’ objective assessment with a large clinical cure sample in the acute phase (Qaseem et al., 2023). Additionally, we conducted network analysis to identify core symptoms, bridge symptoms, and comorbid pathways of anxiety and depression before and after treatment, Furthermore, a longitudinal network analysis was employed to concentrate on individual-specific symptoms rather than aggregate scores or overall diagnoses, providing specific and feasible insights for clinical intervention.
Central symptoms
After a thorough examination of the contemporary and longitudinal network distribution trends, we identified the top 20% of nodes based on their centrality scores for further discussion (Jones et al., 2021; Sun et al., 2023). The contemporaneous network at T1, HA2 (tension), HA7 (somatization), HA8 (anxiety, somatic), HD1 (depressed mood), HD8 (psychomotor retardation), HD12 (somatic symptoms, GI), HD13 (autonomic symptoms), and HD23 (hopelessness) were the nodes with higher centrality. Meanwhile, depressed mood, psychomotor retardation, somatic symptoms (GI), and hopelessness were also bridge nodes associated with psychological and physical symptoms of anxiety. The contemporaneous network at T1 estimation revealed complex interactions in the comorbidity of anxiety and depression, illuminating multiple associations among symptoms related to both domains. Activation of these nodes may increase the likelihood of comorbidity (Jones et al., 2021). For instance, somatic symptoms associated with depression, such as nausea, diarrhea, and constipation, may trigger a cluster of somatic symptoms related to anxiety. Similarly, psychological symptoms linked to depression, such as depressed mood, may elicit psychological symptoms associated with anxiety. In this study, depressed mood emerged as the central symptom in the network, aligning with prior research (Beard et al., 2016; Tao & Hou et al., 2023) and clinical diagnostic criteria that consider it as a prerequisite for major depressive disorder (Tao et al., 2023). Given that data collection occurred during the unprecedented COVID-19 pandemic, previous studies suggest that unexpressed depressed mood can accumulate into depression (Jeong et al., 2019). Fortunately, Tao et al. (Tao et al., 2023) found through network analysis that once lockdowns end or interventions like cognitive therapy reduce the level of sadness, depression levels significantly decrease. Therefore, interventions should consider the interconnectedness between various dimensions to disrupt pathways leading to comorbidity (Bringmann et al., 2022; Sun et al., 2023).
Bridge symptoms and comorbid pathways
We found the comorbid pathways primarily through depressive dimensions of psychomotor retardation (i.e., depressed mood, work and interests, psychomotor retardation, and genital symptoms) and somatization (i.e., somatic symptoms, GI; somatic symptoms, general; hypochondriasis) and diurnal variation, thus enriching previous research (e.g., Kaiser et al., 2021). The interrelations between the two disorders extend beyond emotional symptoms and might involve relationships between physical and psychological symptoms, a circumstance in which comorbidity among patients is more likely to occur once these nodes are activated. Additionally, the results of CLPN showed that all iEI nodes were anxiety items, namely, anxiety symptoms at T2 might be influenced more by the comorbidity of T1, which probably indicated that anxiety is superficially developed and might be easier to cure from specific symptom levels (Salari et al., 2020; Santomauro et al., 2021), and previous research demonstrated that symptoms of anxiety commonly appear after depression symptoms (Long et al., 2018). Given the minor CS coefficients of iEI, the absence of long-term and multipoint data (three or more), and caution is required when interpreting the linkage-directed aspects in the present study.
Assessment of treatment outcomes
Our NCT findings confirmed that there was no significant change in overall network strength during treatment. However, a difference test of total scores indicated a marked improvement in comorbid symptoms following treatment. As Fried and Nesse (2015) highlighted, commonly used total scores may mask the interactions between symptoms. Node strength invariance supports this inference, with the majority of nodes showing significant differences and even a prognostic enhancement. For instance, the community symptom cluster for sleep was a relatively isolated cluster module and did not change significantly before and after treatment, in which the strength of node HD6 (insomnia, late) was markedly increased instead. It may be related to the inpatient setting being group mixed accommodation (see Supplementary Figure S12), or depressive sleep diagnostic symptoms might have been similar to sleep disorder ones that are transdiagnostic (First et al., 2022; McNally, 2021). A previous meta-analysis by Deng et al. (2021) found that the prevalence of anxiety, depression and sleep disturbances in higher education students during the pandemic were homogeneous among them. From a clinical perspective, a short-term treatment focus for physicians should probably be on other targets or changes in current therapies, and patients would be better off being self-accepting of sleep disorders; in the mid to long term perhaps a doctor-patient partnership could be considered, and cognitive behavioral therapy for insomnia would be a good choice (Deng et al., 2021; Qaseem et al., 2016). Simultaneously, clinicians and patients should be aware that side effects induced by antidepressants, such as selective serotonin reuptake inhibitors, may encompass gastrointestinal dysfunction, insomnia, headaches, and sexual dysfunction (Qaseem et al., 2023). It is noteworthy that our research also revealed a significant enhancement in genitourinary symptoms and somatic symptoms (general) at T2.
Furthermore, oEI nodes of genital symptoms, hypochondriasis, paranoid symptoms, and insight at T1 could predict certain symptoms at T2. For those higher oEI nodes that become targets for focused clinical intervention, their cure could be crucial for the rapid elimination of other comorbidity symptoms at T2 (Bringmann et al., 2022; McNally, 2021). Drawing insights from our findings, (a) Genital symptoms exhibit a robust predictive influence on other symptoms from T1 to T2. Considering the potential financial and time costs for hospitalized individuals, a pragmatic approach involving a combination of pharmacological and psychological interventions aligns with treatment guidelines recommendations (First et al., 2022; Wrobel et al., 2023). (b) Hypochondriasis and paranoid symptoms at T1 could predict other symptoms at T2, a study has indicated that mindfulness-based stress reduction can effectively alleviate discomfort in interpersonal relations and paranoid ideation symptoms (Hirshberg et al., 2022). (c) Insight negatively predicted tension, a node with high centrality in the contemporaneous network, and heightened susceptibility. Psychological interventions, such as cognitive-behavioral therapy, aimed at helping patients recognize, acknowledge, and transform negative or unhelpful thinking patterns may be appropriate (Chavez-Baldini et al., 2022). Notably, both genital symptoms and hypochondriasis also served as critical bridge nodes for anxiety disorders and depression at T1, which suggests the need for swift and targeted intervention at these identified nodes is essential for the expeditious mitigation of comorbidity symptoms at T2.
Limitations
Despite the identification of temporal changes in symptom networks in this study, it is important to approach with caution when attempting to assert directional explanations for causal relationships among symptoms. This is because, first, the clinical sample mainly consisted of the pandemic and required a careful extension to non-pandemic periods. Second, the homogeneous country of the patients (from China) also affected the universality of the findings. Finally, the CLPN model confounds intra- and inter-participant effects (Borsboom et al., 2021), which might bias outcomes if the variables contain stable individual differences (McNally, 2021; Wysocki et al., 2022). This means that cross-lagged effects might arise in correlated variables without causality (Borsboom, 2022; Borsboom et al., 2021), and the approach to separate these influences may require at least three waves of data.
Conclusion
In conclusion, our study is grounded in clinicians’ objective assessments of a substantial clinical sample during the acute phase and utilized network analysis to delineate core symptoms, bridge symptoms, and comorbid pathways of anxiety and depression before and after treatment. Findings demonstrate multiple unique associations of nodes within networks and further support the importance of comorbidity interactions over time and between disorders, which provide clinical recommendations for psychological interventions targeted to reduce the development of comorbidity symptoms.
First, our analysis of central symptoms highlighted the significance of certain nodes, such as tension, somatization, anxiety (somatic), depressed mood, psychomotor retardation, somatic symptoms (GI), autonomic symptoms, and hopelessness, with higher centrality in the contemporaneous network at T1. Moreover, depressed mood emerged as a central symptom, aligning with prior research and clinical diagnostic criteria. Second, bridge symptoms and comorbid pathways enriched our understanding. Swift and targeted intervention at identified nodes, particularly higher oEI nodes like genital symptoms, hypochondriasis, paranoid symptoms, and insight at T1, is crucial for the rapid elimination of comorbidity symptoms at T2. Finally, Assessment of treatment outcomes revealed that while overall network strength remained stable during treatment, specific symptoms changed significantly, underscoring the importance of nuanced evaluations beyond total scores. Existing assessment tools face limitations in capturing real symptom changes, and to prompt the integration of network analysis in clinical practice, the reshape2 R can be considered for data integration, extraction, and analysis with the existing Hospital Information System to provide additional symptomatic network features. Additionally, our findings suggested that certain symptoms, such as those related to sleep disturbances, may require a differentiated treatment focus and a long-term doctor-patient partnership. Given the predominant use of pharmacological treatments in this study (see Table 1), targeted interventions such as psychotherapy may enhance treatment outcomes. Existential Group Therapy, for instance, utilizes creative activities like constructing a ‘Tree of Life’ and engaging in psychodrama sessions, coupled with group dynamics, to explore existential sub-themes. This approach aims to facilitate self-identification, enhance the sense of meaning, and alleviate existential anxiety, fear of death, and depression among group members (Ding et al., 2023; Peteet, 2011).
Overall, integrating network analysis into diagnostic, treatment, and follow-up processes offers valuable insights at the symptom level. This integration can inform the development of personalized treatment plans, expediting recovery in comorbid populations.
Data availability
Raw data and R script were uploaded to OSF (https://osf.io/mt9gx/), identifier: DOIhttps://doi.org/10.17605/OSF.IO/MT9GX.
References
Angold, A., Costello, E. J., & Erkanli, A. (1999). Comorbidity. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 40(1), 57–87. https://doi.org/10.1111/1469-7610.00424.
Bao, Y., Li, L., Guan, Y., Wang, W., Liu, Y., Wang, P., Huang, X., Tao, S., & Wang, Y. (2017). Prevalence and associated positive psychological variables of anxiety and depression among patients with central nervous system tumors in China: A cross-sectional study. Psycho-Oncology, 26(2), 262–269. https://doi.org/10.1002/pon.4128.
Beard, C., Millner, A. J., Forgeard, M. J. C., Fried, E. I., Hsu, K. J., Treadway, M. T., Leonard, C. V., Kertz, S. J., & Björgvinsson, T. (2016). Network analysis of depression and anxiety symptom relationships in a psychiatric sample. Psychological Medicine, 46(16), 3359–3369. https://doi.org/10.1017/S0033291716002300.
Borsboom, D. (2022). Reflections on an emerging new science of mental disorders. Behaviour Research and Therapy, 156, 104127. https://doi.org/10.1016/j.brat.2022.104127.
Borsboom, D., & Cramer, A. O. J. (2013). Network analysis: An integrative approach to the structure of psychopathology. Annual Review of Clinical Psychology, 9(1), 91–121. https://doi.org/10.1146/annurev-clinpsy-050212-185608.
Borsboom, D., Deserno, M. K., Rhemtulla, M., Epskamp, S., Fried, E. I., McNally, R. J., Robinaugh, D. J., Perugini, M., Dalege, J., Costantini, G., Isvoranu, A., Wysocki, A. C., van Borkulo, C. D., van Bork, R., & Waldorp, L. J. (2021). Network analysis of multivariate data in psychological science. Nature Reviews Methods Primers, 1(1), 58. https://doi.org/10.1038/s43586-021-00055-w.
Bringmann, L. F., Albers, C., Bockting, C., Borsboom, D., Ceulemans, E., Cramer, A., Epskamp, S., Eronen, M. I., Hamaker, E., Kuppens, P., Lutz, W., McNally, R. J., Molenaar, P., Tio, P., Voelkle, M. C., & Wichers, M. (2022). Psychopathological networks: Theory, methods, and practice. Behaviour Research and Therapy, 149(104011), 104011. https://doi.org/10.1016/j.brat.2021.104011.
Chavez-Baldini, U., Verweij, K., de Beurs, D., Bockting, C., Lok, A., Sutterland, A. L., van Rooijen, G., van Wingen, G., Denys, D., & Vulink, N. (2022). The interplay between psychopathological symptoms: Transdiagnostic cross-lagged panel network model. Bjpsych Open, 8(4), e116. https://doi.org/10.1192/bjo.2022.516.
Cramer, A. O., Waldorp, L. J., van der Maas, H. L., & Borsboom, D. (2010). Comorbidity: A network perspective. Behavioral and Brain Sciences, 33(23), 137–150, 150–193. https://doi.org/10.1017/S0140525X09991567.
Cummings, C. M., Caporino, N. E., & Kendall, P. C. (2014). Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychological Bulletin, 140(3), 816–845. https://doi.org/10.1037/a0034733.
Deng, J., Zhou, F., Hou, W., Silver, Z., Wong, C. Y., Chang, O., Drakos, A., Zuo, Q. K., & Huang, E. (2021). The prevalence of depressive symptoms, anxiety symptoms, and sleep disturbance in higher education students during the COVID-19 pandemic: A systematic review and meta-analysis. Psychiatry Research, 301, 113863. https://doi.org/10.1016/j.psychres.2021.113863.
Ding, F., Li, N., Zhang, S., Li, J., Jing, Z., & Zhao, Y. (2023). Network comparison analysis of comorbid depression and anxiety disorder in a large clinical sample before and after treatment. Current Psychology. https://doi.org/10.1007/s12144-023-05308-3.
Epskamp, S. (2020). Psychometric network models from time-series and panel data. Psychometrika, 85(1), 206–231. https://doi.org/10.1007/s11336-020-09697-3.
Epskamp, S., Cramer, A. O. J., Waldorp, L. J., Schmittmann, V. D., & Borsboom, D. (2012). Qgraph: Network visualizations of relationships in psychometric data. Journal of Statistical Software, 48(4), 1–18. https://doi.org/10.18637/jss.v048.i04.
Epskamp, S., Borsboom, D., & Fried, E. I. (2018). Estimating psychological networks and their accuracy: A tutorial paper. Behavior Research Methods, 50(1), 195–212. https://doi.org/10.3758/s13428-017-0862-1.
First, M. B., Yousif, L. H., Clarke, D. E., Wang, P. S., Gogtay, N., & Appelbaum, P. S. (2022). DSM-5-TR: Overview of what’s new and what’s changed. World Psychiatry, 21(2), 218–219. https://doi.org/10.1002/wps.20989.
Fried, E. I., & Nesse, R. M. (2015). Depression sum-scores don’t add up: Why analyzing specific depression symptoms is essential. BMC medicine, 13(1), 72. https://doi.org/10.1186/s12916-015-0325-4.
Friedman, J., Hastie, T., & Tibshirani, R. (2008). Sparse inverse covariance estimation with the graphical lasso. Biostatistics, 9(3), 432–441. https://doi.org/10.1093/biostatistics/kxm045.
Funkhouser, C. J., Chacko, A. A., Correa, K. A., Kaiser, A. J., & Shankman, S. A. (2021). Unique longitudinal relationships between symptoms of psychopathology in youth: A cross-lagged panel network analysis in the ABCD study. Journal of Child Psychology and Psychiatry, 62(2), 184–194. https://doi.org/10.1111/jcpp.13256.
Furukawa, T. A., Efthimiou, O., Weitz, E. S., Cipriani, A., Keller, M. B., Kocsis, J. H., Klein, D. N., Michalak, J., Salanti, G., Cuijpers, P., & Schramm, E. (2018). Cognitive-behavioral analysis system of psychotherapy, drug, or their combination for persistent depressive disorder: Personalizing the treatment choice using individual participant data network meta-regression. Psychotherapy and Psychosomatics, 87(3), 140–153. https://doi.org/10.1159/000489227.
Heeren, A., Jones, P. J., & McNally, R. J. (2018). Mapping network connectivity among symptoms of social anxiety and comorbid depression in people with social anxiety disorder. Journal of Affective Disorders, 228(1), 75–82. https://doi.org/10.1016/j.jad.2017.12.003.
Hirshberg, M. J., Goldberg, S. B., Rosenkranz, M., & Davidson, R. J. (2022). Prevalence of harm in mindfulness-based stress reduction. Psychological Medicine, 52(PII S00332917200028346), 1080–1088. https://doi.org/10.1017/S0033291720002834.
Jeong, E. J., Ferguson, C. J., & Lee, S. J. (2019). Pathological gaming in young adolescents: A longitudinal study focused on academic stress and self-control in South Korea. Journal of Youth and Adolescence, 48(12), 2333–2342. https://doi.org/10.1007/s10964-019-01065-4.
Jones, P. J., Ma, R., & McNally, R. J. (2021). Bridge centrality: A network approach to understanding comorbidity. Multivariate Behavioral Research, 56(2), 353–367. https://doi.org/10.1080/00273171.2019.1614898.
Kaiser, T., Herzog, P., Voderholzer, U., & Brakemeier, E. L. (2021). Unraveling the comorbidity of depression and anxiety in a large inpatient sample: Network analysis to examine bridge symptoms. Depression and Anxiety, 38(3), 307–317. https://doi.org/10.1002/da.23136.
Kalisch, R., Cramer, A. O., Binder, H., Fritz, J., Leertouwer, I., Lunansky, G., Meyer, B., Timmer, J., Veer, I. M., & Van Harmelen, A. (2019). Deconstructing and reconstructing resilience: A dynamic network approach. Perspectives on Psychological Science, 14(5), 765–777. https://doi.org/10.1177/1745691619855637.
Long, E. E., Young, J. F., & Hankin, B. L. (2018). Temporal dynamics and longitudinal co-occurrence of depression and different anxiety syndromes in youth: Evidence for reciprocal patterns in a 3-year prospective study. Journal of Affective Disorders, 234, 20–27. https://doi.org/10.1016/j.jad.2018.02.074.
Lu, W., Wang, H., Lin, Y., & Li, L. (2020). Psychological status of medical workforce during the COVID-19 pandemic: A cross-sectional study. Psychiatry Research, 288(112936), 112936. https://doi.org/10.1016/j.psychres.2020.112936.
McNally, R. J. (2016). Can network analysis transform psychopathology? Behaviour Research and Therapy, 86, 95–104. https://doi.org/10.1016/j.brat.2016.06.006.
McNally, R. J. (2021). Network analysis of psychopathology: Controversies and challenges. Annual Review of Clinical Psychology, 17, 31–53. https://doi.org/10.1146/annurev-clinpsy-081219-092850.
Opsahl, T., Agneessens, F., & Skvoretz, J. (2010). Node centrality in weighted networks: Generalizing degree and shortest paths. Social Networks, 32(3), 245–251. https://doi.org/10.1016/j.socnet.2010.03.006.
Peteet, J. R. (2011). Approaching emptiness: Subjective, objective, and existential dimensions. Journal of Religion & Health, 50(3), 558–563. https://doi.org/10.1007/s10943-010-9443-7.
Qaseem, A., Kansagara, D., Forciea, M. A., Cooke, M., Denberg, T. D., & Physicians, C. G. C. O. (2016). Management of chronic insomnia disorder in adults: A clinical practice guideline from the American college of physicians. Annals of Internal Medicine, 165(2), 125–133. https://doi.org/10.7326/M15-2175.
Qaseem, A., Owens, D., Etxeandia Ikobaltzeta, I., Tufte, J., Cross, J., & Wilt, T. (2023). Nonpharmacologic and pharmacologic treatments of adults in the acute phase of major depressive disorder: A living clinical guideline from the American college of physicians. Annals of Internal Medicine, 176. https://doi.org/10.7326/M22-2056.
Salari, N., Hosseinian-Far, A., Jalali, R., Vaisi-Raygani, A., Rasoulpoor, S., Mohammadi, M., Rasoulpoor, S., & Khaledi-Paveh, B. (2020). Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: A systematic review and meta-analysis. Globalization and Health, 16(1), 57–67. https://doi.org/10.1186/s12992-020-00589-w.
Santomauro, D. F., Herrera, A. M. M., Shadid, J., Zheng, P., Ashbaugh, C., Pigott, D. M., Abbafati, C., Adolph, C., Amlag, J. O., & Aravkin, A. Y. (2021). Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. The Lancet, 398(10312), 1700–1712. https://doi.org/10.1016/S0140-6736(21)02143-7.
Schlechter, P., Hellmann, J. H., McNally, R. J., & Morina, N. (2022). The longitudinal course of posttraumatic stress disorder symptoms in war survivors: Insights from cross-lagged panel network analyses. Journal of Traumatic Stress, 35(3), 879–890. https://doi.org/10.1002/jts.22795.
Schoevers, R. A., Deeg, D. J. H., van Tilburg, W., & Beekman, A. T. F. (2005). Depression and generalized anxiety disorder: Co-occurrence and longitudinal patterns in elderly patients. The American Journal of Geriatric Psychiatry, 13(1), 31–39. https://doi.org/10.1097/00019442-200501000-00006.
Sun, Y., Zhang, Y., Lu, Z., Yan, H., Guo, L., Liao, Y., Lu, T., Wang, L., Li, J., & Li, W. (2023). Longitudinal network analysis reveals interactive change of schizophrenia symptoms during acute antipsychotic treatment. Schizophrenia Bulletin, 49(1), 208–217. https://doi.org/10.1093/schbul/sbac131.
Tao, Y., Hou, W., Niu, H., Ma, Z., Zheng, Z., Wang, S., Liu, X., & Zhang, L. (2023). Comparing the centrality symptoms of major depressive disorder samples across junior high school students, senior high school students, college students, and elderly adults during city lockdown of COVID-19 pandemic: A network analysis. Journal of Affective Disorders, 324, 190–198. https://doi.org/10.1016/j.jad.2022.12.120.
Tay, J. K., Narasimhan, B., & Hastie, T. (2023). Elastic net regularization paths for all generalized linear models. Journal of Statistical Software, 106(1), 1–31. https://doi.org/10.18637/jss.v106.i01.
van Borkulo, C., Boschloo, L., Borsboom, D., Penninx, B. W. J. H., Waldorp, L. J., & Schoevers, R. A. (2015). Association of symptom network structure with the course of depression. Jama Psychiatry, 72(12), 1219–1226. https://doi.org/10.1001/jamapsychiatry.2015.2079.
van Borkulo, C. D., van Bork, R., Boschloo, L., Kossakowski, J. J., Tio, P., Schoevers, R. A., Borsboom, D., & Waldorp, L. J. (2022). Comparing network structures on three aspects: A permutation test. Psychological Methods. https://doi.org/10.1037/met0000476.
Wolk, C. B., Carper, M. M., Kendall, P. C., Olino, T. M., Marcus, S. C., & Beidas, R. S. (2016). Pathways to anxiety-depression comorbidity: A longitudinal examination of childhood anxiety disorders. Depression and Anxiety, 33(10), 978–986. https://doi.org/10.1002/da.22544.
Wrobel, A. L., Cotton, S. M., Jayasinghe, A., Diaz Byrd, C., Yocum, A. K., Turner, A., Dean, O. M., Russell, S. E., Duval, E. R., & Ehrlich, T. J. (2023). Childhood trauma and depressive symptoms in bipolar disorder: A network analysis. Acta Psychiatrica Scandinavica, 147(3), 286–300. https://doi.org/10.1111/acps.13528.
Wysocki, A., Rhemtulla, M., van Bork, R., & Cramer, A. (2022). Cross-lagged network models. https://doi.org/10.31234/osf.io/vjr8z.
Xiao, Y., Du, N., & Li, Y. (2023). Mental health services in China: Challenges in the context of COVID-19. Asian Journal of Psychiatry, 80(103348). https://doi.org/10.1016/j.ajp.2022.103348.
Yang, W., Zhang, G., Jia, Q., Qian, Z., Yin, G., Zhu, X., Alnatour, O. I., Trinh, T. H., Wu, H. E., Lang, X., Du, X., & Zhang, X. (2019). Prevalence and clinical profiles of comorbid anxiety in first episode and drug naïve patients with major depressive disorder. Journal of Affective Disorders, 257, 200–206. https://doi.org/10.1016/j.jad.2019.06.052.
Zhang, S., Yong, S., Tang, Y., Feng, H., Chen, L., Zhang, L., Deng, Y., & Chen, J. (2023). Intolerance of uncertainty fuels preservice teachers’ smartphone dependence through rumination and anxiety during the COVID-19 pandemic: A cross-sectional study. Heliyon, 9(7), e17798. https://doi.org/10.1016/j.heliyon.2023.e17798.
Acknowledgements
We appreciate the hard work and critical comments of the editors and reviewers. Additionally, we thank all clinical participants for their understanding and selfless support.
Funding
This study was funded by the National Nature Science Foundation of China (32360206).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by S.S.Z., F.Q.D., and J.T.C. The first draft of the manuscript was written by S.S.Z. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Financial interests
The authors have no relevant financial or non-financial interests to disclose.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Ningxia University (protocol code NXU-23-051).
Informed consent
Informed consent was obtained from all subjects involved in the study.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, S., Ding, F. & Chen, J. Comorbidity of anxiety and depression disorder among clinical referral patients: a longitudinal study based on network analysis. Curr Psychol 43, 20655–20667 (2024). https://doi.org/10.1007/s12144-024-05856-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12144-024-05856-2