Abstract
A complex environment requires us to maintain good conflict processing ability at all times, but how sleep deprivation affects conflict processing remains unclear. Here, we sought to use EEG techniques to explore how sleep deprivation affects the interference of stimulus-driven processing in goal-driven processing in individuals. Subjects were randomly assigned to a sleep deprivation group and control group. Behaviorally, there were significant between-group differences in accuracy but not in response time. At the EEG level, the main effect of P3b, Ni latency, and LSP mean amplitude was significant. Furthermore, at the time–frequency level, the interaction between the group and the conflict effect in the lower alpha band in the conflict detection stage, the alpha band and the beta band in the conflict suppression stage, and the alpha band in the conflict resolution stage was significant. These results suggest that sleep deprivation affects the effectiveness of the flanker task without affecting efficiency, mainly because sleep deprivation highlights the subject’s stimulus-driven processing, thereby weakening the subject’s conflict resolution ability. Our findings may provide insights into neural functional changes in the impact of sleep deprivation on conflict processing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Complex environments require people to allocate their attention flexibly. Interfering information leads to a competition for attention, where conflict processing capabilities are highlighted (Siemann et al., 2016; Norman & Shallice, 1986). Emerging literature suggests that working memory and executive functions, including conflict processing, are impaired by sleep deprivation (SD) (Durmer & Dinges, 2005; Killgore, 2010; Cremone et al., 2017), and some error-monitoring literature suggests that after SD and sleep restriction, error-related attention was limited, both unconscious error-detection and conscious error-awareness processes may be impaired (Boardman et al., 2021). Yet, the cognitive neural mechanisms by which SD affects conflict processing are poorly understood.
Researchers have now defined various types of conflict and corresponding behavioral paradigms. For example, when studying the conflict between task-relevant information and task-irrelevant information processing, the classical or adapted flanker paradigm is appropriate (Eriksen, 1974; Larson et al., 2014; Pires et al., 2014; Folstein & Van, 2008; Posner et al., 1980). Take the following scenario: An individual is studying for a math test tomorrow, but his favorite soccer team is playing a crucial game. When considering that an individual is performing a goal-directed task, task-irrelevant stimuli in the external environment may cause individual distraction, which in turn affects performance on the task. In the classical flanker task, participants were asked to ignore task-irrelevant items on the sides (e.g., arrows on the sides) and respond to the target item in the center (e.g., arrows in the center). When task-irrelevant stimuli were congruent with target stimuli, responses were generally faster and more accurate than when they were incongruent (Eriksen, 1974; Larson et al., 2014; Pires et al., 2014). Despite previous systematic reviews and studies suggesting that sleep deprivation can systematically affect goal-driven processing and stimulus-driven processing (Pilcher et al., 2007; Harrison & Horne, 2000; Chee, 2015), the direct behavioral and biological evidence is still limited.
Most researchers prefer to use event-related potentials (ERPs) to study the issues in the field of cognitive processing because their excellent temporal precision ensures insight into the cerebral basis of cognitive processing (Kałamała et al., 2018). The P2 and N2 components are widely used as indicators at the early stage of conflict detection. Frontal-area P2 was found to be an indicator of the selective attention process related to stimulus assessment (Luck & Hillyard, 1994). Studies have shown that N2 components elicited during the flanker task are associated with conflict detection, and the N2 amplitude is larger in incongruent conditions than in congruent conditions (Folstein & Van, 2008; Yeung et al., 2004; Veen & Carter, 2002a, b). Subsequently, Ni and P3b are classical components of conflict suppression; the Ni (N400) component is thought to reflect interference suppression, which is mainly generated by the anterior cingulate cortex and the prefrontal cortex (PFC) (Heidlmayr et al., 2020), and the decline of P3b in the top central area is correlated with resource competition and restraint control (Kok, 2001; Polich, 2007). Finally, the lateral slow potentials (LSP) is believed to reflect the conflict resolution processes (Heidlmayr et al., 2020). Neural oscillations have also been used to explore the neural mechanisms of cognitive control (Cohen & Donner, 2013). When some researchers used narrow-band analysis to explore the electrophysiological (EEG) changes after SD, they found that the EEG power density increased in the frequency band from 1 to 24 Hz (Corsi-Cabrera et al., 1992; Dumont et al., 1999). The abovementioned evidence from time domain and time–frequency domain revealed the neural processing characteristics of individuals when faced with task-relevant and task-irrelevant conflicting information. These important results provided scientific evidence for further exploration of how SD affects flanker task performance.
In the present study, we adopted the classical version of the flanker task, with the seven horizontal arrows displayed in the center of the screen at the same time, including two conditions: congruent (CON) and incongruent (ICO). We hypothesized that at the behavioral level, the SD group might show lower accuracy (ACC) and a longer reaction time (RT) compared with the control group, and the two groups of ICO trials would exhibit lower ACC and longer RT relative to the CON trials. We further hypothesized that the ERP components associated with conflict processing (such as P2, N2, Ni, P3b, and LSP) and the conflict-related neural oscillations might be affected after SD.
Method
Participants
Participants were recruited through advertisements posted by the psychology department of Xinxiang Medical University. Seventy-two individuals (36 females, aged = 18–23 years old; Mage = 20.46; Standard Deviation = 2.39) met all the inclusion criteria: right-handed; healthy (free from medications or drugs); no self-reported history of neurological conditions, sleep-related disorders, or traumatic brain injury; no color blindness or color weakness; normal or corrected-to-normal vision; no major life events in the past month (such as a breakup or death of a relative or friend); scores of 0–10 on the Pittsburgh Sleep Quality Index scale (PSQI) (Buysse et al., 1989); scores of > 59 on the Morningness-Eveningness Questionnaire (MEQ) (Horne & Ostberg, 1976; Kanagarajan et al., 2018); and female scores of < 55 and male scores of < 53 on the Trait Anxiety Inventory (TAI) (Spielberger, 1970; Spielberger, 1983). It should be noted that PSQI, MEQ, TAI were respectively used to measure the subjects’ sleep quality, circadian rhythm and trait anxiety levels. The scores for each of these scales were set to ensure that the subjects had good sleep quality, normal circadian rhythm and no high level of trait anxiety. However, one subject’s data were lost due to overwriting, and four subjects’ EEG data were abandoned due to excessive movement artifacts. Thus, the final data set included 67 participants (sleep deprivation group: 16 females, 15 males, Mage = 21.13; control group: 18 females, 18 males, Mage = 19.86). All participants provided written informed consent in accordance with the Declaration of Helsinki (Association, 1991). They were paid for their participation after the study. The ethics committee of Xinxiang Medical University approved this study.
Procedure
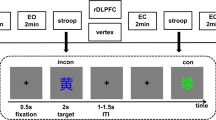
All participants came to the laboratory at 08:00 a.m. on the first day, and the experimental procedures were introduced to the participants, who were instructed not to nap during the day and not to intake any refreshing materials such as caffeine, alcohol, or tea. They were randomly assigned to the control group (sleep normally on the first night) or sleep deprivation (SD) group (stay awake for 24 h on the first day). The SD group was asked to stay awake during the first night (starting at 6 p.m.) under constant supervision from two research assistants in the laboratory, where they could engage in quiet activities such as watching movies or reading. The flanker task for both groups began at 8 a.m. the next day (see Fig. 1A).
Task
There were two conditions for the test: congruent (CON) and incongruent (ICO). The stimulus was represented by a white arrow on a black background in the center of the computer monitor. Participants were asked to press designated keys on the computer keyboard in response to the central arrow. The target arrow was flanked by six other arrows pointing in the same direction as the target (congruent) or in the opposite direction (incongruent). The two types of trials were presented with equal probability, and the trials were presented in random order. Participants pressed the “F” key in response to the target arrow pointing to the left and the “J” key in response to the target pointing to the right. Assignment of response buttons (left and right) was counterbalanced. Each trial began with a central fixation white cross “+,” which lasted for 500–700 ms. The arrow array appeared after the fixation point disappeared, lasted for 1.5 s, and disappeared after the keystroke response. Participants were instructed to value speed as much as accuracy. The task consisted of three blocks of 144 trials each, and between blocks, participants were allowed to take a short break (see Fig. 1B).
Electrophysiological recording
During the task, the EEG was recorded from 64 scalp electrode sites using Ag/AgCl electrodes mounted in an elastic cap, using Cz (the middle point between the ears) as the online reference electrode and the bilateral mastoids as the reference electrode for offline analysis. Horizontal and vertical electrograms were recorded with two pairs of electrodes: One pair of electrodes was placed 10 mm above and 10 mm below the left eye to record the vertical eye activity (VEOG), and the other pair of electrodes was placed 10 mm outside the corner of both eyes to record the horizontal eye activity (HEOG); the scalp electrode impedance was reduced to less than 5 kΩ, the sampling rate was 500 Hz, the band-pass filter frequency was 0.1–100 Hz, and the power frequency interference of 50 Hz was removed.
Data analysis
Behavioral analysis
Reaction time (RT) was measured as the duration between the beginning of the arrow array presentation and the first keystroke. Error t rials and trials with reaction times of less than 200 ms were excluded from the average RT calculation. Experiments with RT of less than 200 ms were not included in the calculation of accuracy. A mixed analyses of variance (ANOVA) was performed for RT and ACC using the two task conditions (CON/ICO) as within-subject factors and group (sleep deprivation/control) as a between-subject factor.
ERP analysis
All ERP data were analyzed using the MATLAB toolbox (R2016b, MathWorks). We checked all of the EEG data, VEO, HEO, CB1 and CB2 channels were excluded from the artifact analysis to ensure that there were no electrodes located on the neck or face. The CB1 and CB2 were two cerebellar electrodes located 2 cm lateral to PO7 and PO8, respectively; the high-pass filter was set to 0.1 Hz, and the low-pass filter was set to 30 Hz; segmented from 200 ms before the onset of the stimulus to 1000 ms after the onset of the stimulus, the 200 ms pre-stimulation period served as the baseline. Eye movement correction was performed using independent component analysis (ICA), followed by manual removal of eye movement and eye drift components using MATLAB loaded with EEGLAB version 4.5; the artifacts with amplitudes exceeding ± 100 µV were removed. Data from no fewer than 40 trials per condition were included in subsequent analyses after artifact control.
To investigate how sleep deprivation affects the interference of stimulus-driven processing in goal-driven processing, we quantified ERP components based on previous studies (Folstein & Van, 2008; Heidlmayr et al., 2020; Kałamała et al., 2018; Polich, 2007) and the electrophysiological properties of the current data set. For the feedback-locked ERPs, we analyzed the peak amplitudes of P2 (160–200 ms) and N2 (220–270 ms) at Fz, the mean amplitude and latency of Ni (270–390 ms) at Fz, the mean amplitude of LSP (600–800 ms) at Fz, and the mean amplitude and latency of P3b (370–550 ms) at Pz using 2 (CON, ICO) × 2 (SD, control) mixed ANOVA.
Time–frequency analysis
We used the short-time Fourier transform (STFT) to calculate the time and frequency distribution of EEG signals in a single period. The frequency range of exploration was 1–30 Hz, and the step size was 1 Hz; the epochs were extracted from 500 ms before stimulus onset to 850 ms after stimulus onset, and the window size was 400 ms; the 500 ms pre-stimulation period served as the baseline. The amplitude of the power spectrum was baseline corrected by subtracting the average power of the signal during the time interval between − 300 ms and − 200 ms. In the delta (1–3 Hz), theta (4–7 Hz), lower alpha (8–10 Hz), upper alpha (11–13 Hz), and beta (14–30 Hz) bands, the data of the corresponding time of the ERP component under each condition were extracted. Then, the 2 (CON, ICO) × 2 (SD, control) mixed ANOVA was performed. Multiple comparisons were performed using Bonferroni-adjusted corrections (p < 0.05). All of the analysis was performed using SPSS Statistics software version 22.0 (IBM, Armonk, NY, USA).
Results
Behavioral results
Accuracy
Table 1; Fig. 1C present the descriptive statistics of the RT and the accuracy (ACC). Table 2 presents results from mixed ANOVA with Groups (Sleep Deprivation vs. Control) and Conflict Effects (Congruent vs. Incongruent) as factors. The mixed ANOVA showed the repeated measures ANOVA showed that the main effect of group was significant [F(1, 65) = 14.170, p < 0.001, ŋ2 = 0.179], with the ACC of the SD group being lower than that of the control group; the main effect of the conflict effect was significant [F(1, 65) = 40.591, p < 0.001, ŋ2 = 0.384], with the ACC being obviously higher in the CON condition than in the ICO condition; the interaction of group and the conflict effect of the ACC was also significant [F(1, 65) = 10.987, p = 0.002, ŋ2 = 0.145]. Then a simple main effect analysis showed that, in both the SD group and the control group, the ACC was higher under the CON condition than under the ICO condition; the ACC of the control group was higher than of the SD group.
Reaction times
The mixed ANOVA showed that the main effect of the conflict effect was significant [F(1, 65) = 391.263, p < 0.001, ŋ2 = 0.858]; the RT was obviously shorter in the CON condition than in the ICO condition.
ERP results
Figures 2 and 3 show the waveforms of ERP, the topographical maps, and the descriptive statistics of the components in each group of channels Fz and Pz under different conditions. Descriptive statistical results for each ERP component are shown in Table 3. Table 4 shows the results of a mixed ANOVA for Groups (Sleep Deprivation vs. Control) and Conflict Effects (Congruent vs. Incongruent) factors on amplitude and latency.
(A) ERP waveforms at electrode sites Fz during flanker task in the SD and control groups. (B) The peak value of the P2/N2 component and the mean amplitude of the Ni/LSP component and the latency of the Ni/LSP component under different conditions. (C) Topographical maps of each group participants for different task types, including P2 (160–200 ms), N2 (220–270 ms), Ni (270–390 ms) and LSP (600–800 ms)
(A) ERP waveforms at electrode sites Pz during flanker task in the SD and control groups. (B) The mean amplitude of the P3b component (up) and the latency of the P3b component (down) under different conditions. (C) topographical maps of each group participants for different conditions at P3b (370–550 ms)
P2 component (160–200 ms)
The mixed ANOVA of P2 peak amplitude did not find any significant main effects or interactions.
N2 component (220–270 ms)
The mixed ANOVA showed that there was a significant main effect of the peak amplitude of N2 in the conflict effect [F(1, 65) = 5.911, p = 0.018, ŋ2 = 0.083]; the peak value under the ICO condition was significantly higher than that under the CON condition.
Ni component (270–390 ms)
The mixed ANOVA showed that the main effect of the mean amplitude of Ni in the conflict effect was significant [F(1,65) = 11.444, p = 0.001, ŋ2 = 0.150]; the mean amplitude under the CON condition was significantly higher than that under the ICO condition.
Furthermore, the mixed ANOVA showed that the main effect of the latency of Ni in the group was significant [F(1, 65) = 10.454, p = 0.002, ŋ2 = 0.139]; the latency in the SD group was significantly longer than that in the control group. There was a significant main effect of the latency of Ni in the conflict effect [F(1, 65) = 33.686, p < 0.001, ŋ2 = 0.341]; the latency under the ICO condition was significantly longer than that under the CON condition. There was also a significant interaction of Ni latency in the group and conflict effect [F(1, 65) = 9.756, p = 0.003, ŋ2 = 0.130]. Then a simple main effects analysis showed that in the SD group, the Ni latency of the ICO condition was significantly longer than the CON condition [F(1, 65) = 37.082, p < 0.001, ŋ2 = 0.363], and in the ICO condition, the SD group was significantly longer than the control group [F(1, 65) = 18.233, p < 0.001, ŋ2 = 0.219].
P3b component (370–550 ms)
The mixed ANOVA showed that there was a significant interaction of P3b mean amplitude in the group and conflict effect [F(1, 65) = 4.678, p = 0.034, ŋ2 = 0.067]. Then a simple main effects analysis showed that the control group had a more positive mean amplitude under the ICO condition than under the CON condition [F(1, 65) = 4.257, p = 0.043, ŋ2 = 0.061].
The mixed ANOVA showed that the main effect of the latency of P3b in the group was significant [F(1, 65) = 13.126, p = 0.001, ŋ2 = 0.168]; the latency in the SD group was significantly longer than that in the control group. There was a significant main effect of the latency of P3b in the conflict effect [F(1, 65) = 33.186, p < 0.001, ŋ2 = 0.338]; the latency under the ICO condition was significantly longer than that under the CON condition.
LSP component (600–800 ms)
The mixed ANOVA showed that the main effect of the mean amplitude of LSP in the group was significant [F(1, 65) = 5.529, p = 0.022, ŋ2 = 0.078]; the mean amplitude in the SD group was significantly lower than that in the control group.
Time–frequency results
Descriptive statistical results for EEG band power at the corresponding time of P2/N2/Ni/LSP components of the Fz electrode point and the corresponding time of P3b components of the Pz electrode point are presented in Table 5. Table 6 presents the results of the mixed ANOVA for Groups (Sleep Deprivation vs. Control) and Conflict Effects (Congruent vs. Incongruent) factors for the different frequency bands of power. Time–frequency maps at the Fz and Pz electrodes and descriptive statistics are shown in Figs. 4 and 5.
Time–frequency at the corresponding time of the P2 component
The main effect of the power in beta (14–30 Hz) of P2 under the group was significant [F(1, 65) = 8.051, p = 0.006, ŋ2 = 0.110]; the power in the control group was significantly higher than that in the SD group. The main effect of the power in beta (14–30 Hz) of P2 under the conflict effect was significant [F(1, 65) = 8.394, p = 0.005, ŋ2 = 0.114]; the power under the CON condition was significantly higher than that under the ICO condition.
Time–frequency at the corresponding time of N2 component
The main effect of the power in the lower alpha (8–10 Hz) of N2 under the group was significant [F(1, 65) = 4.185, p = 0.045, ŋ2 = 0.060]; the power in the control group was significantly higher than that in the SD group. There was also a significant interaction of the power in lower alpha (8–10 Hz) of N2 between the group and conflict effect [F(1, 65) = 4.039, p = 0.049, ŋ2 = 0.059]. Then a simple main effect analysis showed that in the control group, the power under the ICO condition was significantly higher than that under the CON condition [F(1, 65) = 5.726, p = 0.020, ŋ2 = 0.081], and in the ICO condition, the power in the control group was significantly higher than that in the SD group [F(1, 65) = 8.707, p = 0.004, ŋ2 = 0.118].
The main effect of the power in beta (14–30 Hz) of N2 under the group was significant [F(1, 65) = 10.408, p = 0.002, ŋ2 = 0.138]; the power in the control group was significantly higher than that in the SD group. The main effect of the power in beta (14–30 Hz) of the N2 under the conflict effect was also significant [F(1, 65) = 6.449, p = 0.014, ŋ2 = 0.090]; the power in the CON condition was significantly higher than that in the ICO condition.
Time–frequency at the corresponding time of the Ni component
The main effect of the power in theta (4–7 Hz) of Ni under the conflict effect was significant [F(1, 65) = 24.944, p < 0.001, ŋ2 = 0.277]; the power in the ICO condition is significantly higher than that in the CON condition.
The main effect of the power in lower alpha (8–10 Hz) of Ni under the group was significant [F(1, 65) = 4.163, p = 0.045, ŋ2 = 0.060]; the power in the control group was significantly higher than that in the SD group. The main effect of the power in lower alpha (8–10 Hz) of Ni under the conflict effect was significant [F(1, 65) = 13.266, p = 0.001, ŋ2 = 0.169]; the power under the ICO condition was significantly higher than that under the CON condition. There was also a significant interaction of the power in lower alpha (8–10 Hz) of Ni between the group and conflict effect [F(1, 65) = 5.376, p = 0.024, ŋ2 = 0.076]. Then a simple main effect analysis showed that in the control group, the power in the ICO condition was significantly higher than that in the CON condition [F(1, 65) = 19.198, p < 0.001, ŋ2 = 0.228], and in the ICO condition, the power in the control group was significantly higher than that in the SD group [F(1,65) = 7.136, p = 0.010, ŋ2 = 0.099].
There was a significant interaction of the power in upper alpha (11–13 Hz) of Ni between the group and conflict effect [F(1, 65) = 4.564, p = 0.036, ŋ2 = 0.066]. Then a simple main effect analysis showed that under the ICO condition, the power in the control group was significantly higher than that in the SD group [F(1, 65) = 5.120, p = 0.027, ŋ2 = 0.073].
The main effect of the power in beta (14–30 Hz) of Ni under the group was significant [F(1, 65) = 10.337, p = 0.002, ŋ2 = 0.137]; the power in the control group was significantly higher than that in the SD group. The main effect of the power in beta (14–30 Hz) of Ni under the conflict effect was significant [F(1, 65) = 4.950, p = 0.030, ŋ2 = 0.071]; the power in the CON condition was significantly higher than that in the ICO condition. There was also a significant interaction of the power in beta (14–30 Hz) of Ni between the group and conflict effect [F(1, 65) = 4.033, p = 0.049, ŋ2 = 0.058]. Then a simple main effect analysis showed that in the SD group, the power in the CON condition was significantly higher than that in the ICO condition [F(1, 65) = 8.337, p = 0.005, ŋ2 = 0.114], and under both the CON condition and the ICO condition, the power in the control group was significantly higher than that in the SD group [F(1, 65) = 6.753, p = 0.012, ŋ2 = 0.094; F(1, 65) = 10.707, p = 0.002, ŋ2 = 0.141].
Time–frequency at the corresponding time of the P3b component
The main effect of the power in delta (1–3 Hz) of P3b under the conflict effect was significant [F(1, 65) = 6.020, p = 0.017, ŋ2 = 0.085]; the power in the CON condition was significantly higher than that in the ICO condition.
The main effect of the power in theta (4–7 Hz) of P3b under the group was significant [F(1, 65) = 6.351, p = 0.014, ŋ2 = 0.089]; the power in the control group was significantly higher than that in the SD group.
The main effect of the power in beta (14–30 Hz) of P3b under the conflict effect was significant [F(1, 65) = 4.141, p = 0.046, ŋ2 = 0.060]; the power under the CON condition was significantly higher than that under the ICO condition.
Time–frequency at the corresponding time of the LSP component
The main effect of the power in delta (1–3 Hz) of LSP under the group was significant [F(1, 65) = 5.843, p = 0.018, ŋ2 = 0.082]; the power in the SD group was significantly higher than that in the control group.
The main effect of the power in theta (4–7 Hz) of LSP under the conflict effect was significant [F(1, 65) = 4.462, p = 0.038, ŋ2 = 0.064]; the power under the ICO condition was significantly higher than that under the CON condition.
There was a significant interaction of the power in lower alpha (8–10 Hz) of LSP between the group and conflict effect [F(1, 65) = 6.031, p = 0.017, ŋ2 = 0.085]. Then a simple main effects analysis showed that in the SD group, the power under the ICO condition was significantly more negative than that under the CON condition [F(1, 65) = 5.742, p = 0.019, ŋ2 = 0.081].
The main effect of the power in upper alpha (11–13 Hz) of LSP under the conflict effect was significant [F(1, 65) = 8.635, p = 0.005, ŋ2 = 0.117]; the power under the ICO condition was significantly more negative than that under the CON condition. There was also a significant interaction of the power in upper alpha (11–13 Hz) of LSP between the group and conflict effect [F(1, 65) = 6.190, p = 0.015, ŋ2 = 0.087]. Then a simple main effects analysis showed that in the SD group, the power under the ICO condition was significantly more negative than that under the CON condition [F(1, 65) = 13.702, p < 0.001, ŋ2 = 0.174].
The main effect of the power in beta (14–30 Hz) of LSP under the conflict effect was significant [F(1, 65) = 9.010, p = 0.004, ŋ2 = 0.122]; the power under the ICO condition was significantly lower than that under the CON condition.
Discussion
In this study, we used the classical flanker task to study the interference effect of stimulus-driven processing on target-driven processing after sleep deprivation. We found that subjects in the SD group showed poorer conflict processing ability at both behavioral and EEG levels.
Behavioral findings
At the behavioral level, we found that the ACC of the CON condition and the ICO condition in the control group was higher than that in the SD group, which was consistent with the results of previous studies (Tsai et al., 2005; Murphy et al., 2006; Hsieh et al., 2010), indicating that the effectiveness of conflict processing is compromised after SD. Meanwhile, consistent with the results of previous studies on conflict effects (Lavie, 2005; Han, 2015, 2018; Lavie & Robertson, 2001), the RT of ICO conditions was significantly longer than that of CON conditions; however, there was no significant difference in RT between the SD group and control group. This may indicate that SD affects an individual’s ability to inhibit external irrelevant stimuli, but the effect is only reflected in the effectiveness, not the efficiency.
ERP findings
We found that at the ERP level, SD significantly affected the two stages of conflict processing: conflict suppression and conflict resolution.
At the stage of conflict detection, according to previous experiments, the amplitude of the N2 component reflects the conflict detection process under the condition of the ICO flanker task (Tsai et al., 2005). In the versions of the flanker task, the N2 component is consistently considered to be involved in anterior cingulate cortex activation during the conflict assessment phase. N2 is a negative-going deflection that occurs about 250ms to 350ms after stimulus onset, reflecting competing stimulus and/or response selection. Compared with the congruent trials, the amplitude of incongruent trials are larger (i.e. more negative) (Kałamała et al., 2018; Rey-Mermet et al., 2019; Clayson & Larson, 2011; Cheng et al., 2021). Other studies have found that the amplitude of N2 shows greater negative deflection under the condition of increased cognitive control requirements (Forster et al., 2011; Qi & Gao, 2020). Secondly, many recent conflict studies have shifted attention to the frontal P2 component, which is a positive-going deflection occurring approximately 150ms to 250ms after the onset of the stimulus and is generally thought to be involved in the selective attention processing of stimulus evaluation. Some studies using flanker tasks have also found that stimulus-response conflict enhances frontal P2 (Kałamała et al., 2018; Rey-Mermet et al., 2019; Clayson & Larson, 2011; Luck & Hillyard, 1994). In accordance with this, in our experiment, the N2 components also had larger amplitudes under ICO conditions. However, there was no significant difference in the amplitude of the N2 component between the SD group and control group. Moreover, our experiments did not find any significant effect of SD on the P2 component. Based on these findings, SD did not seem to adversely affect stimuli assessment and early selective attention processing in the early stages of conflict processing. This may indicate that SD did not impair the subjects’ ability to detect conflicts.
At the stage of conflict suppression, the Ni component is thought to reflect interference suppression. Ni (N400) refers to the negative component in tasks requiring conflict control and is produced primarily by anterior cingulate cortex and the prefrontal cortex (PFC). It peaks amplitude approximately 400ms after the onset of stimulation, and it is often interpreted as reflecting inhibition processes and interference suppression (Duncan et al., 2009; Heidlmayr et al., 2020; Liotti et al., 2000; Almeida, 2021). The Ni latency in the ICO conditions was significantly longer in the SD group than in the control group; this suggests that subjects needed to spend more time suppressing interference after SD. It is worth noting that SD may impair the interference inhibition ability of subjects to some extent. The mean amplitude of Ni was enhanced under ICO conditions, which further confirms the role of Ni in conflict suppression.
On top of that, P3b is a broad, positive component of ERP, typically peaking in the frontal parietal region 300ms or more after task-related interfering stimuli onset (Duncan et al., 2009). The increase of P3b activity may reflect more neural resource allocation (Clayson & Larson, 2011), and the attenuation of P3b may result from the interruption of attention control process (Shackman et al., 2011). Moreover, the latency of the P3b component usually reflects the time required for the evaluation or classification of stimuli (Polich, 2007). In the present study, the P3b latency of ICO conditions was significantly longer than that of the CON conditions, indicating that subjects needed more time to evaluate stimuli in the face of conflict, and the P3b latency of the SD group was significantly longer than that of the control group, which seems to reflect that the time to suppress irrelevant external stimuli after SD was increased. We have reason to speculate that SD may have impaired participants’ ability to evaluate or classify stimuli. The mean amplitude of the P3b component is related to restraint control (Kok, 2001; Polich, 2007). We only found an average amplitude enhancement under ICO conditions in the control group; after SD, the subjects seemed to expend the same cognitive resources on the inhibition process under the CON and ICO conditions. After sleep deprivation, subjects appeared to be unable to allocate cognitive resources precisely during the inhibition phase of conflict processing compared with having normal sleep.
At the stage of conflict resolution, the LSP component reflects the conflict resolution processes. LSP is a persistent frontal-central negative-going potential found during a time window of about 550-800ms in cognitive control tasks involving linguistic (semantic) components. It is thought to reflect participation in conflict resolution, reactivation of meaning after conflict resolution, or response selection (Heidlmayr et al., 2020; Liotti et al., 2000; Hanslmayr et al., 2008). We found that the average amplitude of the LSP component in the SD group was significantly higher than that in the control group, indicating that, after SD, subjects needed to make more effort in the conflict resolution phase of the flanker task. This also partly reflects that after SD, subjects still have difficulty resolving conflicts even after successfully detecting and suppressing conflicting information.
Time–frequency findings
Third, to further explore how SD affects conflict processing in different periods of time, we conducted a time–frequency analysis. Consistent with previous studies (Corsi-Cabrera et al., 1992; Dumont et al., 1999), after SD, the power density of different bands changed significantly in different time periods.
According to the literature (Leszczyński et al., 2015), the period of delta oscillation is phase-locked with stimulation. In the present study, we found that, only in the conflict resolution stage (time period related to LSP), there was a significant difference in the delta band between groups, where the power of the SD group was significantly higher than that of the control group. These differences may be due to the neural oscillation caused by stimulation after SD is magnified, which seems to be related to the excessive bottom-up processing.
The neural oscillation in the theta band is related to the active maintenance and recall of working memory representation, and the participation of attention is particularly important (Meltzer et al., 2017; Klimesch et al., 1997; Jensen & Tesche, 2002). In the theta band, we found a significant group difference in the conflict suppression stage (time period related to P3b), indicating that after SD, the ability to actively maintain and recall WM representation was affected when suppressing external irrelevant stimuli. It may also suggest that SD may impair an individual’s attention and cognitive abilities in conflict situations. However, how SD affects neuronal activity associated with conflict processing in the theta frequency band warrants further exploration in the future studies as it involves the general activation/inhibition system of the central nervous system.
When task-irrelevant information must be actively suppressed, the power of the occipital alpha oscillation will increase. The lower alpha band is related to attentional and motivational processes (Klimesch et al., 1992), and the upper alpha band is considered to reflect processing related to stimulation (Bonnefond & Jensen, 2012; Jokisch & Jensen, 2007; Pfurtscheller & Klimesch, 1992). In this study, we found that in the lower alpha band of the conflict detection stage (time period related to N2) and the conflict suppression stage (time period related to Ni), in the control group, the power of the ICO condition was significantly higher than that of the CON condition, which suggested that, in both the conflict detection stage and the conflict suppression stage, the attentional processes were disturbed after SD. Furthermore, in the upper alpha band of the conflict suppression stage (time period related to Ni), under the ICO condition, the power of the control group was significantly higher than that of the SD group, indicating that after SD, the processing related to stimulation in the conflict suppression stage was impaired. Moreover, in both the lower alpha band and upper alpha band of the conflict resolution stage (time period related to LSP), the CON condition in the SD group had a more negative power, which seems to indicate that the effect of SD on top-down processing (conflict effect) mainly occurs in the upper alpha band.
The activity in the beta band is related to the maintenance of the current sensorimotor or cognitive state (Engel & Fries, 2010). We only found that, in the conflict suppression stage (time period related to Ni), under both the CON condition and ICO condition, the power of the control group was significantly higher than that of the SD group. It seems that, after SD, the ability to maintain the cognitive state in the conflict inhibition stage is affected.
Conclusions
In conclusion, SD mainly affects the effectiveness (ACC) but not the efficiency (RT) of performance on the flanker task. The results of ERP analysis showed that SD weakens conflict suppression ability, which is mainly reflected in the difference in the latency of Ni and the mean amplitude of P3b. Further time–frequency analysis found that the significant difference between the detection stage and the inhibition stage is reflected in the ICO conditions, which comes from the highlighting of the external irrelevant stimuli. The significant difference in the resolution stage is reflected in the SD group, and the overall conflict resolution ability is weakened, which suggests that SD highlights subjects’ bottom-up processing. Stimulus-driven processing occupies more cognitive resources and weakens top-down processing. It is reasonable to speculate that, in an abnormal environment, SD prevents individuals from regulating their behavior in response to the environment. Our findings may provide insights into neural functional changes in the impact of SD on conflict processing.
Limitations and prospects
There are still some limitations in the current research that need to be addressed in future studies. Firstly, Ru et al. (2019) found that task difficulty selectively modulated the impact of napping on higher working memory and conflict monitoring. However, the Flanker paradigm adopted in the current research is less difficult, and whether the impact of SD on conflict processing efficiency is modulated by different task difficulty needs to be further explored in future study. Secondly, continuous supervision of participants in the SD group and differences in the activities allowed during deprivation may have affected participants’ alertness and performance, which may have introduced bias in the results and may not accurately reflect the true impact of SD on cognitive performance. Thirdly, this research is a short-term acute SD, but people are more troubled by long-term chronic SD in daily life, so before extending our findings to more general sleep problems, it is necessary to further explore the similarities and differences between the effects of chronic SD and acute SD on conflict processing.
Data availability
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
References
Almeida, V. N. (2021). Neurophysiological basis of the N400 deflection, from Mismatch Negativity to Semantic Prediction Potentials and late positive components. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 166, 134–150. https://doi.org/10.1016/j.ijpsycho.2021.06.001.
Association, W. M. (1991). Declaration of Helsinki. Law, medicine & health care: A publication of the American Society of law & medicine. Gastroenterologia Japonica, 26(2), 269–270.
Boardman, J. M., Porcheret, K., Clark, J. W., Andrillon, T., Cai, A. W. T., Anderson, C., & Drummond, S. P. A. (2021). The impact of sleep loss on performance monitoring and error-monitoring: A systematic review and meta-analysis. Sleep Medicine Reviews, 58, 101490. https://doi.org/10.1016/j.smrv.2021.101490.
Bonnefond, M., & Jensen, O. (2012). Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Current Biology: CB, 22(20), 1969–1974. https://doi.org/10.1016/j.cub.2012.08.029.
Buysse, D. J., Reynolds, C. F. 3rd, Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. https://doi.org/10.1016/0165-1781(89)90047-4.
Chee, M. W. L. (2015). Limitations on visual information processing in the sleep-deprived brain and their underlying mechanisms. Curr Opin Behav Sci, 1, 56–63. https://doi.org/10.1016/j.cobeha.2014.10.003.
Cheng, W., Huang, Q., Chen, Y., Dai, W., Cui, L., Shan, S., Chen, Z., & Zhou, S. (2021). Parsing the neural mechanisms of short-term and Long-Term Associations in the Flanker Tasks: An ERP Analysis. Frontiers in Behavioral Neuroscience, 15, 626907. https://doi.org/10.3389/fnbeh.2021.626907.
Clayson, P. E., & Larson, M. J. (2011). Effects of repetition priming on electrophysiological and behavioral indices of conflict adaptation and cognitive control. Psychophysiology, 48(12), 1621–1630. https://doi.org/10.1111/j.1469-8986.2011.01265.x.
Cohen, M. X., & Donner, T. H. (2013). Midfrontal conflict-related theta-band power reflects neural oscillations that predict behavior. Journal of Neurophysiology, 110(12), 2752–2763. https://doi.org/10.1152/jn.00479.2013.
Corsi-Cabrera, M., Ramos, J., Arce, C., Guevara, M. A., Ponce-de León, M., & Lorenzo, I. (1992). Changes in the waking EEG as a consequence of sleep and sleep deprivation. Sleep, 15(6), 550–555. https://doi.org/10.1093/sleep/15.6.550.
Cremone, A., McDermott, J. M., & Spencer, R. (2017). Naps enhance executive attention in preschool-aged children. Journal of Pediatric Psychology, 42(8), 837–845. https://doi.org/10.1093/jpepsy/jsx048.
Dumont, M., Macchi, M. M., Carrier, J., Lafrance, C., & Hébert, M. (1999). Time course of narrow frequency bands in the waking EEG during sleep deprivation. Neuroreport, 10(2), 403–407. https://doi.org/10.1097/00001756-199902050-00035.
Duncan, C. C., Barry, R. J., Connolly, J. F., Fischer, C., Michie, P. T., Näätänen, R., Polich, J., Reinvang, I., & Van Petten, C. (2009). Event-related potentials in clinical research: Guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 120(11), 1883–1908. https://doi.org/10.1016/j.clinph.2009.07.045.
Durmer, J. S., & Dinges, D. F. (2005). Neurocognitive consequences of sleep deprivation. Seminars in Neurology, 25(1), 117–129. https://doi.org/10.1055/s-2005-867080.
Engel, A. K., & Fries, P. (2010). Beta-band oscillations–signalling the status quo? Current Opinion in Neurobiology, 20(2), 156–165. https://doi.org/10.1016/j.conb.2010.02.015.
Eriksen, B. A., & Eriksen, C. W. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics, 16(1), 143–149. https://doi.org/10.3758/BF03203267.
Folstein, J. R., & Van Petten, C. (2008). Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology, 45(1), 152–170. https://doi.org/10.1111/j.1469-8986.2007.00602.x.
Forster, S. E., Carter, C. S., Cohen, J. D., & Cho, R. Y. (2011). Parametric manipulation of the conflict signal and control-state adaptation. Journal of Cognitive Neuroscience, 23(4), 923–935. https://doi.org/10.1162/jocn.2010.21458.
Han, S. W. (2015). Working memory contents enhance perception under stimulus-driven competition. Memory & Cognition, 43(3), 432–440. https://doi.org/10.3758/s13421-014-0460-8.
Han, S. W. (2018). Opposing effects of memory-driven and stimulus-driven attention on distractor perception. Cognitive Processing, 19(1), 117–123. https://doi.org/10.1007/s10339-017-0834-5.
Hanslmayr, S., Past¨otter, B., B¨auml, K. H., Gruber, S., Wimber, M., & Klimesch, W. (2008). The electrophysiological dynamics of interference during the Stroop task. Journal of Cognitive Neuroscience, 20, 215–225. https://doi.org/10.1162/jocn.2008.20020.
Harrison, Y., & Horne, J. A. (2000). The impact of sleep deprivation on decision making: A review. Journal of Experimental Psychology Applied, 6(3), 236–249. https://doi.org/10.1037//1076-898x.6.3.236.
Heidlmayr, K., Kihlstedt, M., & Isel, F. (2020). A review on the electroencephalography markers of Stroop executive control processes. Brain and Cognition, 146, 105637. https://doi.org/10.1016/j.bandc.2020.105637.
Horne, J. A., & Ostberg, O. (1976). A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology, 4(2), 97–110.
Hsieh, S., Li, T. H., & Tsai, L. L. (2010). Impact of monetary incentives on cognitive performance and error monitoring following sleep deprivation. Sleep, 33(4), 499–507. https://doi.org/10.1093/sleep/33.4.499.
Jensen, O., & Tesche, C. D. (2002). Frontal theta activity in humans increases with memory load in a working memory task. The European Journal of Neuroscience, 15(8), 1395–1399. https://doi.org/10.1046/j.1460-9568.2002.01975.x.
Jokisch, D., & Jensen, O. (2007). Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(12), 3244–3251. https://doi.org/10.1523/JNEUROSCI.5399-06.2007.
Kałamała, P., Szewczyk, J., Senderecka, M., & Wodniecka, Z. (2018). Flanker task with equiprobable congruent and incongruent conditions does not elicit the conflict N2. Psychophysiology, 55(2), https://doi.org/10.1111/psyp.12980.
Kanagarajan, K., Gou, K., Antinora, C., Buyukkurt, A., Crescenzi, O., Beaulieu, S., Storch, K. F., & Mantere, O. (2018). Morningness-eveningness questionnaire in bipolar disorder. Psychiatry Research, 262, 102–107. https://doi.org/10.1016/j.psychres.2018.02.004.
Killgore, W. D. (2010). Effects of sleep deprivation on cognition. Progress in Brain Research, 185, 105–129. https://doi.org/10.1016/B978-0-444-53702-7.00007-5.
Klimesch, W., Pfurtscheller, G., & Schimke, H. (1992). Pre- and post-stimulus processes in category judgement tasks as measured by event-related desynchronization (ERD). Journal of Psychophysiology, 6(3), 185–203.
Klimesch, W., Doppelmayr, M., Schimke, H., & Ripper, B. (1997). Theta synchronization and alpha desynchronization in a memory task. Psychophysiology, 34(2), 169–176. https://doi.org/10.1111/j.1469-8986.1997.tb02128.x.
Kok, A. (2001). On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology, 38(3), 557–577. https://doi.org/10.1017/s0048577201990559.
Larson, M. J., Clayson, P. E., & Clawson, A. (2014). Making sense of all the conflict: A theoretical review and critique of conflict-related ERPs. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 93(3), 283–297. https://doi.org/10.1016/j.ijpsycho.2014.06.007.
Lavie, N. (2005). Distracted and confused?: Selective attention under load. Trends in Cognitive Sciences, 9(2), 75–82. https://doi.org/10.1016/j.tics.2004.12.004.
Lavie, N., & Robertson, I. H. (2001). The role of perceptual load in neglect: Rejection of ipsilesional distractors is facilitated with higher central load. Journal of Cognitive Neuroscience, 13(7), 867–876. https://doi.org/10.1162/089892901753165791.
Leszczyński, M., Fell, J., & Axmacher, N. (2015). Rhythmic Working Memory activation in the human Hippocampus. Cell Reports, 13(6), 1272–1282. https://doi.org/10.1016/j.celrep.2015.09.081.
Liotti, M., Woldorff, M. G., Perez, R., & Mayberg, H. S. (2000). An ERP study of the temporal course of the Stroop color-word interference effect. Neuropsychologia, 38(5), 701–711. https://doi.org/10.1016/s0028-3932(99)00106-2.
Luck, S. J., & Hillyard, S. A. (1994). Electrophysiological correlates of feature analysis during visual search. Psychophysiology, 31(3), 291–308. https://doi.org/10.1111/j.1469-8986.1994.tb02218.x.
Meltzer, J. A., Kielar, A., Panamsky, L., Links, K. A., Deschamps, T., & Leigh, R. C. (2017). Electrophysiological signatures of phonological and semantic maintenance in sentence repetition. Neuroimage, 156, 302–314. https://doi.org/10.1016/j.neuroimage.2017.05.030.
Murphy, T. I., Richard, M., Masaki, H., & Segalowitz, S. J. (2006). The effect of sleepiness on performance monitoring: I know what I am doing, but do I care? Journal of Sleep Research, 15(1), 15–21. https://doi.org/10.1111/j.1365-2869.2006.00503.x.
Norman, D., & Shallice, T. (1986). Attention to action: Willed and automatic control of behavior. In R. Davidson, G. Schwartz, & D. Shapiro (Eds.), Consciousness and self regulation: Advances in research and theory (pp. 1–18). Plenum.
Pfurtscheller, G., & Klimesch, W. (1992). Functional topography during a visuoverbal judgment task studied with event-related desynchronization mapping. Journal of Clinical Neurophysiology: Official Publication of the American Electroencephalographic Society, 9(1), 120–131. https://doi.org/10.1097/00004691-199201000-00013.
Pilcher, J. J., Band, D., Odle-Dusseau, H. N., & Muth, E. R. (2007). Human performance under sustained operations and acute sleep deprivation conditions: Toward a model of controlled attention. Aviation Space and Environmental Medicine, 78(5 Suppl), B15–B24.
Pires, L., Leit ~ ao, J., Guerrini, C., & Sim ~ oes, M. R. (2014). Event-related brain potentials in the study of inhibition: Cognitive control, source localization and age-related modulations. Neuropsychology Review, 24(4), 461–490. https://doi.org/10.1007/s11065-014-9275-4.
Polich, J. (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 118(10), 2128–2148. https://doi.org/10.1016/j.clinph.2007.04.019.
Posner, M. I., Snyder, C. R., & Davidson, B. J. (1980). Attention and the detection of signals. Journal of Experimental Psychology, 109(2), 160–174.
Qi, M., & Gao, H. (2020). Acute psychological stress promotes general alertness and attentional control processes: An ERP study. Psychophysiology, 57(4), e13521. https://doi.org/10.1111/psyp.13521.
Rey-Mermet, A., Gade, M., & Steinhauser, M. (2019). Sequential conflict resolution under multiple concurrent conflicts: An ERP study. Neuroimage, 188, 411–418. https://doi.org/10.1016/j.neuroimage.2018.12.031.
Ru, T., Chen, Q., You, J., & Zhou, G. (2019). Effects of a short midday nap on habitual nappers’ alertness, mood and mental performance across cognitive domains. Journal of Sleep Research, 28(3), e12638. https://doi.org/10.1111/jsr.12638.
Shackman, A. J., Maxwell, J. S., McMenamin, B. W., Greischar, L. L., & Davidson, R. J. (2011). Stress potentiates early and attenuates late stages of visual processing. Journal of Neuroscience, 31(3), 1156–1161. https://doi.org/10.1523/JNEUROSCI.3384-10.2011.
Siemann, J., Herrmann, M., & Galashan, D. (2016). fMRI-constrained source analysis reveals early top-down modulations of interference processing using a flanker task. Neuroimage, 136, 45–56. https://doi.org/10.1016/j.neuroimage.2016.05.036.
Spielberger, C. D., Gorsuch, R. L., & Lushene, R. E. (1970). STAI: Manual for the state-trait anxiety inventory (self-Evaluation questionnaire). Palo Alto.
Spielberger, C. D. (1983). Manual for the state-trait anxiety Inventory STAI (form Y) (self-evaluation questionnaire). Consulting Psychologists Press.
Tsai, L. L., Young, H. Y., Hsieh, S., & Lee, C. S. (2005). Impairment of error monitoring following sleep deprivation. Sleep, 28(6), 707–713. https://doi.org/10.1093/sleep/28.6.707.
van Veen, V., & Carter, C. S. (2002a). The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior, 77(4–5), 477–482. https://doi.org/10.1016/s0031-9384(02)00930-7.
van Veen, V., & Carter, C. S. (2002b). The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience, 14(4), 593–602. https://doi.org/10.1162/08989290260045837.
Yeung, N., Botvinick, M. M., & Cohen, J. D. (2004). The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review, 111(4), 931–959. https://doi.org/10.1037/0033-295x.111.4.939.
Acknowledgements
Meng Zhang received support from the National Natural Science Foundation of China (31600927, 81830040), and the Youth Foundation of Social Science and Humanity, China Ministry of Education (21YJC190023). Yufeng Wang received support from the Youth Foundation of Social Science and Humanity, China Ministry of Education (19YJCZH179). Feng Zou received support from the Planning Project of Henan Provincial Philosophy and Social Science (2022BJY028). The funders had no role in the study design, data collection, analysis and interpretation of data, writing of the manuscript, or in the decision to submit the article for publication. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Yunwen Peng: Investigation, Data curation, Methodology, Formal analysis, Writing – original draft, Writing - review & editing. Xirui Zhang: Investigation, Data curation, Methodology. Xiaochen Yang: Investigation, Data curation, Methodology. Shuqing Feng: Investigation, Data curation, Methodology. Mei Du: Investigation, Data curation, Methodology. Rui Zhang: Investigation, Data curation. Jiashan Sima: Investigation, Data curation. Xin Wu: Investigation. Feng Zou: Investigation, Funding acquisition, Writing - review & editing. Yufeng Wang: Investigation, Funding acquisition, Writing - review & editing. Yanyan Luo: Investigation, Writing - review & editing. Meng Zhang: Conceptualization, Methodology, Investigation, Project administration, Funding acquisition, Writing - original draft, Writing - review & editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The studies involving human participants were reviewed and approved by the Ethics Committee of Xinxiang Medical University. Written informed consent to participate in this study was provided by the participant.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yunwen Peng, Xirui Zhang and Xiaochen Yang contributed equally to this work and are Co-first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, Y., Zhang, X., Yang, X. et al. Sleep deprivation weakens goal-driven processing by highlighting stimulus-driven processing in conflict processing. Curr Psychol 43, 10383–10397 (2024). https://doi.org/10.1007/s12144-023-05182-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12144-023-05182-z