Abstract
In the previous 15 years, a variety of experimental paradigms and methods have been employed to study inhibition. In the current review, we analyze studies that have used the high temporal resolution of the event-related potential (ERP) technique to identify the temporal course of inhibition to understand the various processes that contribute to inhibition. ERP studies with a focus on normal aging are specifically analyzed because they contribute to a deeper understanding of inhibition. Three time windows are proposed to organize the ERP data collected using inhibition paradigms: the 200 ms period following stimulus onset; the period between 200 and 400 ms after stimulus onset; and the period between 400 and 800 ms after stimulus onset. In the first 200 ms, ERP inhibition research has primarily focused on N1 and P1 as the ERP components associated with inhibition. The inhibitory processing in the second time window has been associated with the N2 and P3 ERP components. Finally, in the third time window, inhibition has primarily been associated with the N400 and N450 ERP components. Source localization studies are analyzed to examine the association between the inhibition processes that are indexed by the ERP components and their functional brain areas. Inhibition can be organized in a complex functional structure that is not constrained to a specific time point but, rather, extends its activity through different time windows. This review characterizes inhibition as a set of processes rather than a unitary process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Everyday functioning requires the ability to successfully inhibit irrelevant stimuli, thoughts, and behaviors (Logan et al. 1984; Hasher and Zacks 1988). Inhibition has a central role in the organization of various cognitive domains, including attention, memory and language (MacLeod et al. 2003). Furthermore, inhibition may function at different levels of cognitive processing, such as thoughts, verbal responses, visual processing, sounds, actions or semantic processing (Amieva et al. 2004). However, because of the variety of methods, experimental paradigms and contexts in which the concept of inhibition has been studied, it is difficult to fully understand how and when inhibition occurs. In the present review, we demonstrate inhibitory processes are not unitary. Rather, they are multifaceted and entail various functions that can be linked to automatic or controlled processing depending on the context.
Theoretical Issues in Inhibition
Inhibition has received labels such as “interference” (Piai et al. 2012) and “suppression” (Ludowig et al. 2010) to highlight its automatic nature (implicit or unintentional inhibitory processes) and controlled nature (explicit or intentional inhibitory processes), respectively (Nigg 2000; Friedman and Miyake 2004; Andres et al. 2008; Collette et al. 2009). This theoretical construct of the level of control that is needed in a cognitive process, in this case inhibition, was initially proposed by Shiffrin and colleagues (for a review, see Shiffrin and Schneider 1977). According to this model, automatic processes typically occur without intention and conscious awareness. As a result, these processes are quick and can occur in parallel with other operations without impairment. Perhaps the most relevant characteristic of an automatic process is that it can occur without the subject’s conscious control. In contrast, controlled processes require intention and awareness. Therefore, these processes are slow and have limited capacity, which reduces the possibility to simultaneously perform other operations (Posner and Snyder 1975). However, controlled processes can be easily changed and applied to novel situations when an automatic sequence cannot be applied (Shiffrin and Schneider 1977). In this theoretical framework, controlled inhibition is the conscious and deliberate suppression of irrelevant stimuli or responses. An example of a laboratory controlled inhibition task is the Stroop task (Stroop 1935). In this task, colored words are presented and the participant must consciously inhibit the tendency to produce a more dominant automatic response (i.e., naming the color word) to be capable of naming the color of the ink in which the word is printed. Automatic inhibition occurs without the subject’s awareness and appears to be involuntary. An example of a laboratory automatic inhibition task is the negative priming (NP; Tipper 1985) paradigm. In a typical NP task, the participant views two images and must respond to a target, thereby inhibiting the distractor (prime trial). In a subsequent trial (probe trial), the distractor of the previous trial becomes the target. In the probe trial, the reaction times are prolonged because of the residual inhibition from the prime display.
Other distinctions among types of inhibition have emerged. A number of studies have established and examined specific categories of inhibitory phenomena, such as response or motor inhibition (the process of inhibiting a planned response or movement; Robinson et al. 2013), lateral inhibition (the capacity of an excited neuron to reduce the activity of its neighbors; Bridgeman 2006), prepulse inhibition (when a stimulus inhibits the startle blink reflex to a subsequent stronger startle stimulus; Dawson et al. 2004), inhibition of return (inhibition produced by a peripheral cue or target; Possin et al. 2009), knowledge or semantic inhibition (inhibition responsible for reducing the activation of the inappropriate knowledge for the context; Debruille 2007), and proactive interference (i.e., the disruption of behaviour due to the influence of antecedent inforation that is no longer relevant and has to be inhibited; Yi and Friedman 2011). In opposition to these types of inhibition, several authors (Hasher and Zacks 1988; Collette et al. 2009) have proposed that inhibition is a unitary process that integrates the following three different but related functions: the access function (responsible for the prevention of irrelevant information entry); the deletion function (responsible for the suppression of information that either is or has become irrelevant); and the restrain function (responsible for the prevention of access to relevant but contextually inappropriate responses).
As a final point in this overview of the conceptualizations of inhibition, we highlight the literature’s general acceptance of the distinction between cognitive and behavioral inhibitory processes. Cognitive inhibition is responsible for the suppression of previously activated cognitive contents, the clearing of non-relevant information and the resistance to interference of information from a potentially attention-capturing stimulus or cognitive content that is contextually inadequate (Koch et al. 2010; Bjorklund and Harnishfeger 1995). Harnishfeger (1995) defined behavioral inhibition in terms of overt behavior control, such as resistance of a prepotent response, delay of a reward, motor inhibition, and impulse control.
Measuring the Time Course of Inhibition
Some of the most important inhibitory processes occur within the first second after the presentation of the stimuli or information that must be inhibited (Kok 1999; Amieva et al. 2004; Huster et al. 2013). To study inhibitory processes in the narrow time window when they occur, event-related brain potentials (ERPs) have been used. The ERP technique has a high temporal resolution, which therefore enables neural activity to be tracked on a millisecond time scale (Albert et al. 2013) and represents a continuous measure of processing (Luck 2005). An ERP is a measured brain electrical response that is directly the result of sensory, motor or cognitive processes. It is a voltage fluctuation, which is derived from the ongoing electroencephalogram (EEG), that is time-locked to a specific event (Kuperberg 2004). These voltage fluctuations are represented in the ERP waveform as a series of positive and negative peaks that vary in amplitude and latency (Dauwels et al. 2010). The amplitude can be measured as the difference between the maximum peak of the ERP waveform over a period of time and the mean baseline voltage (which occurs prior to the stimulus) (Polich 2007). The latency is defined as the interval from the stimulus onset to the point of highest amplitude within a time window. As Kappenman et al. (2012) noted, the characteristics of the ERP waveform do not reflect a specific brain process. To understand the voltage deflections that occur in an ERP waveform (i.e., different peaks and troughs), the term ERP component has been proposed. An ERP component can be described as a scalp-recorded voltage change that reflects a specific neural or psychological process (Luck 2005). ERP components have traditionally been classified as exogenous components, which depend on external factors (i.e., determined by the physical nature of the eliciting stimulus and generally occur within the first 200 ms after stimulus onset), or endogenous components, which primarily depend on internal factors (i.e., sensitive to proprieties, such as the meaning of the stimulus and/or the processing required to accomplish the task) (Picton et al. 2000). An ERP component can be sensitive to different cognitive processes. For example, P3 modulations induced by an oddball paradigm can index attentional processes responsible for updating stimulus representations, while P3 modulations induced by a memory recall task can index encoding mechanisms and P3 modulations observed while a Go/No-go task is performed can index inhibition mechanisms (for a review see Polich 2007). Throughout this review we will focus only on ERPs observed in inhibition studies.
A variety of paradigms have been employed to study inhibition with ERPs (for a review, see Kok 1999), such as location and identity NP, Stop-signal, Go/No-go, Stroop effect, Task Switching, the Eriksen Flanker Task, Spatial cueing tasks, Antisaccade, Proactive Interference and Direct Forgetting. Figure 1 presents a schematic display of the most commonly used inhibition-related paradigms in ERP research.
It is widely accepted that these different paradigms can be related to different types of inhibition. For example, the Stop-signal, Go/No-go and Eriksen Flanker tasks have been related to behavioral inhibition (specifically, motor inhibition), whereas the NP, Stroop and Direct Forgetting paradigms have been related to cognitive inhibition. The nature of inhibition, as an automatic or controlled process, can also be modulated by the paradigm that is used to evoke the inhibition ERPs. According to Nigg (2000), the Stroop and Stop-signal tasks, for example, engage controlled inhibition, whereas the NP and Spatial cueing tasks engage automatic inhibition (see also, Andres et al. 2008). In addition, the effectiveness of inhibition may largely depend on sensory or bottom-up processing associated with the modality of the paradigm (e.g., auditory versus visual). For example, Ramautar et al. (2006) suggested that an auditory version of a paradigm allows for faster processing than the visual version of the same paradigm. Regardless of this variability, the electrophysiological responses that are evoked during inhibition paradigms have been used to clarify the temporal course of inhibition and highlight the differences in the temporal course of different types of inhibition (see Fig. 2 for a schematic illustration of the ERP components that are linked to inhibition processes within different paradigms).
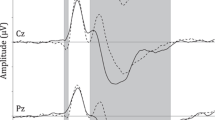
Schematic illustration of grand average event-related potential waveforms linked to inhibition in different paradigms: Go/No-go (Tian and Yao 2008; Thomas et al. 2009); Stop-signal (Bekker et al. 2005; van Boxtel et al. 2001); Eriksen Flanker (Wild-Wall et al. 2008; Neuhaus et al. 2007); Stroop (Hanslmayr et al. 2008); and NP (Gibbons et al. 2006; Kathmann et al. 2006). The P1, P2 and N1 were located at posterior electrode sites (i.e., O1, O2, T5, T6, P7, and P8); the N2 and P3 were located at fronto-central electrode sites (i.e., FC1, FC2, F3, F4, CZ, PZ, FZ, and FCz); the N400 was located at central electrode sites (i.e., Cz and CPz); the N450 was located at fronto-central electrode sites (FC1, FCz, FC2, C1, CZ, C2, CP1, CPz, and CP2); and the LPC was located at central-parietal sites (i.e., P3, P4, Pz, Cz, and Pz)
Recently, there has been an increasing interest in inhibition, which has specifically focused on the neural underpinnings of inhibitory processes and the role of inhibition in cognitive domains, such as memory, language and attention (Verhoef et al. 2009; Neuhaus et al. 2010; Yi and Friedman 2011; Albert et al. 2013). Several of these studies have examined the relevance of inhibitory processes in normal aging (Mayas et al. 2012; Turner and Spreng 2012; Haring et al. 2013; Wostmann et al. 2013), as well as a variety of clinical conditions, such as Alzheimer’s disease (AD; Collette et al. 2009; Thomas et al. 2010; Cheng et al. 2012), mild cognitive impairment (MCI; Belleville et al. 2007), traumatic brain injury (TBI; Dimoska-Di Marco et al. 2011), depression (Dai and Feng 2011; Bobb et al. 2012), anxiety (Robinson et al. 2013), schizophrenia (Hughes et al. 2012), fibromyalgia (Mercado et al. 2013), attention deficit-hyperactivity disorder (ADHD; Senderecka et al. 2012), alcoholism (Padilla et al. 2011) and psychopathy (Verona et al. 2012).
Inhibition and the Aging Process
A decrease in inhibition capacities has been proposed to be one of the main factors that underlies age-related cognitive decline (Andres and Van der Linden 2000). To explain this idea, Hasher and Zacks (1988) proposed the inhibition deficit theory. According to this theory, the aging process weakens inhibition, which is responsible for the suppression and the clearing of non-relevant information, as well as the resistance to interference of information that is contextually inadequate. Consequently, a greater amount of irrelevant information is not restrained and/or deleted, which produces more interference. These inhibition deficits have been used to explain various impairments in older adults’ cognition, such as increased distractibility (Wascher et al. 2012), time needed for an appropriate response (Anguera and Gazzaley 2012), forgetting because of codification inefficiency and competition of related concepts (Raaijmakers and Jakab 2013), difficulty in understanding speech when background speech or noise is present (Tun et al. 2002), and difficulty in ignoring visually distracting information while reading (Li et al. 1998). Despite this decline in the efficiency of inhibitory processes with cognitive aging, not all inhibitory processes are impaired. Specifically, older adults are impaired in inhibition processes that involve controlled or top-down mechanisms (e.g. with impaired performance in Stroop or Stop-Signal paradigms when compared with young adults; Andres et al. 2008) but not in processes that can be considered more automatic or unintentional (e.g. equal performance when compared with young adults in NP or Spatial Cueing paradigms; Amieva et al. 2002; Andres et al. 2008; Collette et al. 2009).
Aim and Rationale of the Review
The aim of the present article is to critically review the published research that has probed the fine-grained temporal course of inhibition, with a particular emphasis on ERP studies. Because most of these studies have not intended to provide a timeline for the entire unfolding of an inhibitory processing event, we attempt to reconstruct this timeline by abstracting it away from a larger set of studies and then using it to frame the information in individual studies. This review attempts to clarify inhibition as a complex process that can be automatically initiated in the first 100 ms post-stimulus and extend its action through both automatic and controlled processes until 800 ms. The recurrent question regarding the existence of one general or different types of inhibition is also addressed. A distinctive interest of this review is the effects of normal aging on inhibition, as reflected by changes in processing that occur at a fine-grained temporal scale. As previously discussed, normal aging selectivity affects some inhibitory processes while sparing other processes (Andres et al. 2008; Collette et al. 2009), and temporally detailed analyses of inhibitory processing may greatly enhance the characterization of these differential effects. Thus, the study of the temporal course of inhibition in normal aging can facilitate the clarification of both the overall nature of cognitive aging and the complex nature of inhibition, which we consider to be crucial. Furthermore, several issues that pertain to the distinction of types and subprocesses in inhibition can be significantly clarified by considering the patterning of hindered/spared inhibitory processes with other age-related changes in cognitive function and brain structure.
A straightforward approach to gather and systematize information about the timing of inhibitory processes is to examine the ERPs observed in inhibition studies. As previously described, the ERP technique has a high temporal resolution (in the order of a few milliseconds). Therefore, it is possible to capture the various processes that contribute to inhibition. This emphasis on the temporal course of inhibition is related to the hypothesis that the time of activation of different brain structures related to inhibition is as important as the level of activity of these brain structures to accomplish the inhibition process. To link the time of activation of inhibitory processes to the brain structures that underlie inhibition, studies that explored the anatomical substrates of inhibition with electroencephalography (EEG) are addressed in this review. Specifically, we focus on studies that used source localization analysis of ERP data, which were collected with high-density EEG or magnetoencephalography (MEG).
The present review will focus on three time windows where inhibition ERP correlates have been found: 0–200 ms; 200–400 ms; and 400–800 ms. This article structure is based on the current ERP literature and facilitates an understanding of inhibition as it unfolds in real time, highlighting the plurality of processes that may correspond to the term “inhibition” in different tasks and moments. Furthermore, it highlights the automatic and controlled nature of different types of inhibition or different processes that contribute to inhibition because we hypothesize that the automatic processes (i.e., fast and unconscious processes) will occur in the first and possibly the second but not in the third window. These three time windows are used mainly as a means to organize the information that we will present and discuss; we do not intend to imply that there are three types of inhibition, one for each time window, or that there is a general process of inhibition that necessarily spans over the three time windows. Occasionally inhibition can be completed before 200 ms and other times it can be extended until after 400 ms. For each time window (0–200 ms; 200–400 ms; 400–800 ms) a description of the main inhibition-related paradigms yielding ERP modulations therein will be provided as well as a discussion of those modulations, addressing systematically the brain sources involved and the nature of inhibition as an automatic or controlled process. Finally, the age-related changes in inhibition are addressed.
Literature Search
A literature search was performed using the Web of Science, Proquest, Ovid, Science Direct and PubMed databases. The search included internationally published peer-reviewed research papers through August 15, 2014. Additional studies were identified by hand-searching the references that were cited in the previously collected articles. The main keywords that were used in this literature search were’inhibition’,’suppression’,’interference’ as well as ‘event-related potentials’ and terms labelling different inhibitory paradigms. Within this first level of literature analysis, we conducted a second search that identified the articles that contain the term ‘Aging’. Fifty ERP studies that used paradigms like ‘Stop-signal’, ‘Go/No-go’, ‘Eriksen Flanker Task’, ‘Stroop Task’ and ‘proactive interference resolution’ are examined in this review. Whenever possible, depending on the information made available in the original articles, we provide a detailed description of the sample that was used in each study we review, comprising sample size, age (mean, standard deviation or range), years of education (mean, standard deviation or range) and gender-balance. Since the amount of information concerning the sample and the specific parameters used to convey that information may vary from study to study, our rendering of that information will vary accordingly.
Inhibition in the First 200 ms
ERPs for Inhibition in the First 200 ms
In this early time window, ERP components, such as the N1 and P1, have been associated with the ability to inhibit responses to incoming sensory information (Di Russo et al. 2003). N1 and P1 effects have primarily been identified in behavioral inhibition paradigms, such as the Stop-signal (Bekker et al. 2005), Go/No-go (Thomas et al. 2009; Tian and Yao 2008; Kirmizi-Alsan et al. 2006; Lavric et al. 2004; Bokura et al. 2002; Filipovic et al. 2000), Eriksen Flanker (Abad-Rodriguez et al. 2004; Hsieh and Fang 2012; Johnstone et al. 2009; Wild-Wall et al. 2008) and Spatial cueing (Fu et al. 2005; McDonald et al. 1999; Wascher and Tipper 2004) tasks. However, there is also involvement of these early ERP components in cognitive paradigms, such as Location-based Priming (Gibbons et al. 2006; Kathmann et al. 2006) and emotional Stroop (Thomas et al. 2007) tasks. To better understand the inhibitory processes that are linked to this early time window, we analyzed data from the ERP studies that were conducted with inhibitory paradigms, such as the Go/No-go, Stop-signal and Eriksen Flanker paradigms.
In the Go/No-go paradigm, participants are asked to respond to a type of stimulus (Go stimuli) and withhold the response to a different type of stimulus (No-go stimuli). Several studies have shown the importance of the first 200 ms after the stimulus onset for the No-go processing (Hoshiyama et al. 1996; Schluter et al. 1998; Filipovic et al. 2000). E. Kirmizi-Alsan et al. (2006) studied the electrophysiological markers of response inhibition in a sample of young adults (N = 24; M ± SD = 25.8 ± 5.6 years old; M ± SD = 17.8 ± 3.3 years of education; 11 women) who participated in a visual Go/No-go task. They observed a significant N1 amplitude increase in the No-go ERPs compared with the Go ERPs. Because the participant must recruit inhibitory processes to withhold the No-go response, the N1 was indicated as an ERP component associated with inhibition despite its early onset (E. Kirmizi-Alsan et al. 2006). Thomas et al. (2009) also used a visual Go/No-go task to study inhibition in healthy adults (N = 20; 13 women). The level of inhibition required to withhold the No-go trials was manipulated by varying the number of immediately preceding Go trials. A greater number of consecutive Go trials before a No-go trial increased the inhibitory load. The authors demonstrated an increased latency of N1 and P2 in the first 200 ms in the No-go trials preceded by a greater number of consecutive Go trials, which supports a potential relationship between these components and inhibition (Fallgatter and Strik 1999; Thomas et al. 2009). Tian and Yao (2008) used ERPs with a peripheral cued Go/No-go task to study the neural mechanism of Inhibition of Return (IOR), which represents an inhibitory effect produced by a peripheral cue or target that hinders the accuracy and speed of response to targets that appear on the peripherally cued locations. Twelve young adults (M = 21.4 years old, range 18–25; 2 women) participated in this experiment, in which the stimulus (Go and No-go stimulus) was designed to appear with equal probability at the cued and uncued locations. This study identified a smaller and earlier P1 and a larger and earlier N1 in valid (i.e., the stimulus was preceded by a valid cue) compared with invalid (i.e., the stimulus was preceded by an invalid cue) trials regardless of the Go/No-go response. These observations confirmed that these early components were associated with the IOR effect on sensory/perceptual processes (McDonald et al. 1999; Wascher and Tipper 2004).
In the Stop-signal paradigm, participants are asked to respond to a stimulus (Go stimuli). However, when these Go stimuli are followed by a stop-signal, participants must withhold the response. Bekker et al. (2005) studied the electrophysiology of an auditory Stop-signal task in a sample of young adults (N = 20; M ± SD = 21.4 ± 5.6 years old; 16 women) and identified a larger N1 for successful compared with failed stops. This ERP component was interpreted as reflecting the amount of attention that is paid to (or switched to) the stop-signal, which is partially determinative of the subsequent success of inhibition in stopping the response. Thus, Bekker et al. (2005) suggested the strength of the inhibitory control on the Stop-signal paradigm might be determined, in part, by the ability to switch attention to the stop-signal. Complementing these results, Ramautar et al. (2006) suggested the N1 was associated with exogenous/sensory aspects of the stop signal. In their experiment, 15 young adults (M = 21.2 ± 1.78 years old; 8 women) participated in a bimodal Stop-signal task (with 12 visual and 12 auditory stop blocks of 120 trials each). The researchers identified an N1 component that did not differentiate between successful and unsuccessful stopping and was therefore associated with sensory processing of the stop-signal.
In the Eriksen Flanker Task (1974), a central target (e.g., letter or arrow) is flanked at both sides by items that indicate a response that is the same (congruent condition), opposite (incongruent condition) or neutral in relation to the response that is required by the target. For example, if participants are instructed to press a left button every time they view the letter “H” in a central position and a right button every time they view the letter “C” in a central position, the two main conditions are as follows: a congruent condition includes the same letter “H” or “C” for both flankers and the central target (e.g., HHHHH or CCCCC), whereas an incongruent condition includes opposite letters for the flankers and central target (e.g., HHCHH or CCHCC). In the incongruent condition, the incongruent flankers cause interference, which leads to slower and more inaccurate responses compared with the congruent condition. This effect is known as the flanker congruency effect (FCE) (White et al. 2011). Wild-Wall et al. (2008) conducted an ERP study using two variants of a Flanker Task with two age groups: a younger group (N = 15; M ± SD = 23.7 ± 3.7 years old; 7 women) and an older group (N = 15; M ± SD = 60.9 ± 6.5 years old; 7 women). In the first variant of the task, the flankers were presented 100 ms before the target (Experiment 1). In the second variant, the flankers were presented at the same time as the target (Experiment 2). Both experiments included congruent, incongruent and neutral conditions. The researchers’ main goals were to identify the temporal course of the FCE and the differences between the two age groups in the flanker and target processing. In both experiments, the P1 and N1 ERP components were identified in the first 200 ms. In Experiment 1, the onset of the two ERPs preceded the appearance of the target stimulus. Therefore, the authors suggested that P1 and N1 are primarily associated with flanker processing. The aging effects that were identified in this research on flanker and target processing are discussed later in this paper. These studies suggest that both N1 and P1 are associated with sensory information processing regardless of the task type. In particular, the P1 and N1 effects reflect the inhibition and enhancement of sensory information.
Automatic and Controlled Nature of Inhibition in the First 200 ms
The nature of the inhibitory processing in this early time window can easily be related to automatic processing. The fact that automatic processing has a short duration and can be elicited without the subject’s awareness supports this assumption. As previously described, Ramautar et al. (2006) studied the ERPs that were elicited during a Stop-signal task in a sample of young adults. They did not identify changes in the amplitude or latencies of the N1 component between successful and unsuccessful stopping. As a result, Ramautar et al. (2006) proposed that this ERP component was more strongly linked with exogenous sensory aspects of the stop-signal and, therefore, with automatic processing. Several studies have suggested that even this automatic processing may entail some executive control because a higher N1 amplitude for successful than for failed Stop-signal/Flanker conditions has been identified (Bekker et al. 2005; Wild-Wall et al. 2008). Despite these results, inhibition has been more frequently associated with automatic processing in the first 200 ms (Roche et al. 2005).
Source Localization of ERPs Associated with Inhibition in the First 200 ms
Some studies have attempted to better characterize the neural basis and dynamics of inhibition by exploiting the high temporal resolution of ERPs and the advances in source localization (Scherg 1990). Applying the Low Resolution Brain Electromagnetic Tomography method (LORETA; Pascual-Marqui et al. 1994) to ERP data that were collected during a cued Continuous Performance Test (CPT), Strik et al. (1998) reported that the main source of the P1 component was in the occipital area in both the Go and No-go conditions. Consistent with these results, using LORETA, Bokura et al. (2002) did not identify differences in the P1 component sources between the Go and No-go conditions and demonstrated that the P1 component for both the Go and No-go trials has generators that are located in the occipital lobes. Bokura et al. (2002) demonstrated, in both Go and No-go trials, an N1 component with bilateral brain generators in the occipito-temporal lobes, which likely encompass the primary and secondary visual areas. Tian and Yao (2008) studied the neural mechanisms of inhibition of return with a cued Go/No-go task. The 3D scalp topographic maps and LORETA images indicated that P1 and N1, which are linked to the inhibition of return processing, were localized in occipito-parietal regions, specifically, the P1 on the middle occipital gyrus and the N1 on the cuneus.
In summary, the P1 component that is elicited when inhibition processes are triggered may represent the visual processing of the stimulus, whereas the N1 may be related to the orientation of attention via the fronto-parietal attention network (Natale et al. 2006).
Age-Related Inhibition Changes in the First 200 ms
A limited number of studies have investigated age-related differences in these early ERP components in the context of inhibition. As previously discussed, Wild-Wall et al. (2008) studied inhibition in two age groups with two variations of the Flanker Task. In the first 200 ms, they identified a P1 and subsequent N1 components in the two variations of the Flanker Task for both groups. In Experiment 1 (when flankers appeared 100 ms before the target), the P1 and N1 onset was prior to the presentation of the target; therefore, both components were associated with flanker processing. Because both the P1 and N1 exhibited similar latencies and amplitudes for both groups, age does not appear to affect flanker processing. In Experiment 2 (when the flankers appeared at the same time as the target), the P1 latency and amplitude were similar in both groups; however, the N1 amplitude was markedly larger in the older group. This increased N1 amplitude in the older group was interpreted as an increased processing of the target. In Experiment 1, this target-related processing in the older group appears to be indexed by a negativity that appears after the N1. This result suggests the N1 amplitude increase in Experiment 2 is the result of a superposition of the flanker-related N1 activity with this dissociable target-specific signature. Behaviorally, an age-related slowing was identified and the older group exhibited surprisingly lower error rates compared with the younger group in the incongruent condition in both Experiments 1 and 2. Hence, it appears that the older participants do not exhibit inhibitory deficits in flanker processing, even though it is well known that this population displays a lower processing speed. However, in Experiment 2, a higher N1 amplitude during target presentation was identified in the older participants. Wild-Wall et al. (2008) proposed that during information processing, older participants pay greater attention to the target compared with younger participants. These enhancement processes, which are related to the target information, are complementary to the inhibition processes, which are related to the flanker information. The increased attention to the target might explain the lower error rates that are present in the older participants because they focus on the target and, therefore, reduce flanker interference.
Similar results were identified by Hsieh and Fang (2012), who investigated ERP correlates of the Flanker Task and potential compensatory strategies that older adults use to maintain the ability to inhibit irrelevant information. To achieve these goals, they compared young and older adults in three experiments in which the probability of congruent, incongruent and neutral trials in the Flanker Task was manipulated. A group of young adults (N = 16; M ± SD = 20.44 ± 1.71 years old; 10 females; M ± SD = 14.25 ± 1.24 years of education) and a group of older adults (N = 16; M ± SD = 64.63 ± 4.13 years old; 7 females; M ± SD = 14 ± 1.93 years of education) participated in the first experiment, in which the number of congruent trials was greater than incongruent trials. In the second experiment, a group of young adults (N = 16; M ± SD = 21.06 ± 1.61 years old; 9 females; M ± SD = 14.81 ± 1.05 years of education) and a group of older adults (N = 16; M ± SD = 64.13 ± 2.47 years old; 7 females; M ± SD = 13.81 ± 1.80 years of education) completed a Flanker Task with the same number of congruent and incongruent trials. Finally, a group of young adults (N = 16; M ± SD = 21.19 ± 2.20 years old; 7 females; M ± SD = 15.188 ± 1.40 years of education) and a group of older adults (N = 16; M ± SD = 64.19 ± 5.72 years old; 8 females; M ± SD = 13 ± 1.26 years of education) participated in the third experiment, in which the number of incongruent trials was greater than congruent trials. Consistent with Wild-Wall et al.’s (2008) findings, Hsieh and Fang (2012) did not observe an increased flanker effect in older adults compared with young adults across the three experiments. Additionally, throughout the three experiments, the older adults exhibited greater N1 amplitudes compared with the young adults during target presentation, which suggests the older adults engaged in increased top–down visual processing of the central target.
Gazzaley et al. (2008) compared young adults (N = 20; M = 23.1 years old, range 19–30; 10 women) and older adults (N = 26; M = 65.7 years old, range 60–72; 13 women) in the selective attention delayed-recognition task that was developed to measure both inhibition and enhancement. In this task, the participants viewed sequences of two faces and two natural scenes structured in three conditions presented in a randomized order. In one condition, the participants had to remember the faces (attend condition) and ignore the scenes (ignore condition). In a second condition, the participants had to remember the scenes (attend condition) and ignore the faces (ignore condition). In the third condition, the participants did not have to ignore any of the images (passive condition). Within the first 200 ms, the young adults exhibited the largest P1 amplitude and earliest N1 latency for the attended faces, followed by passive faces and then ignored faces, whereas the older adults only exhibited the largest P1 amplitude and earliest N1 latency for the attended faces compared with passive faces. Gazzaley et al. (2008) interpreted these results as an indication of sensory suppression deficits in older adults (because there were no differences between the passive and ignore conditions), as well as an indication of preserved enhancement processes (the same change in young and older adults between the passive and attend conditions). In an additional experiment with the selective attention delayed-recognition task, Anguera and Gazzaley (2012) studied the neural markers of inhibition in the first 200 ms (P1, N170) in a sample of older adults (N = 16; M ± SD = 70.6 ± 6.7 years old; 7 women). ERP age-related modulation analyses for face stimuli were conducted that focused on P1 amplitude and N170 latency as indices of top-down enhancement (attend vs. passive) and inhibition (ignore vs. passive). The authors demonstrated that older adults did not exhibit the signatures of early neural inhibition (reflected by the absence of differences in N170 latency and P1 amplitude) when viewing irrelevant visual stimuli. However, there was neural enhancement for the relevant stimuli, which was reflected by the early N170 latency for attended versus passively viewed faces.
Summary
In the preceding section, we summarized ERP research results that provide significant insights regarding inhibition processing during the first 200 ms post-stimulus in a variety of paradigms. Despite the limited number of ERP studies in the context of inhibition that have addressed this early time window, the P1 and N1 ERP components have consistently been found to reflect inhibition-related phenomena. As early as 100 ms post-stimulus, these components index sensory information processing and have primarily been associated with automatic processing. The P1 has been associated with the inhibition of irrelevant sensory information and linked to the occipital lobes. The N1 has been associated with a complementary process that facilitates or enhances relevant sensory information (Hillyard et al. 1994) and has been linked to the frontal and parietal components of the attention network. The age-related differences that have been identified in the ERP components support this dissociation. Specifically, the N1 is related to enhancement processes, which are preserved in older adults compared with young adults, and the P1 is related to the onset of inhibition processes, which are less effective in older adults compared with young adults.
Inhibition Between 200 and 400 ms
ERPs for Inhibition Between 200 and 400 ms
ERP research has identified two components within this time window that might be related to inhibition: the N2, which represents a pronounced fronto-central negativity that peaks approximately 200–350 ms post-stimulus, and the P3, which peaks at approximately 250–500 ms and exhibits a fronto-central to centro-parietal scalp topography (Johnstone et al. 2007; Polich 2007; Folstein and Van Petten 2008). In early research, these two components were often referred to together as the “N2-P3 complex” (Folstein and Van Petten 2008; Huster et al. 2013).
The N2 is an endogenous ERP component and can be separated into the following subcomponents according to Folstein and Van Petten (2008) review: (i) a fronto-central component that is associated with novelty detection (N2a); (ii) a second fronto-central component that is associated with executive control (which encompasses motor inhibition, response conflict and error monitoring) (N2b); (iii) and a posterior N2 that is associated with stimulus classification operations related to target processing (N2c). Furthermore, there is an attention-related ERP, the N2-posterior-contralateral (N2pc), which is typically observed in the N2 time window at posterior scalp sites that are contralateral to the position of a potential target item on which attention is focused (Patel and Azzam 2005). The N2a that is elicited by deviant auditory stimuli, attended or unattended, is referred to as mismatch negativity (MMN; for a review, see Naatanen et al. 2012). P3 is an umbrella term that encompasses at least two functionally distinct subcomponents with different scalp distributions, P3a and P3b (Polich 2007; O'Connell et al. 2012). P3a and P3b differ in terms of latency (P3a has a shorter latency) and topography (P3a has a fronto-central distribution compared with the more parietal distribution of P3b) (Fjell et al. 2009). Polich and Comerchero (2003) have suggested that P3a and P3b are connected to a circuit pathway between the frontal and temporal/parietal brain areas. The P3a reflects involuntary, transient allocation of attention to salient changes in stimuli and novel stimuli, which is linked to frontal lobe activity. The P3b is related to a controlled cognitive attentional process that is tied to the stimulus evaluation process, which is linked to temporal/parietal areas (Kirino et al. 2000; Polich 2007).
For both the N2 (typically the N2b) and P3 (typically the P3a) components, larger amplitudes have been identified when inhibiting a response compared with executing a response (Maguire et al. 2009). The relationship between the N2, the P3, and inhibitory processing remains a matter of debate (Bruin et al. 2001; Smith et al. 2007). Some experts have argued that inhibitory processes are associated with the N2 (Kopp et al. 1996; Van Veen and Carter 2002; Falkenstein et al. 2002; Roche et al. 2005), whereas other experts have argued that the P3 has an association with inhibition (i.e., the N2 is associated with other processes, such as recognition of the need for inhibition or even response conflict) (Bruin et al. 2001; Smith et al. 2008). There is, however, a general consensus that both components are associated with inhibition to some degree (van Boxtel et al. 2001; Kok et al. 2004; Kirmizi-Alsan et al. 2006; Dimoska et al. 2006; Smith et al. 2006, 2007; Maguire et al. 2009).
The N2 and P3 ERP components have predominantly been studied in inhibitory paradigms, such as the Stop-signal, Go/No-go and Eriksen Flanker tasks. Both the Stop-signal and Go/No-go paradigms elicit inhibitory processes that can be explained by the well-established horse-race model (Logan 1994). In this model, the “Go” process races against the “No-go/Stop-signal/Inhibition” process. If the “No-go/Stop-signal/Inhibition” process is completed before the “Go” process, this finding signifies inhibition of the response. Typically, in the Stop-signal and Go/No-go tasks, although the latency and variability of the Go response can be observed directly, the inhibition response that is observed in the No-go/Stop-signal trial is internally generated; therefore, it cannot be directly observed. However, in the Stop-signal task, it is possible to quantify the latency of the inhibition mechanism with the Stop-signal Reaction Time (SSRT; Logan et al. 1984), which can be estimated using the assumptions of the race model (Logan 1994; Logan et al. 1984). Some authors have suggested the Go/No-go and Stop-signal paradigms involve equivalent inhibitory processes (Verbruggen and Logan 2008a). In both paradigms, participants are instructed to respond to the Go stimuli and to withhold a response when a No-go/Stop-signal is presented. To be successful, participants must identify the strategy that optimally balances the following two goals: respond as quickly and as accurately as possible to the Go stimuli and withhold the response to the No-go or Stop-signal as effectively as possible.
In accordance with this assumption, van Boxtel et al. (2001) identified similar ERP patterns in No-go and Stop-signal trials, which suggests the underlying mechanisms of these two paradigms are similar. They examined a sample of young adults (N = 10; M = 22.2 years old, range 19–28) in a combined visual Stop-signal and visual Go/No-go task in which 20 % of the trials included a Stop-signal and 10 % were No-go trials. Following the combined Stop-signal and Go/No-go task, van Boxtel et al. (2001) divided the young adult group into efficient and less efficient inhibitors using a median split of the SSRT. A larger N2 amplitude was identified for the efficient inhibitors, which suggests inhibition bears a N2 signature in both the Stop-signal and Go/No-go paradigms. Despite this association between the No-go and Stop signal N2s, to our knowledge, only the van Boxtel et al. (2001) study directly compared the Go/No-go and Stop-signal paradigms. Therefore, we cannot undoubtedly declare that the inhibition processes that are recruited during No-go and Stop-signal trials are the same. Additionally, according to Folstein and Van Petten (2008), the Stop-signal N2, in contrast with the No-go N2, might comprise various subcomponents that are associated with inhibition and evaluation of the stop-signal. Therefore, we review the Stop-signal and Go/No-go ERP studies that have identified N2 and P3 modulations related to inhibition independently.
ERP correlates of inhibition processes that are recruited in the Stop-signal task have been extensively studied (Kok et al. 2004; Ramautar et al. 2004; Bekker et al. 2005; Ramautar et al. 2006; Luus et al. 2007; Dimoska and Johnstone 2008; Knyazev et al. 2008). Luus et al. (2007) conducted an MEG study of inhibition elicited by a visual Stop-signal paradigm (with 25 % Stop-signal trials) in a sample of young adults (N = 11; M ± SD = 28 ± 5.3 years old; 5 women). The results indicated greater differences between successful stop-signal responses and fail stop-signal responses in the 100–220 ms range of the grand average waveforms. Specifically, the researchers identified an earlier and larger N2 in successful stop-signal responses compared with failed responses, which suggests the association of N2 amplitude and latency with successful inhibition. Knyazev et al. (2008) contributed to the understanding of successful and unsuccessful stopping performance in young adults (N = 51; M ± SD = 20 ± 2.6 years old; 35 females) through a study of the ERP correlates of an auditory Stop-signal task with a fixed stop-signal delay. As Knyazev et al. (2008) noted, failed stop responses are typically associated with a longer stop-signal delay, which has been conceptualized as an explanation for failure. Comparing successful and unsuccessful stop-signal responses with a fixed stop-signal delay, they identified differences not only in the Stop-signal trial but also in the preceding Go trial. Specifically, they identified smaller N2 and P3 amplitudes in Go trials that preceded successful Stop-signal trials, a larger P3 amplitude in successful Stop-signal trials and shorter latencies for both N2 and P3 in successful, relative to failed, Stop-signal trials. Knyazev et al. (2008) interpreted these results as evidence for a direct relation between the level of attention toward the stop-signal and the success in stopping.
Kok et al. (2004) examined the ERP correlates of inhibition in a sample of young adults (N = 12; M ± SD = 23 ± 7 years old; 6 women) using a visual Stop-signal task in which the Stop-signal and Go trials had equal probabilities of occurrence (see Fig. 1 for a schematic display of the task). They identified a larger N2 followed by a larger P3 in Stop-signal trials compared with Go trials. Therefore, both N2 and P3 appear to be related to the processing that occurs in the Stop-signal trials, particularly inhibition. A deeper analysis of the Stop-signal trials that contrasted successful and unsuccessful responses revealed higher amplitudes for the N2 and P3 in unsuccessful compared with successful stop-signal responses. Kok et al. (2004) interpreted this amplitude difference in the N2 as reflecting aspects of response monitoring and conflict. The P3 exhibited different scalp distributions for successful and unsuccessful stop-signal responses. Therefore, the authors formulated two interpretations of this result. The P3 fronto-central distribution in successful responses might reflect inhibition processes that are triggered by the stop-signal appearance, whereas a more posterior distribution of the P3 in unsuccessful responses might reflect response monitoring.
As previously discussed, Bekker et al. (2005) examined the ERP correlates of an auditory Stop-signal task with a 40 % probability of occurrence of Stop-signal trials. They identified a larger P3 amplitude in successful compared with unsuccessful stop-signal responses. Therefore, the P3 amplitude change was interpreted as an index of inhibition processes. These similar results identified in both visual and auditory Stop-signal tasks suggest that the processes that are indexed by the P3 in the stop-signal processing are endogenous (i.e., independent of the modality). Ramautar et al. (2006) specifically studied the effects of modality in a sample of young adults (N = 15; M ± SD = 21.2 ± 1.78 years old; 8 women) using a mixed Stop-signal task with auditory and visual Stop-signal trials, which had the same probability of occurrence as go trials. Concerning N2 modulations in the Stop-signal trials, they identified a smaller N2 amplitude in the auditory Stop-signal trials compared with the visual trials. Longer N2 and P3 latencies were identified for unsuccessful Stop-signal trials, regardless of the modality. Regarding the N2 and P3 amplitudes, a different pattern was identified. The authors identified a larger N2 in unsuccessful compared with successful Stop-signal trials, regardless of the stop-signal modality, and suggested that this result reflects conflict detection. Regarding the P3, they identified a larger amplitude in successful compared with unsuccessful Stop-signal trials, regardless of the stop-signal modality. Therefore, these authors concluded that the P3 appears to be an index of modality-unspecific inhibition processes.
The effects of stop-signal probability are also important in the study of ERP correlates of inhibition using the Stop-signal paradigm. Ramautar et al. (2004) examined the ERP correlates of a visual Stop-signal task in a sample of young adults (N = 14; M ± SD = 20.14 ± 1.99 years old; 7 women) to specifically explore the effects of stop-signal probability. There were two conditions in this experiment: one condition in which the Stop-signal trials had a probability of 20 % (low probability condition) and a second condition in which the Stop-signal trials had the same probability as the Go trials (i.e., 50 %; high probability condition). The results were similar to Kok et al. (2004) concerning the dissociation between the successful and unsuccessful stop-signal responses. With respect to their stop-signal probability manipulation, Ramautar et al. (2004) identified a larger P3 amplitude for low compared with high probability stop-signals. In addition, the P3 that was elicited during successful stop-signals had a more anterior distribution in the low probability condition. These findings were interpreted as a reflection of increased inhibitory load in the low probability condition. However, these ERP modulations that reflect the stop-signal probability manipulation may, in fact, be novelty effects (i.e., stop-signal presented rarely) (Dimoska and Johnstone 2008).
To determine whether a low probability condition is related to an increase in inhibitory load, Dimoska and Johnstone (2008) examined not only the effects of varying stop-signal probabilities on ERP correlates of an auditory Stop-signal task but also the effects of varying the probability of a task-irrelevant ignore-signal. In their experiment, young adults (N = 30; M ± SD = 22.1 ± 3.3 years old; 20 women) performed the Stop-signal task with frequent and rare stop-signal conditions. In the frequent condition, the stop-signal was presented in 42 % of the trials and the ignore-signal (i.e., a tone that differed from the stop-signal that participants were instructed to ignore) was presented in 18 % of the trials. In the rare condition, the stop-signal was presented in 18 % of the trials and the ignore-signal was presented in 42 % of the trials. The authors identified an increased P3 amplitude in the rare compared with frequent conditions, but this amplitude difference did not differ between the stop and ignore-signal trials. These findings suggest the larger P3 amplitude in successful responses may reflect novelty effects. Nevertheless, Dimoska and Johnstone (2008) suggested an activation of inhibitory processes in the Stop-signal trials that was indexed by the P3 amplitude change, regardless of the probability differences effect, which results from the different topographic distributions of P3 identified in stop and ignore-signal trials.
ERP research using the Go/No-go task has also yielded results that are relevant to understanding the N2 and P3 association with inhibition (Falkenstein et al. 1999; Bruin and Wijers 2002; Nieuwenhuis et al. 2003; Roche et al. 2005; Folstein et al. 2008; Smith et al. 2008). Falkenstein et al. (1999) studied the ERP correlates of inhibition in a sample of young adults (N = 10; M = 24.1 years old, range 18–33; 4 women) using visual and auditory versions of the same Go/No-go task to determine the modality effects on the ERPs. The authors divided the participants into the following two groups based on their performance: the “Good” group, with low error rates in the No-go trials, and the “Poor” group, with high error rates. They identified a larger amplitude and earlier latency of the No-Go N2 for the “Good” compared with the “Poor” participants, which supports the hypothesis that the No-go N2 reflects inhibition, which is better in the “Good” group. In contrast, the No-go P3 amplitude and latency were similar for both the “Good” and “Poor” groups. Falkenstein et al. (1999) suggested that this component is not related to inhibition processes. A smaller No-go N2 amplitude after auditory compared with visual stimuli was identified, which suggests the inhibition processes likely indexed by the No-go N2 are modality-specific and, therefore, occur at earlier non-motor processing stages.
Roche et al. (2005) suggested that the latency of the N2 and P3 might determine the success or failure of inhibitory control. Their experiment used a visual Go/No-go task (see Fig. 1 for a schematic display of the task) in which the letter X and the letter Y were presented sequentially at the center of the screen. The participants (N = 20; M = 21.5 years old, range 17–31; 17 women) were asked to press a button every time the letters appeared (Go condition - 94 % of the trials), with the exception of when two identical stimuli followed each other (e.g., an X followed an X); in this condition, they were required to withhold the response (No-go condition – 6 % of the trials). Roche and colleagues (2005) identified a larger amplitude and later latency for the No-go N2 and P3 compared with the Go N2 and P3. Additionally, they identified a shorter latency of the N2 and the P3 for successful No-go responses compared with unsuccessful responses, which suggests the relevance of the latency of these two ERP components for successful inhibition. Roche et al. (2005) suggested that the No-go N2 onset is the most valid index of active inhibitory processes. They also interpreted the No-go P3 onset for errors that were more than 100 ms higher than the corresponding mean response latency as a reflection of No-go P3’s role in performance evaluation, error detection and/or preparation for future trials.
Bruin and Wijers (2002) also examined the ERP correlates evoked in a visual Go/No-go task and specifically addressed the response mode and Go/No-go stimulus probability effects. In their experiment, young adults (N = 12; M = 21.5 years old, range 19–28; 8 women) participated in a visual Go/No-go task with two response mode conditions, including a manual condition (i.e., lifting their right or left index finger from a response panel in Go trials) and a mental count condition (i.e., count the total number of go stimuli in each task block and report the answer following the block). The stimulus probability effect had the following three conditions per response mode: 25, 50 and 75 % No-go trials. As expected, the authors identified smaller N2 and P3 amplitudes for the high probability condition compared with the lower probability conditions. Concerning the different response modes, they identified larger N2 and P3 amplitudes in the No-go compared with Go trials in both response modes. However, the No-go P3 was smaller in the counting condition compared with the manual condition. Bruin and Wijers (2002) interpreted their results as supportive of Pfefferbaum et al. (1985) study in which similar results were identified, which indicates both N2 and P3 reflect both cognitive and motor inhibition processes. The authors interpreted the smaller No-go P3 that was identified in the counting condition as a reflection of a smaller level of inhibition needed to withhold a response compared with the manual condition.
Smith et al. (2008) further explored the contribution of movement-related potentials to N2 and P3 modulations within the Go/No-go paradigm while controlling for stimulus probability. In their study, a sample of young adults (N = 20; M ± SD = 22.4 ± 5.6 years old; 12 women) participated in an auditory Go/No-go task with rare (20 %) No-go, rare (20 %) Go, and frequent (60 %) Go stimuli (a different tone than a rare Go stimulus). The participants pressed a response button (overt condition) or counted (covert condition) if either rare or frequent go stimuli appeared. The authors compared the No-go and Go trials with the same probability (20 %) to ensure that the effect identified in the N2 and P3 could not be explained by differences in stimulus probability. The No-go P3 effect (i.e., the No-go P3 higher than the Go P3) was identified in both response conditions, but it was reduced in magnitude in the covert condition. Smith et al. (2008) suggested that the No-go P3 reflects inhibition and movement-related potentials that are responsible for the difference identified between overt and covert versions of the Go/No-go task. In respect to the No-go N2 effect (the No-go N2 higher than the Go N2), they identified the same effect in overt and covert versions of the Go/No-go task. Therefore, Smith et al. (2008) suggested the No-Go N2 effect does not reflect motor inhibition, but it may reflect recognition that no response is needed or the conflict between executing and withholding the response.
Nieuwenhuis et al. (2003) investigated the conflict hypothesis in a sample of young adults (N = 12; M = 20.9 years old, range 18–24; 9 women) using a visual Go/No-go task. In their experiment, the following three conditions were used to manipulate the No-go and Go stimulus probability: rare No-go trials (20 %), frequent No-go trials (80 %) and equally frequent No-go and Go trials (50 %). Nieuwenhuis et al. (2003) identified the traditional No-go N2 effect in the 20 % and 50 % (with smaller magnitude) No-go trial conditions. However, in the 80 % No-go trial condition, the No-go N2 amplitude was slightly smaller than the Go N2 (Go trials were less frequent in this condition). The hypothesis defending an association between the No-go N2 and inhibition processes cannot easily explain why a small N2 amplitude increase can be observed in infrequent Go trials relative to the amplitude in frequent No-go trials because no inhibition is needed in Go trials. Additionally, a source localization analysis revealed that the localization of the No-go N2 might be in the anterior cingulate cortex (ACC), which has been associated with conflict processing (Botvinick et al. 2001). Based on these ERP results and source localization analyses, Nieuwenhuis et al. (2003) suggested that the N2 observed in Go/No-go tasks reflects response conflict.
Donkers and van Boxtel (2004) also tested the conflict hypothesis in a sample of young adults (N = 13; M = 21 years old, range 18–32; 6 women) with two tasks, including visual Go/No-go and visual go/GO tasks. In the Go/No-go task, the participants were asked to withhold the response to the “No-go” stimuli. In contrast, in the go/Go task, the participants were asked to respond with maximal force to the “GO” stimuli. In both tasks, the participants were asked to respond to the “go” stimulus with “nominal” force. The “go” probability varied between 80 % and 50 % to test the hypothesis of higher conflict levels for low compared with high frequency stimuli. They identified a larger N2 and P3 for both “No-go” and “GO” trials compared with “go” trials. The “No-go” P3 amplitude was larger than the “GO” P3 amplitude. Therefore, Donkers and van Boxtel (2004) suggested that the “No-go” P3 might index response inhibition. Consistent with Nieuwenhuis et al.’s (2003) results, the “No-go” N2 and the “GO” N2 amplitudes were higher in the 80 % “go” probability condition compared with the 50 % “go” probability. Therefore, Donkers and van Boxtel (2004) suggested that the No-go N2 is primarily associated with conflict monitoring and any association of the No-go N2 with inhibition is limited.
Smith et al. (2007) suggested that the No-go N2 is not related to inhibition or conflict processes. In their experiment, young adults (N = 26; M ± SD = 22.6 ± 7.2 years old; 15 women) participated in a cued auditory Go/No-go task (adapted from Bruin et al. 2001) with three different targets, which included Go Left (i.e., tone presented in the left ear, which required a left button press), Go right (tone presented in the right ear, which required a right button response), and a No-go (tone presented binaurally, which required a withheld response). The Go targets were preceded by cues that were valid (e.g., left tone preceded a left target), invalid (e.g., left tone preceded a right target) or non-specific (e.g., binaural tones preceded a left target). There was a specific No-go cue that was always valid. These informative cues were used to examine variations in response inhibition and conflict when the planned response was inappropriate. The authors identified a larger N2 amplitude in No-go compared with Go targets, regardless of whether the cue that preceded the Go target was specific (i.e., valid or invalid) or non-specific. Despite this significant No-go N2 effect, a larger N2 amplitude was identified after No-go cues (when participants knew no response was needed, which reduced response preparation at minimum) compared with after Go cues. Furthermore, larger N2 amplitudes were identified for invalid compared with valid cues, which is in contrast to the response conflict theory. Accordingly, Smith et al. (2007) suggested that the No-go N2 was not related to inhibition or conflict. In contrast, these results concerning P3 amplitude suggest that the No-go P3 effect may be associated with inhibitory and/or conflict processes.
To distinguish between inhibition and conflict accounts for both N2 and P3 components, Smith et al. (2010) studied the sequence effects of a visual Go/No-go task in a sample of young adults (N = 23; M ± SD = 22.5 ± 8.1 years old; 17 women). As previously described by Nieuwenhuis et al. (2003) and Donkers and van Boxtel (2004), greater inhibition and/or conflict occur with unexpected stimuli. In a Go/No-go task, even when the sequence of Go and No-go stimuli is randomized, participants can spontaneously generate expectancies for the upcoming stimulus based on the previous sequence of stimuli. Therefore, if the N2 and P3 reflect inhibition in No-go trials, there must be an increase in their amplitudes in unexpected compared with expected No-go stimuli beyond the typical increase of these amplitudes in No-go compared with Go trials. However, if the N2 and P3 amplitude is higher for all unexpected stimuli, regardless of whether Go or No-go, then it must reflect conflict. Smith et al.’s (2010) results supported the conflict interpretation for both N2 and P3.
An additional paradigm that is used to study inhibition and response conflict is the Eriksen Flanker Task. In this task, a prominent N2 component is observed after the incongruent condition (incongruent flankers surround the target) compared with the congruent condition (congruent flankers surround the target) (Wild-Wall et al. 2008). The frontal negative component that is observed in the incongruent condition of the Eriksen Flanker Task is likely to correspond with the N2 that is observed after No-go stimuli in the Go/No-go task or after the stop-signal in the Stop-signal task (Kopp et al. 1996; Van Veen and Carter 2002; Bartholow et al. 2005). Van Veen and Carter (2002) studied the ERP correlates of the Eriksen Flanker Task in a sample of young adults (N = 12; M ± SD = 23.4 ± 2.8 years old; 6 women). This experiment included the following three conditions: a congruent condition (50 %), in which the flankers were equal to the target; a stimuli incongruent condition (25 %), in which the flankers were different but mapped onto the same response hand; and a response incongruent condition (25 %), in which the flankers were mapped onto the opposite response hand than the target stimulus. The researchers identified a fronto-central N2 enhanced only to the response incongruent condition and a N2 dipole located in the ACC, which suggests the N2 that is elicited in the Eriksen Flanker Task is sensitive to response conflict. Supporting the same conflict interpretation, Bartholow et al. (2005) also identified an enhanced N2 in the incongruent condition of the Eriksen Flanker Task in a sample of young adults (N = 45; range 21–30 years old; 21 women). However, in contrast to the conflict interpretation of N2 in this task, Bartholow et al. (2005) identified a larger N2 when the incongruent trials were highly probable (80 %) in contrast with low (20 %) or equally probable (50 %) incongruent trials. This finding questions the association between the N2 and conflict because conflict prior to the response should be less in the highly probable incongruent trials condition; therefore, the N2 amplitude elicited therein should be smaller.
Purmann et al. (2011) identified a larger N2 in low frequency incongruent trials of the Eriksen Flanker Task. In their study, participants (N = 12; M = 25 years old, range 22–38; 2 women) responded to frequent (75 %) and rare (25 %) incongruent blocks. Consistent with conflict theory, the authors identified a larger N2 in incongruent compared with congruent trials, and this difference in amplitude was larger with infrequent conflict (i.e., in the rare incongruent blocks). Additionally, they identified a longer P3 latency for incongruent compared with congruent stimuli, which suggests the evaluation of incongruent stimuli requires more time.
Tillman and Wiens (2011) challenged the notion that the N2 that is elicited in the Eriksen Flanker Task is a valid index of response conflict in a study that yielded results consistent with Bartholow et al. (2005). In Tillman and Wiens’ (2011) experiment (see Fig. 1 for a schematic display of the task), young adults (N = 27; M ± SD = 27.22 ± 5.96 years old; 16 women) responded to a Flanker Task that was presented in two blocks: one block with low (20 %) and one block with high (80 %) probable incongruent trials. The authors identified a larger N2 in the 80 % compared with 20 % incongruent trial condition. As an alternative to the conflict hypothesis, Tillman and Wiens (2011) suggested that the N2 might index attentional control or inhibition processes. Neuhaus et al. (2010) studied the ERP correlates of the Attention Network Test, addressing both visual attention in a cued detection task and inhibition in an Eriksen Flanker Task. In the Eriksen Flanker Task, the participants (N = 44; M ± SD = 30.39 ± 7.1 years old; M ± SD = 15.16 ± 2.1 years of education; 22 women) were instructed to indicate the direction of a central arrow while ignoring the flanking stimuli (lines in the neutral condition; congruent or incongruent flankers). They identified a frontal P3 amplitude increment and parietal P3 amplitude decrement following incongruent targets. The authors interpreted the frontal P3 amplitude increment as an index of response inhibition and suggested that because of its frontal distribution, it is likely the same modulation that is present in Go/No-go tasks (i.e., the No-go P3 effect).
Automatic and Controlled Nature of Inhibition Between 200 and 400 ms
Several authors have assumed that inhibition in this time window is a top-down executive control process (Ridderinkhof et al. 1999; Enriquez-Geppert et al. 2010). As previously described, the Go/No-go and Stop-signal paradigms are frequently used to study inhibition. Both paradigms appear to imply the use of controlled processes to proactively change between goals for an optimal performance, i.e., to respond as quickly as possible to the Go stimuli and withhold the response to the No-go stimuli or when the stop-signal is present. However, stimulus repetition may also be a crucial variable that affects performance in these motor inhibition paradigms. In support of this possibility, Shiffrin and Schneider’s (1977) theory proposes that automatic processing may develop with practice.
In the Go/No-go paradigm, stimuli are consistently associated with going and stopping (i.e., there is a Go and a different No-go stimulus, and this functional distinction remains the same throughout the entire experiment); thus, automatic inhibition is likely to develop after many repetitions. In contrast, if the stimulus is inconsistently mapped onto different responses, such as in a typical Stop-signal task in which the stop-signal is not associated with a specific stimulus, automatic processing is unlikely to develop. However, even in the Stop-signal task, the stimuli can be associated with stopping. Verbruggen and Logan (2008b) studied a Stop-signal task in which the participants viewed words that represented living and nonliving objects. Each word was presented once or twice, and a random selection of the words was repeated after a variable number of trials (i.e., the word from trial n was repeated on trial n + 1, n + 5, n + 10 or n + 20). The participants responded by pressing one key for “living” and a different key for “nonliving” (Go trials). On some trials, an auditory tone was presented as a stop-signal and the participants were required to withhold the response. After a first successful stop, a longer RT was identified in the Go trial that repeated the same target compared with the Go RT that followed a first presentation of the target coupled with either a successful Go response or an unsuccessful stop. This inhibition aftereffect was significant up to the n + 20 repetition lag condition. In a separate experiment, Verbruggen and Logan (2008a) developed a modified Stop-signal task using the same stimuli (i.e., words that represented living and non-living objects) divided in training and test phases. The authors varied the stimulus-stop mapping and hypothesized that automaticity in the Stop-signal task may develop when there is consistent stimulus-stop mapping (i.e., in both the training and test phases, the living stimuli were associated with the go response and the non-living stimuli were associated with the stop-signal). In the test phase, a slower response to go stimuli was identified when the same type of stimuli was consistently associated with stopping in the training phase. Additionally, consistent with the authors’ hypothesis, response inhibition benefited when the stimuli that were associated with stopping were the same in the training and test phases. In the Stop-signal task, the mapping between stimulus and stop-signal is typically inconsistent, which hinders the development of automaticity. Regarding the Go/No-go task, Verbruggen and Logan (2008a) suggested that the development of automaticity may be avoided using a large set of No-go and Go stimuli to avoid repetitions.
Source Localization of ERPs Associated With Inhibition Between 200 and 400 ms
In one of the first experiments conducted to understand brain sources of inhibition processes, Kiefer et al. (1998) conducted a source analysis of the N2 and P3 that were elicited by No-go trials using Brain Electrical Source Analysis (BESA), a spatio-temporal dipole fit model, in an auditory Go/No-go task. They reported an inferior prefrontal cortex (PFC) generator for the N2 and a fronto-central P3 source located in the ACC and left motor and premotor sources. Bokura et al. (2002) also conducted an experiment to understand the anatomical structures that are involved in N2 and P3 generation in a Go/No-go paradigm, but they used a visual modality of the paradigm and a different source localization technique referred to as LORETA. They identified right lateral orbitofrontal and cingulate generators for the N2 and left lateral orbitofrontal sources for the P3. In an MEG study of inhibition elicited by a visual Stop-signal paradigm, Luus et al. (2007) identified a main source for success-related N2 modulation located in the dorsal ACC using BESA. Van Veen and Carter (2002) used source localization analysis with BESA to study inhibition and response conflict in the Eriksen Flanker Task. They determined that the N2 amplitude associated with incongruent trials (i.e., both inhibition and response conflict occur) can be explained by a dipole that is located in the ACC. These experiments with different modality Go/No-go tasks, a visual Stop-signal task and an Eriksen Flanker Task suggest that the orbitofrontal area and the ACC (in both hemispheres) are important regions for No-go, Stop-signal and incongruent flanker processing. Other brain areas have also been associated with these paradigms. Recently, Albert et al. (2013) used a modified visual Go/No-go task to dissociate brain electrical activity related to motor inhibition from the processing of infrequent stimuli (via the contrast of infrequent No-go with infrequent Go). Source localization data, which were obtained using LORETA, revealed increased activation for No-go compared with Go trials in the pre-supplementary motor areas (preSMA) during the P3 time range, but not the N2 time range. At the scalp level, the authors also determined that only brain electric activity associated with P3 exhibited differences between No-go and Go trials. Therefore, Albert et al. (2013) suggested that the preSMA plays an important role in motor inhibition.
These source localization studies suggest related but different brain generators for the inhibition reflections on the N2 and P3 components. The orbitofrontal cortex, the ACC, and the preSMA have been suggested as the core regions associated with inhibition (Albert et al. 2013; Bokura et al. 2002; Kiefer et al. 1998; Luus et al. 2007). It has been suggested that during the first 200 ms in a No-go or Stop-signal trial, a posterior portion of the pre-SMA, the right orbitofrontal and the ACC are activated to resolve the conflict between the execution and inhibition of a motor response. After this process and before 400 ms post-stimulus, the left orbitofrontal cortex and the anterior portion of the pre-SMA are activated to yield a successful inhibition (Kok et al. 2004; Lavric et al. 2004; Nieuwenhuis et al. 2003; Ramautar et al. 2006; Falkenstein et al. 2002; Vallesi et al. 2009). In unsuccessful inhibition trials, during the first 200 ms, supplementary motor areas are activated to permit response execution rather than inhibition (Lavric et al. 2004; Zhang and Lu 2012).
Age-related Inhibition Changes Between 200 and 400 ms
ERP studies of age-related inhibition changes with the Go/No-go task have consistently demonstrated longer latencies for both the No-go N2 and No-go P3 components in older adults (Pfefferbaum and Ford 1988; Tachibana et al. 1996; Fallgatter et al. 1999; Horvath et al. 2009). Tachibana et al. (1996) studied ERP age-related changes in a visual Go/No-go task in participants (N = 29) who ranged in age from 21–74 years old. Two classes of stimuli, semantic and physical, were presented. The authors identified longer latencies for both No-go N2 and P3 for the group over 40 years of age (N = 14; M ± SD = 56.4 ± 12.2 years old) compared with the group under 40 years of age (N = 15; M ± SD = 26.9 ± 5.1 years old). However, this aging effect was only present with semantic stimuli. Tachibana et al. (1996) interpreted this result within Shiffrin and Schneider’s (1977) model. Specifically, they suggested that semantic stimuli processing involves controlled processes, and therefore, it is more sensitive to aging; in contrast, physical stimuli processing involves automatic processes, which are less sensitive to aging. Horvath et al. (2009) compared behavioral and ERP measures of inhibition in children (N = 18; M = 6 years old; 9 girls), young adults (N = 9; M = 21.2 years old, range 19–24; 5 women) and older adults (N = 9; M = 68.4 years old, range 62–82; 7 women) using an auditory Go/No-go task. They identified a longer latency for the No-go N2b and a longer latency and higher amplitude for the No-go P3 with a more parietal distribution in older compared with young adults. It has been suggested that this age-related effect (i.e., latency increased for N2 and P3 with age) may represent a general slowing rather than a selective slowing, which only affects inhibition processes (Falkenstein et al. 2002; Vallesi et al. 2009).
Falkenstein et al. (2002) studied aging effects on inhibition with a speeded (maximum reaction time of 400 ms) Go/No-go task with both visual and auditory stimuli. In their study, older adults (N = 12; M = 58.3 years old, range 54 to 65; 6 women) required more time than young adults (N = 12; M = 22.5 years old, range 19 to 25; 6 women) to decide whether to press a key (as reflected in the latency of the Go P3) or to inhibit the response (as likely reflected in the latency of the No-go P3). The No-go N2 was also delayed in the elderly adults, but to a lesser extent than the No-go P3 and only after visual stimuli. The No-go N2 results demonstrate that age effects in the No-go N2 are modality-specific and affect inhibition after visual but not auditory stimuli. In contrast, the comparable No-go P3 and Go P3 results suggest that the final decision process, i.e., whether to respond or inhibit, is modality-unspecific and affected by age. These results concerning both N2, which reflect modality-specific processes, and P3, which reflect modality-unspecific processes, are in accordance with the Falkenstein et al. (1999) study with a Go/No-go task and the Ramautar et al. (2006) study with a Stop-signal task.
To investigate age-related changes in inhibition, Vallesi et al. (2009) compared young adults (N = 14; M = 27 years old, range 20–34 years; 8 women) and older adults (N = 14; M = 71 years old, range 60–80 years; 9 women) on two Go/No-go tasks, including a simple and a complex task, that were designed to control for conflict level. The simple task comprised a Go condition (a red O and a blue X), a conflict No-go condition, in which the No-go stimuli were defined by combinations of colors and letters that corresponded to the stimuli used in the Go stimuli (a blue O and a red X), and an irrelevant condition, in which the identity of the No-go stimuli differed from the target stimuli (colored numbers rather than colored letters). In the complex task, there were 8 different Go and No-go stimuli rather than the 2 different stimuli per condition in the simple task. Vallesi et al. (2009) identified a longer P3 latency for the “conflict No-go” and a larger No-go P3 amplitude in older adults, regardless of the No-go and Go stimuli similarity, which suggests an age-related change in No-go stimuli processing. Additionally, they identified greater reaction times to both No-go and Go trials in older adults, which supports the age-related general slowing hypothesis (Salthouse 1996). Although these results point to a general slowing that affects inhibition processes, Vallesi (2011) experiment that tested age-related changes in inhibition did not identify changes in the No-go P3 latency, which suggests not all inhibition processes become slower with age. Vallesi (2011) tested young (N = 14; M = 25 years old, range 19–34; 8 women) and older adults (N = 14; M = 73 years old, range 65–81; 8 women) on a visual Go/No-go task. There was no age difference for the latency of the P3, but the results indicated a larger No-go P3 for older adults. These results suggest that older adults must devote considerably more resources (enhanced No-go P3 amplitude) to suppress the processing of non-target information compared with young adults.
In the Stop-Signal paradigm, a greater SSRT has been identified for older adults, which suggests an age-related deficit in inhibition that is not explained by a general decline in processing speed (Kramer et al. 1994; Andres et al. 2008). In an ERP experiment, Anguera and Gazzaley (2012) studied age-related modulations of the N2 and P3 associated with inhibition in a visual Stop-signal task and determined that the older adults’ greater SSRT was associated with the P3 latency but not with the P3 amplitude or the N2 latency or amplitude. In their experiment with older adults (N = 20; M ± SD = 70.6 ± 6.7 years old; 9 women), the Stop-signal N2 and P3 were qualitatively comparable to previous ERP studies that used a visual Stop-signal task with young adults (van Boxtel et al. 2001; Kok et al. 2004; Ramautar et al. 2004). However, in contrast with the results that have generally been reported for young adults, the N2 and P3 amplitude was not greater in successful compared with unsuccessful inhibition trials. Anguera and Gazzaley (2012) identified a later latency peak onset for unsuccessful inhibition trials in both N2 and P3. Only the P3 latency correlated with the SSRT, which is similar to other studies of age-related effects that used the Stop-signal task (Kramer et al. 1994). These results suggest the latency of the P3 might be an index of age-related inhibition changes.
As previously discussed, the Eriksen Flanker Task has also been used to study inhibition within this time window. ERP studies have been conducted to establish age-related modulations of the ERPs that are associated with the inhibitory processes elicited by the Flanker Task. In Wild-Wall et al. (2008) experiment, inhibition differences between two age groups, a younger and an older adults group, were explored using a Flanker Task. In addition to the modulation of the N1 and P1 components, Wild-Wall and colleagues (2008) identified a frontal N2 that was substantially smaller for the older group. This smaller N2 amplitude in the older group may reflect reduced flanker conflict in the incongruent condition. The reduced flanker conflict identified in the older group may explain the inferior error rate in the incongruent condition in older compared with younger adults. In Hsieh and Fang (2012) study, young and older adults’ inhibitory processes were compared using a Flanker Task in three experiments. Throughout the three experiments, and beyond the N1 modulations previously described, a decreased N2 in incongruent trials and a prolonged P3 peak latency to the central target were identified. These results support the notion that older adults use compensatory strategies (e.g., paying more attention to the central target) to be as capable as young adults in reducing flanker impact (Wild-Wall et al. 2008; Hsieh and Fang 2012).
Summary
In the preceding section, we summarized the ERP research that investigated the interval between 200 and 400 ms post-stimulus with respect to the processing events that may be involved in the instantiation of inhibition. The experimental paradigms utilized to examine the 0–200-ms time window were again considered. The following two ERP components have been consistently hypothesized to reflect inhibition in this time window, regardless of the paradigm used: The N2 and the P3. To understand the processes that are reflected by these components, studies have addressed the effects of different modalities (i.e., visual and auditory), different response modes (i.e., covert and overt), different stimulus probabilities (high, equal or low), successful and unsuccessful inhibition-related trials, sequence effects and age-related modulations. Taken together, these studies suggest that the N2 and P3 reflect different processes; however, both N2 and P3 processes contribute to successful inhibition and are associated with the orbitofrontal cortex, the ACC and the pre-supplementary motor areas. The N2 reflects conflict processes that are modality-specific and independent of motor processing, whereas the P3 reflects inhibition processes that are modality-unspecific and reflect motor processing. These components have primarily been associated with controlled processing. However, with stimulus repetition, automaticity may develop.
Inhibition Between 400 and 800 ms
ERPs for Inhibition Between 400 and 800 ms
At times, inhibitory processes operate between 400 and 800 ms after stimulus onset. Knowledge or semantic inhibition is a type of cognitive inhibition that has been suggested to occur in this late time window. In our daily living, in a particular context, knowledge is activated; however, only a portion of this knowledge is integrated in the representation of the context. An inhibition process is responsible for reducing the activation of unsuited knowledge (Debruille et al. 2008). For example, the presentation of a lexically ambiguous word (i.e., an instance of two or moremeanings being mapped onto identical phonological forms in the mental lexicon) has been shown to unconsciously trigger the activation of all of that word’s lexical meanings, even when the previous context is compatible with only one of them (Ihara et al. 2007). As a consequence, inhibition, which yields the selective activation of the appropriate meaning, must occur.
Recent consideration has been given to the possibility that the N400, an ERP component with a negative polarity that reaches its maximum approximately 400 ms after stimulus onset and is typically observed when meaningful stimuli are processed (e.g., words), indexes semantic inhibitory processes (Barber et al. 2004; Debruille 2007; Barber and Kutas 2007). The N400 is typically considered to reflect the processing effort associated with the integration of new semantic content. However, the types of processes that it indexes remain under debate. Debruille et al. (1996) tested the hypothesis that the N400 is an index of inhibition. They examined N400 amplitude differences for famous and unknown faces in a sample of young adults (N = 12, range 20–30 years old, 6 women). The participants signaled whether the face that they were viewing was known or unknown to them. The task was divided in three blocks that contained different percentages of famous faces (33, 50 and 67 %). Unknown faces are stimuli that, similar to infrequent words (or pseudo words), are new and entail the activation of previous knowledge that must inhibited. Therefore, if the N400 indexes inhibition, its amplitude should be larger for unknown compared with known faces (because more irrelevant knowledge is activated while initially attempting to match the new face to stored representations of known faces); it should also be larger for the blocks with higher percentages of famous faces (because in these contexts, the expectation of knowing the new face is higher, which stimulates an increased search effort, as well as more irrelevant knowledge activation that must be inhibited). Consistent with the hypothesis, unknown faces elicited larger N400 activity compared with known faces, especially in the presence of a higher percentage of famous faces. It was proposed that the N400 amplitude would depend on the amount of knowledge that must be inhibited and the strength of its previous activation. Thus, as the activation becomes stronger, the amount of inhibition that is required increases, which elicits larger N400 amplitudes (Debruille 2007).
Inhibition might also occur when words activate the representation of similar words (Debruille 1998; Holcomb et al. 2002). In Debruille's (1998) study, participants (N = 26; range 19–30 years old; 12 women) responded to a single-item lexical decision task, in which they identified pseudo-words by pressing a left button and real words by pressing a right button. The real words included look-alike words (i.e., low frequency words that can trigger the representations of the higher frequency words that they resemble), eccentric words (i.e., low frequency words that do not resemble higher frequency words) or frequent words, which were used as fillers. The author identified larger N400s for look-alike compared with eccentric words. He concluded that low frequency words with high frequency orthographic neighbors (i.e., look-alike words) triggered more inhibition than low frequency words with no such neighbors (i.e., eccentric words). Further evidence for the N400 as an index of inhibition was identified in a recent study by Shang and Debruille (2013). In their experiment, each trial consisted of three written words that were serially presented to the participants (N = 20; M ± SD = 27.7 ± 5 years old; M ± SD = 15.6 ± 1.8 years of education). In one block, the participants were asked to judge whether the meaning of the first word was related to the meaning of the third word, thus ignoring the second word. In the other block, the participants were asked to determine whether the meaning of the second word was related to the meaning of the third word. The researchers studied the N400 that was elicited by the second word in both conditions (i.e., the conditions in which the meaning of the second word was inhibited versus not inhibited). The results demonstrated a small but significant N400 effect associated with the second word processing. Specifically, the N400 that was elicited when the meaning of the second word was ignored was larger than when the meaning of the second word had to be attended. Therefore, the authors concluded that the results support the inhibition N400 hypothesis.
An additional type of cognitive inhibition that has been suggested to occur in this time window is known as interference control and has been studied using the Stroop task. In this task, the subject is asked to name the print color of a color-word (e.g., BLUE printed in red, which requires the name red to be pronounced while controlling the interference from the word meaning, yielded by automatized reading). The critical condition is composed of incongruent trials in which the print color and the meaning of the word mismatch, such as in the previous example. Typically, there is a control condition with congruent trials in which the meaning of the word and its color are the same (e.g., the word BLUE printed in blue). At times, other control conditions are used, including conditions in which only the colors (e.g., XXX printed in blue, with color-naming instructions) or color-words (e.g., BLUE written in black, with simple reading instructions) are presented. Behaviorally, the Stroop color-word interference effect refers to an increased response latency in incongruent compared with congruent or neutral trials.
In ERP research that has employed the Stroop task, the N450 and the late positive complex (LPC) components have reflected this interference effect (West and Alain 1999; Liotti et al. 2000; Hanslmayr et al. 2008; Tillman and Wiens 2011; Li et al. 2013). West and Alain (1999) investigated the temporal course of the Stroop effect, as reflected in ERP waveforms, in a sample of young adults (N = 12; range 24–31 years old; 6 women). In their task (see Fig. 1 for a schematic display of the task), in addition to the common incongruent, congruent and neutral conditions, there was a word identification condition in which the participants named the four color-words presented in light grey. This additional condition enhanced the Stroop effect by creating a context that increased the competition between color and word processing in the incongruent trials. It also allowed an additional comparison between the congruent and incongruent trials with a condition in which word information guided the response in contrast to the neutral condition in which color guided the response. West and Alain (1999) identified a larger N450, a fronto-central slow wave with an onset of approximately 500 ms, in incongruent compared with other trials. Therefore, they interpreted the N450 as an index of inhibition processes that are involved in word processing suppression.
Liotti et al. (2000) studied the temporal course of the Stroop color-word interference effect in a Stroop task with three response modalities, including overt, covert and manual. In the overt condition, the participants (N = 8; M ± SD = 27.6 ± 6.8 years old; 5 women) were asked to speak aloud the color of the word. In the covert condition, they were asked to speak the color of the word silently in their mind. In the manual condition, the participants were asked to press a designated button for each color. Liotti et al. (2000) created five control conditions to analyze the effects of color-word incongruence and response modality. They identified an increased N450 amplitude in incongruent trials, with an anterior medial and mid-dorsal scalp distribution and maximum amplitude at 410 ms. This result was interpreted as a reflection of the processes related to the suppression of word information. This effect was present for the three response modalities, but with a different scalp distribution for speech (i.e., overt and convert) and manual responses. Additionally, the authors identified a left-lateralized LPC effect with a maximum amplitude at 600 ms in incongruent compared with congruent trials, regardless of the response modality. This finding was interpreted as a reflection of the semantic processing of the word.
Li et al. (2013) investigated the functional meaning of this LPC in the Stroop task. In their experiment, young adults (N = 22; M = 21 years old, range 19–24; 13 women) participated in a traditional Stroop task and a rotation judgment task, in which they were asked to judge the rotation state of words equal to those found in the incongruent and congruent conditions of the Stroop Task (i.e., the participants pressed designated buttons to indicate upright, left or right rotation states). Consistent with previous studies, the researchers identified a larger N450 and LPC in incongruent compared with congruent trials. In the rotation judgment task, there was no response conflict because the resolution of perceptual conflict between the print color and the word meaning was not necessary to successfully respond. However, Li et al. (2013) identified a larger LPC in incongruent compared with congruent trials in the rotation judgment task, which suggests the LPC is sensitive to perceptual conflict. The N450 effects that were identified in the Stroop task were not identified in the Rotation task.
Hanslmayr et al. (2008) also investigated the temporal dynamics of the Stroop effect in a sample of young adults (N = 21; M = 24.9 years old, range 20 to 33; 16 women). In addition to the traditional conditions of the Stroop task, they studied a fourth condition in which they manipulated the order of incongruent trials to create an NP condition. This manipulation was performed to determine whether NP effects strengthen the Stroop color-word interference effect. Behaviorally, the participants were slower in the NP compared with non-primed incongruent trials and in both the NP and non-primed incongruent trials relative to the neutral and congruent trials. The ERP data analysis indicated increased N450 amplitude in NP and non-primed incongruent trials over fronto-central regions with a maximum amplitude at approximately 400 ms, which suggests this ERP effect reflects interference detection. This negativity over fronto-central regions was also evident later in time at approximately 600 ms post-stimulus. Hanslmayr et al. (2008) suggested that it might reflect the elicitation of central executive processes to overcome interference at that stage.
Tillman and Wiens (2011) used ERPs to study the effects of varying the probability of incongruent and congruent trials in the Stroop and Eriksen Flanker tasks. In each task, participants (N = 27; M ± SD = 27.22 ± 5.96 years old; 16 women) performed two blocks, including one block with rare incongruent trials (20 %) and a second block with frequent incongruent trials (80 %). The analysis of behavioral data demonstrated similar results in the Stroop and Eriksen Flanker tasks, with slower RTs and less accurate results on incongruent compared with congruent trials and on rare compared with frequent incongruent trials. With respect to the ERP measures, the researchers identified a modulation of the N450 in the Stroop task, with a larger amplitude on incongruent than congruent trials. However, this effect was only identified when the incongruent trials were rare. The authors suggested that the N450 is a measure of response conflict because it was only enhanced when conflict was high (i.e., with rare incongruent trials). In the Eriksen Flanker Task, a larger N2 amplitude was identified for frequent compared with rare incongruent trials, which replicated Bartholow et al.’s (2005) findings. Tillman and Wiens (2011) proposed that Flanker N2 reflects attentional control processes; therefore, it indexes a process that differs from the process reflected by the N450 observed in the Stroop task.
The temporal course of interference has also been studied using working memory paradigms that are known to elicit proactive interference (PI). PI is a type of interference in which previously memorized information is no longer relevant and must be inhibited. A task that is commonly used to elicit PI is the Sternberg’s working memory task (Sternberg 1966). In this task, the subject is first asked to memorize several lists of items. Following each of the to-be-memorized lists of items, there is a delay in which the list is not accessible. The subject is subsequently presented with one item and must decide whether it belongs to the to-be-memorized list. The behavioral finding that defines PI is a longer reaction time to decide whether the item belongs to the list when the item was presented in previous lists (i.e., familiar probe) compared with when the item was not presented in previous lists (i.e., non-familiar probe).
In ERP research that used modified versions of the Sternberg’s working memory paradigm, two ERP components have been identified as related to the PI effect, the N450 (Tays et al. 2009; Yi and Friedman 2011) and the LPC (Zhang et al. 2010). This finding is similar to the Stroop interference effect. Yi and Friedman (2011) examined the temporal course of the processes that contribute to the PI effect with a cued Sternberg’s Task. In this task, participants (N = 20; M = 24.1 years old; 11 women) viewed target sets with four digits, two on the right and two on the left. After the target set, a cue appeared (i.e., an arrow) that was either relevant, which pointed to the two to-be-memorized digits (thereby defining the two digits to-be-ignored), or irrelevant, which pointed in both directions (all digits must be in memory). Three probes existed, which included a “positive probe” (i.e., matched to the to-be-memorized digits), a “non-intrusion probe” (i.e., did not match any of the digits in the target set) and an “intrusion probe” (i.e., matched with the to-be-ignored digits). After a relevant cue, inhibition can occur for the irrelevant digits. Consistent with this view at the cue stage, Yi and Friedman (2011) identified a larger N450 after relevant compared with irrelevant cues (for which inhibition processes are not necessary). At the probe stage, they identified an N450 that was larger for “intrusion probes” compared with “non-intrusion probes”, which likely reflects PI triggered by the familiar but now irrelevant intrusion probe.
Zhang et al. (2010) designed an experiment to identify the ERP effects associated with PI. In their experiment, young adults (N = 19; M ± SD = 22.9 ± 1.9 years old; 9 women) participated in a “recent probe task” in which the target set (to-be-memorized) consisted of four consonants. Two consonants were “recent”, which were presented in a preceding target set, and two consonants were “not recent”, which were absent in the two preceding target sets. The researchers identified a larger LPC amplitude when the probe was present in the preceding target set than when it was not present. This effect was modulated by recency effects, with a smaller LPC amplitude for recently encountered probes. Based on these modulations, Zhang et al. (2010) suggested that the LPC is an electrophysiological signature of the PI effect. Tays et al. (2009) studied the effects of stimulus repetition on the PI effect and compared ERP waveforms in two variations of Sternberg’s Task. In their experiment, participants (N = 21; M = 1.94 years old, range 18–23; 15 women) completed two counterbalanced tasks, including one task with a small stimulus pool (i.e., 20 words) and a second task with a large stimulus pool (i.e., 750 words). The authors identified a smaller difference in N450 amplitude between the baseline and experimental PI conditions for the small compared with large stimulus pool tasks, in which the typical N450 amplitude difference was identified. Therefore, the authors suggested that repetition (inherent to the use of a small stimulus pool) can result in an attenuation of PI effects.
Automatic and Controlled Nature of Inhibition Between 400 and 800 ms
Initial studies that investigated the controlled or automatic nature of semantic inhibitory processes demonstrated that the N400 was modulated by task demands (Chwilla et al. 1995), selective attention, and pattern masking. These findings led to a view that associated the N400 with controlled processing. Congruent with this view, McCarthy and Nobre (1993) observed semantic and identity priming effects on the N400 only for words that appeared in the attended spatial location. However, subsequent studies that manipulated the likelihood of intervention of controlled processes demonstrated that N400 amplitude modulations were clearly observed in the experimental conditions that minimized controlled processes (e.g., used low stimulus-onset asynchronies, low proportions of related stimuli, or shallow levels of processing). Although typically larger when the instructions explicitly required semantic analyses, reliable N400 effects were observed in situations in which semantic processing was not necessary or even beneficial. In all of these types of studies, participants directed their attention to the stimuli (if not to the semantic level of analysis), which appears to be important for N400 elicitation.
The Stroop color-word interference effect indexed by the N450 and LPC can be classified as controlled processing. As previously described, the Stroop task is a classic example of a controlled processing task (Nigg 2000) because the participant must consciously inhibit the meaning of a color-word to identify the print color. However, Li et al.’s (2013) findings suggest that some processes involved in the Stroop interference effect can have an automatic nature, such as the perceptual conflict processing indexed by the LPC that can be elicited even when the perceptual conflict is irrelevant for task performance. Regarding the proactive interference effect, no experiments have been developed to address the nature of the involved inhibition processes.
Source Localization of ERPs Associated with Inhibition Between 400 and 800 ms
To the best of our knowledge, no ERPs studies have addressed the source localization of the ERP correlates of semantic inhibition processes. However, Shang and Debruille (2013) examined the scalp distribution of the N400 amplitude related to semantic inhibition and identified maximal effects in centro-parietal regions and a slightly larger effect in the right compared with left hemisphere. As Debruille’s inhibition hypothesis did not refer to a specific N400, as opposed to the N400 linked to semantic integration, we briefly address the results of source localization studies conducted with paradigms that did not directly address the inhibition hypothesis. These studies used MEG (Halgren et al. 2002; Lau et al. 2009) and high density EEG (Silva-Pereyra et al. 2003; Kuperberg et al. 2003) and identified generators for the N400 effect in temporal (i.e., Wernicke’s area and anterior temporal cortex) and frontal (i.e., dorsolateral, orbital and anterior prefrontal cortices) areas.
Concerning the Stroop color-word interference effect, source localization studies have suggested different but related brain generators for the N450 and LPC (Liotti et al. 2000; Markela-Lerenc et al. 2004; Hanslmayr et al. 2008). Liotti et al. (2000) used dipole source analysis with BESA and identified two independent generators in the ACC for the N450 in speech and manual versions of the Stroop task. Hanslmayr et al. (2008) also identified a source in the ACC for the N450 effect in a manual Stroop task using BESA. In an examination of a young adults sample (N = 16; M ± SD = 26 ± 5.4 years old; 12 women), Markela-Lerenc et al. (2004) conducted dipole source analysis with BESA for both N450 and LPC that were larger in incongruent compared with congruent trials in a manual Stroop task. The researchers identified a generator localized in the left PFC that contributed to the N450 effect and a generator in the right ACC that contributed to the LPC effect. The authors suggested that the PFC signals to the ACC when executive control is required, and the ACC is responsible for the ensuing executive control elicitation. In a proactive interference study that utilized dipole source analysis, Tays et al. (2008) identified two major ACC activation peaks (at approximately 340 and 440 ms during the N450 in interference conditions) and a left inferior frontal cortex activation (at approximately 420 ms following the probe in interference conditions). The authors suggested that both the ACC and the inferior frontal cortex contribute to the proactive interference resolution.
Age-Related Inhibition Changes Between 400 and 800 ms
To our knowledge, only one study has focused on age-related changes in the N400 as an index of semantic inhibition. In Cameli and Phillips (2000) study, older adults (N = 20; M ± SD = 71.5 ± 6.4 years old; M ± SD = 12.4 ± 1.7 years of education; 12 women) and young adults (N = 20; M ± SD = 23 ± 2.3 years old; M ± SD = 15.8 ± 1.8 years of education; 12 women) were asked to read sentences and word pairs. In each sentence or word pair, the final word was preceded by a context (i.e., the previous word in the word pair or the previous words in the sentence). The experiment comprised three conditions, which included unrelated, moderately and highly related conditions in which the context was not semantically related, moderately related or highly related to the final word, respectively. Cameli and Phillips (2000) demonstrated that young adults exhibited a higher N400 amplitude for the unrelated condition, followed by a smaller N400 amplitude for the moderately related condition, and an even smaller N400 amplitude for the highly related condition in both sentences and word pairs. However, older adults did not display this pattern. Older adults exhibited a similar N400 amplitude in all conditions (unrelated, moderately and highly related) in relation to sentences and a slightly higher N400 amplitude in the unrelated compared with highly related condition in relation to word pairs. These results were interpreted as a reflection of an age-related semantic inhibition deficit.
Concerning the Stroop interference effect, several behavioral studies have shown an increased Stroop effect in older adults (see for example Mayas et al. 2012); however, this age-related modulation has received little attention from ERP researchers. A rare example is West and Alain (2000), who used ERPs to test whether the increased Stroop effect identified in older adults is because of a general slowing or an inhibition deficit. In their study, young adults (N = 12; M ± SD = 27.08 ± 2.35 years old; 6 women) and older adults (N = 12; M ± SD = 69.50 ± 3.48 years old; 6 women) who differed on years of education (i.e., young adults had two additional years of education, on average) participated in a Stroop Task that was similar to West and Alain (1999). Specifically, neutral, congruent, incongruent and word identification trials were presented. Behaviorally, they identified an increased Stroop effect in older adults even after controlling for age-related differences in reaction times to neutral trials. The analysis of the ERP data revealed a smaller N450 (labeled as N500 by West and Alain 2000) amplitude in incongruent trials over the midline fronto-central in older adults, which likely reflects deficits in the suppression of word information. Following this modulation of the N450, no age-related modulations were identified in a negative slow wave, which likely reflects response selection processes, or in an enhanced positivity over the temporoparietal region, which likely reflects the perceptual processing of the color information used to guide the response. These findings are consistent with the proposal that an age-related decline in the efficiency of inhibition of word information contributes to the increased Stroop interference effect observed in older adults.
This inhibition age-related decline has also been identified in proactive interference studies (Tays et al. 2008; Yi and Friedman 2014). Tays et al. (2008) used high density electrophysiology to examine differences between older (N = 18; M = 72.4 years old, range 65–87; 14 women) and young (N = 16; M = 20 years old, range 18–26; 10 women) adults in a Sternberg Task. They identified a frontal negativity at 450 ms. This negativity was labeled medial frontal negativity (MFN) and had characteristics that were similar to the N450 identified in Stroop task studies. The MFN was observed in the interference conditions only for young adults. Older adults exhibited a large frontal positivity that was associated with poorer behavioral performance. The authors interpreted the absence of the frontal negativity in older adults as an indication of a proactive interference processing deficit in older adults. Yi and Friedman (2014) studied these age-related differences in greater detail via comparisons of young adults (from Yi and Friedman’s 2011 study) to two groups of older adults, which included a group with older adults ranging in age from 60 to 70 years old (N = 20; 15 women) and a yet older group ranging age from 71 to 82 years old (N = 20; 15 women) adults. They used the cued Sternberg’s Task from Yi and Friedman’s (2011) study and examined both cue- and probe-related inhibition ERP correlates. They identified a larger N450 amplitude after a relevant cue (i.e., that points to the left or right, thereby indicating the relevant digits) than after an irrelevant cue (i.e., that points in both directions), which likely reflects the inhibition mechanisms that are responsible for removing irrelevant digits from the focus of attention. With respect to age-related differences, they identified a delayed latency for this activity in the 71–82 age-range group . Similar to Yi and Friedman’s (2011) finding, Yi and Friedman (2014) identified an N450 at the probe stage that was larger for “intrusion probes” compared with “non-intrusion probes”, which likely reflects processes that enable proactive interference resolution. They identified a delayed latency for this activity in both of the older adults’ groups.
Summary
In the preceding section, we summarized the ERP research that addressed the occurrence and nature of inhibition processing activity between 400 and 800 ms post-stimulus. In this time window, the following two types of cognitive inhibition have been studied: semantic inhibition and interference. Semantic inhibition has been related to processes indexed by the N400. These semantic inhibition processes cannot be precisely mapped onto the automatic or controlled processing categories because they have characteristics that are associated with both categories. Semantic inhibition may be associated with controlled processing because it can be modulated by controlled attention processes (e.g., selective attention). However, it may also be linked to automatic processes because it can be elicited with even low awareness levels (Kutas and Federmeier 2011). The temporal course of interference has been studied in this time window with two paradigms, the Stroop and Sternberg’s paradigms. ERP studies that have investigated the Stroop interference effect have identified two ERP components with larger amplitudes in incongruent relative to congruent or neutral trials. These components are the N450, which likely reflects the suppression of word information, and the LPC, which reflects semantic processing of the word meaning or perceptual conflict. To understand the processes that are reflected by these ERP components, studies have been conducted to address the ERP effects of different response modes (i.e., speech and manual), sequence effects (i.e., NP), stimulus probabilities (high or low), and age-related modulations. Taken together, these studies suggest that the N450 and LPC reflect different processes; however, both components index the Stroop interference effect that is related to the activation of the ACC and the PFC. The N450 and the LPC have also been identified in ERP studies that used Sternberg’s paradigm, which suggests these components reflect processes that are related to proactive interference resolution. For this time window, the small number of ERP studies that have explored age-related modulations in semantic inhibition and interference have identified an age-related deficit in both types of inhibition, which is indexed by the N450 and the LPC modulations.
Discussion
The present review aimed to clarify not only the temporal course of inhibition but also its nature as a complex process that entails both automatic and controlled processing. The reviewed ERP data, which were collected using different experimental paradigms, illustrate different inhibition processes, starting as early as 100 ms after stimulus onset and extending their activity beyond 400 ms post-stimulus (for a summary, see Table 1). One of our main goals was to contribute to the understanding of the temporal course and nature of inhibition; therefore, we proceed to discuss sensory, motor and cognitive inhibition-related processes that were identified in the reviewed ERP research. Additionally, we address the brain areas that were activated during inhibition processes and the automatic and controlled nature of inhibition across the three time windows proposed. Despite the scarce ERP research on age-related inhibition changes to date, we also discuss the experiments that have been conducted thus far. Finally, special attention is given to the conflict theory as an alternative explanation for ERP modulations that are typically interpreted as reflections of inhibition.
Inhibition and Sensory Processing
The early inhibitory processes in the 200 ms after stimulus onset have been associated with sensory processing of the stimulus (e.g., flankers in the Eriksen Flanker Task). Therefore, they are referred to as sensory inhibition processes. These processes are reflected by the P1 and N1 ERP components (Roche et al. 2005; Johnstone et al. 2009). Despite this association, the P1 and especially the N1 have been linked to inhibitory events that occur later and may therefore signal the early stages of the processes that subsume these events. Filipovic et al. (2000) suggested that the P1-N1-P2 complex early time window might be as important as the N2-P3 complex time window to the Go/No-Go decision (i.e., decision to withdraw attentional resources from the task on No-go trials). In addition, in a Go/No-go task, Lavric et al. (2004) identified an association between the N1 modulation and an early signal from visual processing areas that triggers later inhibitory processes, which was reflected by the N2. In the Stop-signal task, Bekker et al. (2005) identified a larger N1 for successful compared with failed stops. This result suggests that the N1 may reflect the orienting of attention toward the stop-signal, thereby determining the success of inhibition in the Stop-signal trials.
The inhibition ERP studies that have addressed the N1 and P1 age-related modulations have shown a clear dissociation between the processes that are linked to P1 and N1. The P1 can be linked to the onset of inhibition processes with the detection of irrelevant information that is not affected by age; the age-related amplitude enhancement in N1 can be interpreted as increased attention to relevant information, such as a compensatory mechanism that older adults employ to reduce interference from irrelevant information (Wild-Wall et al. 2008; Hsieh and Fang 2012). The data that pertain to the aging effects on inhibition provide further support for the hypothesis that inhibition processes triggered within sensory processing are essential for later cognitive and motor inhibition processes. Namely, if this first level of processing is delayed because of sensory impairments (e.g., less visual or auditory acuity), the subsequent temporal course of inhibition will also be delayed, even though later inhibition processes may not be impaired. Therefore, it is indispensable to control for the sensory abilities that are required to perform the task. This concept is particularly important in aging studies because the aging process changes sensory abilities. However, as Picton et al. (2000) suggested, even in studies with healthy young adults, a proper sensory abilities questionnaire must be administered to improve the accuracy of self-report and ensure the normal function of the sensory abilities that the task requires. Only then can we accurately interpret changes in processes that occur after sensory processing. These early sensory processes are also influenced by the experimental task modality. For example, ERP studies that have compared auditory and visual modalities of the Stop-signal and Go/No-go tasks have suggested that the N2 reflects modality specific processes (e.g., smaller N2 amplitude in auditory compared with visual Stop-signal trials), whereas the P3 reflects processes that are independent of stimulus modality (Falkenstein et al. 1999, 2002; Ramautar et al. 2006).
Motor and Cognitive Inhibition
ERP studies that used the Go/No-go task have compared overt and covert response modes and have identified No-go N2 and No-go P3 effects in both response modes, which suggests the processes that these components reflect occur at motor and non-motor processing stages. However, slightly different results have been demonstrated for N2 and P3. The same No-go N2 effect has been identified in both covert and overt conditions, which suggests the N2 reflects non-motor processes. The No-go P3 effect is smaller for the covert compared with overt conditions, which suggests the No-go P3 reflects, at least in part, movement-related potentials (Van’t Ent and Apkarian 1999; Bruin and Wijers 2002; Smith et al. 2008). ERP studies that have manipulated the probability of inhibition-related stimuli (e.g., compared conditions with low, equal and high probability Stop-signal trials) and explored the sequence effects in inhibition paradigms have also helped to characterize the processes that are associated with N2 and P3 (Bruin and Wijers 2002; Nieuwenhuis et al. 2003; Donkers and van Boxtel 2004; Ramautar et al. 2004; Smith et al. 2007; Dimoska and Johnstone 2008; Smith et al. 2010). Together, these ERP studies suggest that the N2 may be associated with premotor inhibition processes and conflict detection, whereas the P3 may be associated with motor and non-motor inhibition processes, such as conflict resolution (e.g., withhold/execute the response) and evaluation processes (e.g., if the inhibition was correctly performed and appropriate to the context).
Between 400 and 800 ms post-stimulus, the following two types of cognitive inhibition have been reported: semantic inhibition and interference. Semantic inhibition has been related to the N400 component; however, the specific processes that N400 indexes (e.g., semantic integration or inhibition) remain under debate. The few ERP studies that have addressed the inhibitory account of the N400 suggest that this component reflects semantic inhibition processes rather than semantic integration per se. Thus, semantic integration processing deploys semantic inhibition, which is the process that the N400 specifically indexes. The ERP correlates of interference are observed in all three time windows considered in this review (Table 1). However, the interference that is reflected in the first two windows, as summarized in Table 1, pertains to flanker processing in the Eriksen Flanker Task. In contrast, the last time window interference pertains to color-word interference in the Stroop task and proactive interference in Sternberg’s working memory task. Tillman and Wiens (2011) compared interference ERP correlates found in the Eriksen Flanker and Stroop tasks and determined that the results (namely, the flanker N200 and the Stroop N450) differed according to the task. Thus, it appears that the resolution of interference in the Eriksen Flanker and Stroop tasks did not trigger the same inhibition processes.
Brain Structure Activation During Inhibition: Evidence from Source Localization Analysis
An ERP effect may be generated by one or several electrical sources in the brain (Otten and Rugg 2005). The problem of reconstructing the brain localization of the electrical activity responsible for an observed topography of scalp voltages is known as the "inverse problem"(Srinivasan 2005). This problem is intrinsically ill-posed: An infinite number of patterns of local brain activations could be responsible for the same scalp voltage topography. In order to obtain increasingly precise approximate solutions to the “inverse problem”, several source localization algorithms have been developed. These methods have become reasonably accurate in locating the neural activity associated with the scalp EEG topography on a given instant (i.e., with approximately 1 cm of possible error, using a four-shell spherical head model, and improving upon that mark when electrode arrays with at least 64 sensors are employed), but far more coarse in locating brain activity than direct imaging methods, such as fMRI, which, using a 7 tesla MRI machine, can map activity with an accuracy down to 1 mm. However, the temporal resolution of fMRI is inherently limited by the slow blood flow response to increased localized brain metabolism (i.e., one sec in the best case). Therefore, unlike measurements directly derived from the brain’s electrical activity, such as the EEG and ERPs, fMRI cannot track the dynamics of mental activity on the sub-millisecond timescale on which neurons operate. EEG and ERP data have a temporal grain-size of a few milliseconds and therefore approach the real-time scale of neural dynamics. Such data, obtained with high-density electrode arrays and in conjunction with source localization analysis allows for the identification of the brain areas where the successive segments of an ERP were generated, with enough spatial resolution for meaningful interpretation and, crucially, granting information about the temporal succession of active areas, unparalleled with respect to its grain size.
With respect to inhibition, across the three time windows that we have examined, the frontal cortex has the most prevalent involvement, and particularly, the ACC and the PFC. To the best of our knowledge, no studies have included source localization analyses of semantic inhibition ERP correlates. Recent studies have adopted a multimodal approach, which has coupled EEG and fMRI recordings to study brain generators of inhibition triggered during a Stop-signal task (Huster et al. 2013) and combined Eriksen Flanker and Go/No-go tasks (Baumeister et al. 2014). These studies have combined the advantages that are offered by the high spatial resolution of fMRI techniques and the high temporal resolution of EEG techniques. Future studies that combine EEG and fMRI can aid in differentiating between the types of inhibition processes involved in interference resolution in the Eriksen Flanker and Stroop tasks and can decisively contribute to the debate over the conflict/inhibition relationship.
Automatic and Controlled Inhibition Processing
Consistent with our hypothesis, in the first 200 ms, inhibitory processing is primarily an automatic process that is associated with the exogenous sensory aspects of the stimuli. However, automatic processing may also occur between 200 and 400 ms and between 400 and 800 ms following stimulus onset. The different paradigms used across the three time windows can modulate the automatic and controlled nature of the inhibition processes that are evoked. However, even in a classic example of a controlled inhibition task, such as the Stroop task, automatic processes (e.g., the detection of perceptual conflict) can occur. The nature of the stimuli that are used in the task can also modulate the nature of the inhibitory processes that are involved in the task. As Tachibana et al. (1996) suggested, semantic stimuli involve greater executive control compared with physical stimuli. Finally, automaticity may develop with practice in paradigms such as the Go/No-go and Stop-signal tasks.
Age-related Inhibition Changes
Age-related deficits in inhibition can be observed across the three time windows, with the extent of deficits depending on the paradigm used and the type of inhibition under study. In the first 200 ms, the performance of older adults on the Eriksen Flanker Task is similar to young adults. However, ERP studies suggest that older adults invest extra attentional resources on relevant information and are therefore less sensitive to the interference of irrelevant information. Some ERP studies support the hypothesis of a slowing down in inhibitory processing with aging; however, this slowing may be general and affect all cognitive processes (Salthouse 1996) rather than specific to inhibition. ERP studies of motor inhibition suggest older adults require more time not only to inhibit but also to execute responses (Falkenstein et al. 2002; Vallesi et al. 2009). ERP studies of interference that used the Stroop and Sternberg tasks, have identified a slowing down of interference resolution in older adults that appears to be specific to inhibition processing (Yi and Friedman 2011; West and Alain 2000). Regarding semantic inhibition, only one study has been conducted (Cameli and Phillips 2000). Therefore, additional research is needed to understand age-related modulations in this type of cognitive inhibition.
Inhibition and Conflict
Although the N2 and P3 effects in the Eriksen Flanker Task and Go/No-go and Stop-signal paradigms can be attributed to inhibitory processes, they can also be interpreted in light of the conflict hypothesis. In the Eriksen Flanker Task, a conflict between the responses that are required by the target and the flanker stimuli can occur. A similar conflict, which involves the act of withholding the response and the act of executing the response, can arise in the Go/No-go task (i.e., between the Go and No-go responses) and the Stop-signal task (i.e., between the Go and Stop responses) (Van Veen and Carter 2002; Nieuwenhuis et al. 2003; Donkers and van Boxtel 2004; Yeung et al. 2004). Recently, using a Go/No-go task, Smith et al. (2010) demonstrated that N2 and P3 amplitudes increased for unexpected stimuli regardless of whether that stimuli belonged to Go or No-go trials. To reflect inhibition, N2 and P3 should exhibit enhancements only for No-go compared with Go trials. Thus, the results of Smith et al. (2010) support a conflict interpretation of the N2 and P3 effects because conflict can occur in both Go and No-go trials when they are unexpected. In an attempt to reconcile these two alternative explanations for the N2 effects that are present in the inhibition-eliciting paradigms, Falkenstein (2006) proposed that the N2 is related to both conflict and inhibition, and these two processes may be sequentially ordered, i.e., conflict may precede inhibition. The same reasoning can be extended to the P3 interpretation, but its relation to inhibition may be stronger than the relation of the N2 (Smith et al. 2008).
Conclusion
Throughout the reviewed time windows, inhibition emerged as a set of processes that represented a complex functional structure, as opposed to a unitary process or a single processing event. The temporal resolution of the ERP technique helped to reveal the different processes that directly contribute to the success of inhibition, such as the detection of conflict and the investment of extra attentional resources on relevant information. Additionally, the relative autonomy and the structured interplay of these processes were highlighted by the ERP technique, in particular, by its use in conjunction with brain source localization analyses. These latter results permitted the mapping of various processes involved in inhibition, across time and tasks, onto different brain areas, which significantly advanced the understanding of the manifold nature of inhibitory processing. In particular, a more fine-grained understanding of time-scale has been added to the previous fMRI studies, which suggests the involvement of different brain areas in different inhibition paradigms.
References
Abad-Rodriguez, J., Ledesma, M. D., Craessaerts, K., Perga, S., Medina, M., Delacourte, A., et al. (2004). Neuronal membrane cholesterol loss enhances amyloid peptide generation. The Journal of Cell Biology, 167(5), 953–960. doi:10.1083/jcb.200404149.
Albert, J., Lopez-Martin, S., Hinojosa, J. A., & Carretie, L. (2013). Spatiotemporal characterization of response inhibition. NeuroImage, 76, 272–281. doi:10.1016/j.neuroimage.2013.03.011.
Amieva, H., Lafont, S., Auriacombe, S., Le Carret, N., Dartigues, J. F., Orgogozo, J. M., et al. (2002). Inhibitory breakdown and dementia of the Alzheimer type: a general phenomenon? Journal of Clinical and Experimental Neuropsychology, 24(4), 503–516. doi:10.1076/jcen.24.4.503.1034.
Amieva, H., Phillips, L. H., Della Sala, S., & Henry, J. D. (2004). Inhibitory functioning in Alzheimer’s disease. Brain, 127(5), 949–964. doi:10.1093/brain/awh045.
Andres, P., & Van der Linden, M. (2000). Age-related differences in supervisory attentional system functions. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 55(6), 373–380.
Andres, P., Guerrini, C., Phillips, L. H., & Perfect, T. J. (2008). Differential effects of aging on executive and automatic inhibition. Developmental Neuropsychology, 33(2), 101–123. doi:10.1080/87565640701884212.
Anguera, J. A., & Gazzaley, A. (2012). Dissociation of motor and sensory inhibition processes in normal aging. Clinical Neurophysiology, 123(4), 730–740. doi:10.1016/j.clinph.2011.08.024.
Barber, H. A., & Kutas, M. (2007). Interplay between computational models and cognitive electrophysiology in visual word recognition. Brain Research Reviews, 53(1), 98–123. doi:10.1016/j.brainresrev.2006.07.002.
Barber, H., Vergara, M., & Carreiras, M. (2004). Syllable-frequency effects in visual word recognition: evidence from ERPs. Neuroreport, 15(3), 545–548.
Bartholow, B. D., Pearson, M. A., Dickter, C. L., Sher, K. J., Fabiani, M., & Gratton, G. (2005). Strategic control and medial frontal negativity: beyond errors and response conflict. Psychophysiology, 42(1), 33–42. doi:10.1111/j.1469-8986.2005.00258.x.
Baumeister, S., Hohmann, S., Wolf, I., Plichta, M. M., Rechtsteiner, S., Zangl, M., et al. (2014). Sequential inhibitory control processes assessed through simultaneous EEG-fMRI. NeuroImage, 94, 349–359. doi:10.1016/j.neuroimage.2014.01.023.
Bekker, E. M., Kenemans, J. L., Hoeksma, M. R., Talsma, D., & Verbaten, M. N. (2005). The pure electrophysiology of stopping. International Journal of Psychophysiology, 55(2), 191–198. doi:10.1016/j.ijpsycho.2004.07.005.
Belleville, S., Chertkow, H., & Gauthier, S. (2007). Working memory and control of attention in persons with Alzheimer’s disease and mild cognitive impairment. Neuropsychology, 21(4), 458–469. doi:10.1037/0894-4105.21.4.458.
Bjorklund, D. F., & Harnishfeger, K. (1995). The evolution of inhibition mechanisms and their role in human cognition and behavior. In F. Dempster & C. Brainerd (Eds.), Interference and inhibition in cognition (pp. 142–169). San Diego: Academic.
Bobb, D. S., Jr., Adinoff, B., Laken, S. J., McClintock, S. M., Rubia, K., Huang, H. W., et al. (2012). Neural correlates of successful response inhibition in unmedicated patients with late-life depression. The American Journal of Geriatric Psychiatry, 20(12), 1057–1069. doi:10.1097/JGP.0b013e318235b728.
Bokura, H., Yamaguchi, S., Matsubara, M., & Kobayashi, S. (2002). Frontal lobe contribution to response inhibition process—an ERP study and aging effect. International Congress Series, 1232(0), 17–20. doi:10.1016/S0531-5131(01)00677-X.
Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S., & Cohen, J. D. (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–652.
Bridgeman, B. (2006). Contributions of lateral inhibition to object substitution masking and attention. Vision Research, 46(24), 4075–4082. doi:10.1016/j.visres.2006.08.012.
Bruin, K. J., & Wijers, A. A. (2002). Inhibition, response mode, and stimulus probability: a comparative event-related potential study. Clinical Neurophysiology, 113(7), 1172–1182. doi:10.1016/s1388-2457(02)00141-4.
Bruin, K. J., Wijers, A. A., & van Staveren, A. S. (2001). Response priming in a go/nogo task: do we have to explain the go/nogo N2 effect in terms of response activation instead of inhibition? Clinical Neurophysiology, 112(9), 1660–1671.
Cameli, L., & Phillips, N. A. (2000). Age-related differences in semantic priming: evidence from event-related brain potentials. Brain and Cognition, 43(1–3), 69–73.
Cheng, C. H., Wang, P. N., Hsu, W. Y., & Lin, Y. Y. (2012). Inadequate inhibition of redundant auditory inputs in Alzheimer’s disease: an MEG study. Biological Psychology, 89(2), 365–373. doi:10.1016/j.biopsycho.2011.11.010.
Chwilla, D. J., Brown, C. M., & Hagoort, P. (1995). The N400 as a function of the level of processing. Psychophysiology, 32(3), 274–285.
Collette, F., Schmidt, C., Scherrer, C., Adam, S., & Salmon, E. (2009). Specificity of inhibitory deficits in normal aging and Alzheimer’s disease. Neurobiology of Aging, 30(6), 875–889. doi:10.1016/j.neurobiolaging.2007.09.007.
Dai, Q., & Feng, Z. (2011). Deficient interference inhibition for negative stimuli in depression: an event-related potential study. Clinical Neurophysiology, 122(1), 52–61. doi:10.1016/j.clinph.2010.05.025.
Dauwels, J., Vialatte, F., & Cichocki, A. (2010). Diagnosis of Alzheimer’s Disease from EEG Signals: Where Are We Standing? Current Alzheimer Research, 7(6), 487–505.
Dawson, M. E., Oray, S., Lu, Z. L., & Schell, A. M. (2004). Prepulse inhibition of event-related brain potentials and startle eyeblink. Advances in Psychology Research, 29, 57–70.
Debruille, J. B. (1998). Knowledge inhibition and N400: A study with words that look like common words. Brain and Language, 62(2), 202–220. doi:10.1006/brln.1997.1904.
Debruille, J. B. (2007). The N400 potential could index a semantic inhibition. Brain Research Reviews, 56(2), 472–477. doi:10.1016/j.brainresrev.2007.10.001.
Debruille, J. B., Pineda, J., & Renault, B. (1996). N400-like potentials elicited by faces and knowledge inhibition. Cognitive Brain Research, 4(2), 133–144. doi:10.1016/0926-6410(96)00032-8.
Debruille, J. B., Ramirez, D., Wolf, Y., Schaefer, A., Nguyen, T. V., Bacon, B. A., et al. (2008). Knowledge inhibition and N400: a within- and a between-subjects study with distractor words. Brain Research, 1187, 167–183. doi:10.1016/j.brainres.2007.10.021.
Di Russo, F., Martinez, A., & Hillyard, S. A. (2003). Source analysis of event-related cortical activity during visuo-spatial attention. Cerebral Cortex, 13(5), 486–499.
Dimoska, A., & Johnstone, S. J. (2008). Effects of varying stop-signal probability on ERPs in the stop-signal task: do they reflect variations in inhibitory processing or simply novelty effects? Biological Psychology, 77(3), 324–336. doi:10.1016/j.biopsycho.2007.11.005.
Dimoska, A., Johnstone, S. J., & Barry, R. J. (2006). The auditory-evoked N2 and P3 components in the stop-signal task: Indices of inhibition, response-conflict or error-detection? Brain and Cognition, 62(2), 98–112. doi:10.1016/j.bandc.2006.03.011.
Dimoska-Di Marco, A., McDonald, S., Kelly, M., Tate, R., & Johnstone, S. (2011). A meta-analysis of response inhibition and Stroop interference control deficits in adults with traumatic brain injury (TBI). Journal of Clinical and Experimental Neuropsychology, 33(4), 471–485. doi:10.1080/13803395.2010.533158.
Donkers, F. C. L., & van Boxtel, G. J. M. (2004). The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain and Cognition, 56(2), 165–176. doi:10.1016/j.bandc.2004.04.005.
Enriquez-Geppert, S., Konrad, C., Pantev, C., & Huster, R. J. (2010). Conflict and inhibition differentially affect the N200/P300 complex in a combined go/nogo and stop-signal task. NeuroImage, 51(2), 877–887. doi:10.1016/j.neuroimage.2010.02.043.
Eriksen, B. A., & Eriksen, C. W. (1974). Effects of noise letters upon identification of a target letter in a nonsearch task. Perception & Psychophysics, 16(1), 143–149. doi:10.3758/Bf03203267.
Falkenstein, M. (2006). Inhibition, conflict and the Nogo-N2. Clinical Neurophysiology, 117(8), 1638–1640. doi:10.1016/j.clinph.2006.05.002.
Falkenstein, M., Hoormann, J., & Hohnsbein, J. (1999). ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychologica (Amsterdam), 101(2–3), 267–291.
Falkenstein, M., Hoormann, J., & Hohnsbein, J. (2002). Inhibition-related ERP components: variation with modality, age, and time-on-task. Journal of Psychophysiology, 16(3), 167–175. doi:10.1027//0269-8803.16.3.167.
Fallgatter, A. J., & Strik, W. K. (1999). The NoGo-anteriorization as a neurophysiological standard-index for cognitive response control. International Journal of Psychophysiology, 32(3), 233–238.
Fallgatter, A. J., Mueller, T. J., & Strik, W. K. (1999). Age-related changes in the brain electrical correlates of response control. Clinical Neurophysiology, 110(5), 833–838.
Filipovic, S. R., Jahanshahi, M., & Rothwell, J. C. (2000). Cortical potentials related to the nogo decision. Experimental Brain Research, 132(3), 411–415.
Fjell, A. M., Rosquist, H., & Walhovd, K. B. (2009). Instability in the latency of P3a/P3b brain potentials and cognitive function in aging. Neurobiology of Aging, 30(12), 2065–2079. doi:10.1016/j.neurobiolaging.2008.01.015.
Folstein, J. R., & Van Petten, C. (2008). Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology, 45(1), 152–170. doi:10.1111/j.1469-8986.2007.00602.x.
Folstein, J. R., Van Petten, C., & Rose, S. A. (2008). Novelty and conflict in the categorization of complex stimuli. Psychophysiology, 45(3), 467–479. doi:10.1111/j.1469-8986.2007.00628.x.
Friedman, N. P., & Miyake, A. (2004). The relations among inhibition and interference control functions: a latent-variable analysis. Journal of Experimental Psychology: General, 133(1), 101–135. doi:10.1037/0096-3445.133.1.101.
Fu, S., Greenwood, P. M., & Parasuraman, R. (2005). Brain mechanisms of involuntary visuospatial attention: an event-related potential study. Human Brain Mapping, 25(4), 378–390. doi:10.1002/hbm.20108.
Gazzaley, A., Clapp, W., Kelley, J., McEvoy, K., Knight, R. T., & D’Esposito, M. (2008). Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proceedings of the National Academy of Science of the United States of America, 105(35), 13122–13126. doi:10.1073/pnas.0806074105.
Gibbons, H., Rammsayer, T. H., & Stahl, J. (2006). Multiple sources of positive- and negative-priming effects: an event-related potential study. Memory & Cognition, 34(1), 172–186.
Halgren, E., Dhond, R. P., Christensen, N., Van Petten, C., Marinkovic, K., Lewine, J. D., et al. (2002). N400-like magnetoencephalography responses modulated by semantic context, word frequency, and lexical class in sentences. NeuroImage, 17(3), 1101–1116.
Hanslmayr, S., Pastotter, B., Bauml, K. H., Gruber, S., Wimber, M., & Klimesch, W. (2008). The electrophysiological dynamics of interference during the Stroop task. Journal of Cognitive Neuroscience, 20(2), 215–225. doi:10.1162/jocn.2008.20020.
Haring, A. E., Zhuravleva, T. Y., Alperin, B. R., Rentz, D. M., Holcomb, P. J., & Daffner, K. R. (2013). Age-related differences in enhancement and suppression of neural activity underlying selective attention in matched young and old adults. Brain Research, 1499, 69–79. doi:10.1016/j.brainres.2013.01.003.
Harnishfeger, K. (1995). Development of cognitive inhibition. In F. Dempster & C. Brainerd (Eds.), Interference and inhibition in cognition (pp. 175–204). San Diego: Academic.
Hasher, L., & Zacks, R. T. (1988). Working memory, comprehension, and aging: A review and a new view. In G. H. Bower (Ed.), The psychology of learning and motivation (Vol. 22, pp. 193–225). New York: Academic.
Hillyard, S. A., Luck, S. J., & Mangun, G. R. (1994). The cueing of attention to visual field locations: Analysis with ERP recordings. In H. J. Heinze & G. R. Mangun (Eds.), Cognitive electrophysiology (pp. 1–25). Boston: Birkhauser.
Holcomb, P. J., Grainger, J., & O’Rourke, T. (2002). An electrophysiological study of the effects of orthographic neighborhood size on printed word perception. Journal of Cognitive Neuroscience, 14(6), 938–950. doi:10.1162/089892902760191153.
Horvath, J., Czigler, I., Birkas, E., Winkler, I., & Gervai, J. (2009). Age-related differences in distraction and reorientation in an auditory task. Neurobiology of Aging, 30(7), 1157–1172. doi:10.1016/j.neurobiolaging.2007.10.003.
Hoshiyama, M., Koyama, S., Kitamura, Y., Shimojo, M., Watanabe, S., & Kakigi, R. (1996). Effects of judgement process on motor evoked potentials in Go/No-go hand movement task. Neuroscience Research, 24(4), 427–430.
Hsieh, S. L., & Fang, W. H. (2012). Elderly adults through compensatory responses can be just as capable as young adults in inhibiting the flanker influence. Biological Psychology, 90(2), 113–126, doi:DOI 10.1016/j.biopsycho.2012.03.006
Hughes, M. E., Fulham, W. R., Johnston, P. J., & Michie, P. T. (2012). Stop-signal response inhibition in schizophrenia: behavioural, event-related potential and functional neuroimaging data. Biological Psychology, 89(1), 220–231. doi:10.1016/j.biopsycho.2011.10.013.
Huster, R. J., Enriquez-Geppert, S., Lavallee, C. F., Falkenstein, M., & Herrmann, C. S. (2013). Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. International journal of psychophysiology : official journal of the International Organization of Psychophysiology, 87(3), 217–233. doi:10.1016/j.ijpsycho.2012.08.001.
Ihara, A., Hayakawa, T., Wei, Q., Munetsuna, S., & Fujimaki, N. (2007). Lexical access and selection of contextually appropriate meaning for ambiguous words. NeuroImage, 38(3), 576–588. doi:10.1016/j.neuroimage.2007.07.047.
Johnstone, S. J., Dimoska, A., Smith, J. L., Barry, R. J., Pleffer, C. B., Chiswick, D., et al. (2007). The development of stop-signal and Go/Nogo response inhibition in children aged 7–12 years: performance and event-related potential indices. International Journal of Psychophysiology, 63(1), 25–38. doi:10.1016/j.ijpsycho.2006.07.001.
Johnstone, S. J., Barry, R. J., Markovska, V., Dimoska, A., & Clarke, A. R. (2009). Response inhibition and interference control in children with AD/HD: a visual ERP investigation. International Journal of Psychophysiology, 72(2), 145–153. doi:10.1016/j.ijpsycho.2008.11.007.
Kappenman, Emily, S., & Luck, S. J. (2012). ERP Components: The ups and downs of brainwave recordings. In S. J. Luck, Kappenman, & S. Emily (Eds.), Oxford handbook of event-related potential components (pp. 3–30). New York: Oxford University Press
Kathmann, N., Bogdahn, B., & Endrass, T. (2006). Event-related brain potential variations during location and identity negative priming. Neuroscience Letters, 394(1), 53–56. doi:10.1016/j.neulet.2005.10.001.
Kiefer, M., Marzinzik, F., Weisbrod, M., Scherg, M., & Spitzer, M. (1998). The time course of brain activations during response inhibition: evidence from event-related potentials in a go no go task. Neuroreport, 9(4), 765–770. doi:10.1097/00001756-199803090-00037.
Kirino, E., Belger, A., Goldman-Rakic, P., & McCarthy, G. (2000). Prefrontal activation evoked by infrequent target and novel stimuli in a visual target detection task: an event-related functional magnetic resonance imaging study. The Journal of Neuroscience, 20(17), 6612–6618.
Kirmizi-Alsan, E., Bayraktaroglu, Z., Gurvit, H., Keskin, Y. H., Emre, M., & Demiralp, T. (2006). Comparative analysis of event-related potentials during Go/NoGo and CPT: Decomposition of electrophysiological markers of response inhibition and sustained attention. Brain Research, 1104, 114–128. doi:10.1016/j.brainres.2006.03.010.
Knyazev, G. G., Levin, E. A., & Savostyanov, A. N. (2008). A failure to stop and attention fluctuations: an evoked oscillations study of the stop-signal paradigm. Clinical Neurophysiology, 119(3), 556–567. doi:10.1016/j.clinph.2007.11.041.
Koch, I., Gade, M., Schuch, S., & Philipp, A. M. (2010). The role of inhibition in task switching: a review. Psychonomic Bulletin & Review, 17(1), 1–14. doi:10.3758/PBR.17.1.1.
Kok, A. (1999). Varieties of inhibition: manifestations in cognition, event-related potentials and aging. Acta Psychologica (Amsterdam), 101(2–3), 129–158.
Kok, A., Ramautar, J. R., De Ruiter, M. B., Band, G. P., & Ridderinkhof, K. R. (2004). ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology, 41(1), 9–20. doi:10.1046/j.1469-8986.2003.00127.x.
Kopp, B., Rist, F., & Mattler, U. (1996). N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology, 33(3), 282–294.
Kramer, A. F., Humphrey, D. G., Larish, J. F., Logan, G. D., & Strayer, D. L. (1994). Aging and inhibition: beyond a unitary view of inhibitory processing in attention. Psychology and Aging, 9(4), 491–512.
Kuperberg, G. R. (2004). Electroencephalography, Event-Related Potentials, and Magnetoencephalography. In D. D. Dougherty, S. L. Rauch, & J. F. Rosenbaum (Eds.), Essentials of NeuroImaging for Clinical Practice (1st ed., pp. 117–129): American Psychiatric Publishing, Inc.
Kuperberg, G. R., Holcomb, P. J., Sitnikova, T., Greve, D., Dale, A. M., & Caplan, D. (2003). Distinct patterns of neural modulation during the processing of conceptual and syntactic anomalies. Journal of Cognitive Neuroscience, 15(2), 272–293. doi:10.1162/089892903321208204.
Kutas, M., & Federmeier, K. D. (2011). Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP). Annual Review of Psychology, 62, 621–647. doi:10.1146/annurev.psych.093008.131123.
Lau, E., Almeida, D., Hines, P. C., & Poeppel, D. (2009). A lexical basis for N400 context effects: evidence from MEG. Brain and Language, 111(3), 161–172. doi:10.1016/j.bandl.2009.08.007.
Lavric, A., Pizzagalli, D. A., & Forstmeier, S. (2004). When ‘go’ and ‘nogo’ are equally frequent: ERP components and cortical tomography. European Journal of Neuroscience, 20(9), 2483–2488. doi:10.1111/j.1460-9568.2004.03683.x.
Li, K. Z., Hasher, L., Jonas, D., Rahhal, T. A., & May, C. P. (1998). Distractibility, circadian arousal, and aging: a boundary condition? Psychology and Aging, 13(4), 574–583.
Li, G., Wang, S., Duan, Y., & Zhu, Z. (2013). Perceptual conflict-induced late positive complex in a modified Stroop task. Neuroscience Letters, 542, 76–80. doi:10.1016/j.neulet.2013.01.056.
Liotti, M., Woldorff, M. G., Perez, R., & Mayberg, H. S. (2000). An ERP study of the temporal course of the Stroop color-word interference effect. Neuropsychologia, 38(5), 701–711.
Logan, G. D. (1994). On the ability to inhibit thought and action: A users’ guide to the stop signal paradigm. In D. Dagenbach & T. H. Carr (Eds.), Inhibitory processes in attention, memory, and language. San Diego: Academic.
Logan, G. D., Cowan, W. B., & Davis, K. A. (1984). On the ability to inhibit simple and choice reaction time responses: a model and a method. Journal of Experimental Psychology: Human Perception and Performance, 10(2), 276–291.
Luck, S. J. (2005). An introduction to the event-related potential technique. Cambridge: MIT Press.
Ludowig, E., Moeller, J., Bien, C. G., Muente, T. F., Eiger, C. E., & Rosburg, T. (2010). Active suppression in the mediotemporal lobe during directed forgetting. Neurobiology of Learning and Memory, 93(3), 352–361. doi:10.1016/j.nlm.2009.12.001.
Luus, B. M., Van Snellenberg, J. X., & Liotti, M. (2007). To stop or not to stop: a high spatio-temporal resolution study of response inhibition using MEG. International Congress Series, 1300(0), 425–428. doi:10.1016/j.ics.2007.03.016.
MacLeod, C., Dodd, M., Sheard, E., Wilson, D., & Bibi, U. (2003). In opposition to inhibition. In B. Ross (Ed.), The psychology of learning and motivation (pp. 163–214). San Diego: Elsevier.
Maguire, M. J., Brier, M. R., Moore, P. S., Ferree, T. C., Ray, D., Mostofsky, S., et al. (2009). The influence of perceptual and semantic categorization on inhibitory processing as measured by the N2-P3 response. Brain and Cognition, 71(3), 196–203. doi:10.1016/j.bandc.2009.08.018.
Markela-Lerenc, J., Ille, N., Kaiser, S., Fiedler, P., Mundt, C., & Weisbrod, M. (2004). Prefrontal-cingulate activation during executive control: which comes first? Cognitive Brain Research, 18(3), 278–287.
Mayas, J., Fuentes, L. J., & Ballesteros, S. (2012). Stroop interference and negative priming (NP) suppression in normal aging. Archives of Gerontology and Geriatrics, 54(2), 333–338. doi:10.1016/j.archger.2010.12.012.
McCarthy, G., & Nobre, A. C. (1993). Modulation of semantic processing by spatial selective attention. Electroencephalography and Clinical Neurophysiology, 88(3), 210–219.
McDonald, J. J., Ward, L. M., & Kiehl, K. A. (1999). An event-related brain potential study of inhibition of return. Perception & Psychophysics, 61(7), 1411–1423.
Mercado, F., Gonzalez, J. L., Barjola, P., Fernandez-Sanchez, M., Lopez-Lopez, A., Alonso, M., et al. (2013). Brain correlates of cognitive inhibition in fibromyalgia: emotional intrusion of symptom-related words. International Journal of Psychophysiology, 88(2), 182–192. doi:10.1016/j.ijpsycho.2013.03.017.
Naatanen, R., Kujala, T., Escera, C., Baldeweg, T., Kreegipuu, K., Carlson, S., et al. (2012). The mismatch negativity (MMN)–a unique window to disturbed central auditory processing in ageing and different clinical conditions. Clinical Neurophysiology, 123(3), 424–458. doi:10.1016/j.clinph.2011.09.020.
Natale, E., Marzi, C. A., Girelli, M., Pavone, E. F., & Pollmann, S. (2006). ERP and fMRI correlates of endogenous and exogenous focusing of visual-spatial attention. European Journal of Neuroscience, 23(9), 2511–2521. doi:10.1111/j.1460-9568.2006.04756.x.
Neuhaus, A. H., Koehler, S., Opgen-Rhein, C., Urbanek, C., Hahn, E., & Dettling, M. (2007). Selective anterior cingulate cortex deficit during conflict solution in schizophrenia: an event-related potential study. Journal of Psychiatric Research, 41(8), 635–644. doi:10.1016/j.jpsychires.2006.06.012.
Neuhaus, A. H., Urbanek, C., Opgen-Rhein, C., Hahn, E., Ta, T. M., Koehler, S., et al. (2010). Event-related potentials associated with attention network test. International Journal of Psychophysiology, 76(2), 72–79. doi:10.1016/j.ijpsycho.2010.02.005.
Nieuwenhuis, S., Yeung, N., van den Wildenberg, W., & Ridderinkhof, K. R. (2003). Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cognitive, Affective, & Behavioral Neuroscience, 3(1), 17–26.
Nigg, J. T. (2000). On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin, 126(2), 220–246.
O’Connell, R. G., Balsters, J. H., Kilcullen, S. M., Campbell, W., Bokde, A. W., Lai, R., et al. (2012). A simultaneous ERP/fMRI investigation of the P300 aging effect. Neurobiology of Aging, 33(10), 2448–2461. doi:10.1016/j.neurobiolaging.2011.12.021.
Otten, L. J., & Rugg, M. D. (2005). Event-related potentials: A methods handbook. In T. C. Handy (Ed.), Interpreting event-related brain potentials In (pp. 3–16). Cambridge: MIT Press.
Padilla, M. L., Colrain, I. M., Sullivan, E. V., Mayer, B. Z., Turlington, S. R., Hoffman, L. R., et al. (2011). Electrophysiological evidence of enhanced performance monitoring in recently abstinent alcoholic men. Psychopharmacology, 213(1), 81–91. doi:10.1007/s00213-010-2018-1.
Pascual-Marqui, R. D., Michel, C. M., & Lehmann, D. (1994). Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. International Journal of Psychophysiology, 18(1), 49–65.
Patel, S. H., & Azzam, P. N. (2005). Characterization of N200 and P300: selected studies of the event-related potential. International Journal of Medical Sciences, 2(4), 147–154.
Pfefferbaum, A., & Ford, J. M. (1988). ERPs to stimuli requiring response production and inhibition: effects of age, probability and visual noise. Electroencephalography and Clinical Neurophysiology, 71(1), 55–63.
Pfefferbaum, A., Ford, J. M., Weller, B. J., & Kopell, B. S. (1985). ERPs to response production and inhibition. Electroencephalography and Clinical Neurophysiology, 60(5), 423–434.
Piai, V., Roelofs, A., & van der Meij, R. (2012). Event-related potentials and oscillatory brain responses associated with semantic and stroop-like interference effects in overt naming. Brain Research, 1450, 87–101. doi:10.1016/j.brainres.2012.02.050.
Picton, T. W., Bentin, S., Berg, P., Donchin, E., Hillyard, S. A., Johnson, R., et al. (2000). Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology, 37(2), 127–152. doi:10.1017/S0048577200000305.
Polich, J. (2007). Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology, 118(10), 2128–2148. doi:10.1016/j.clinph.2007.04.019.
Polich, J., & Comerchero, M. D. (2003). P3a from visual stimuli: typicality, task, and topography. Brain Topography, 15(3), 141–152.
Posner, M. I., & Snyder, C. R. R. (1975). Attention and cognitive control. In R. L. Solso (Ed.), Information Processing and Cognition: The Loyola Symposium: Lawrence Erlbaum.
Possin, K. L., Filoteo, J. V., Song, D. D., & Salmon, D. P. (2009). Space-based but not object-based inhibition of return is impaired in Parkinson’s disease. Neuropsychologia, 47(7), 1694–1700. doi:10.1016/j.neuropsychologia.2009.02.006.
Purmann, S., Badde, S., Luna-Rodriguez, A., & Wendt, M. (2011). Adaptation to frequent conflict in the eriksen flanker task: an ERP study. Journal of Psychophysiology, 25(2), 50–59. doi:10.1027/0269-8803/a000041.
Raaijmakers, J. G. W., & Jakab, E. (2013). Rethinking inhibition theory: On the problematic status of the inhibition theory for forgetting. Journal of Memory and Language, 68(2), 98–122. doi:10.1016/j.jml.2012.10.002.
Ramautar, J. R., Kok, A., & Ridderinkhof, K. R. (2004). Effects of stop-signal probability in the stop-signal paradigm: the N2/P3 complex further validated. Brain and Cognition, 56(2), 234–252. doi:10.1016/j.bandc.2004.07.002.
Ramautar, J. R., Slagter, H. A., Kok, A., & Ridderinkhof, K. R. (2006). Probability effects in the stop-signal paradigm: the insula and the significance of failed inhibition. Brain Research, 1105(1), 143–154. doi:10.1016/j.brainres.2006.02.091.
Ridderinkhof, K. R., Band, G. P. H., & Logan, G. D. (1999). A study of adaptive behavior: effects of age and irrelevant information on the ability to inhibit one’s actions. Acta Psychologica (Amsterdam), 101(2–3), 315–337.
Robinson, O. J., Krimsky, M., & Grillon, C. (2013). The impact of induced anxiety on response inhibition. Frontiers in Human Neuroscience, 7, 69. doi:10.3389/fnhum.2013.00069.
Roche, R. A., Garavan, H., Foxe, J. J., & O’Mara, S. M. (2005). Individual differences discriminate event-related potentials but not performance during response inhibition. Experimental Brain Research, 160(1), 60–70. doi:10.1007/s00221-004-1985-z.
Salthouse, T. A. (1996). The processing-speed theory of adult age differences in cognition. Psychological Review, 103(3), 403–428.
Scherg, M. (1990). Fundamentals of dipole source potential analysis. In G. L. Romani (Ed.), Auditory evoked magnetic fields and electric potentials (pp. 40–69). Basel: Karger.
Schluter, N. D., Rushworth, M. F., Passingham, R. E., & Mills, K. R. (1998). Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation. Brain, 121(Pt 5), 785–799.
Senderecka, M., Grabowska, A., Szewczyk, J., Gerc, K., & Chmylak, R. (2012). Response inhibition of children with ADHD in the stop-signal task: an event-related potential study. International Journal of Psychophysiology, 85(1), 93–105. doi:10.1016/j.ijpsycho.2011.05.007.
Shang, M., & Debruille, J. B. (2013). N400 processes inhibit inappropriately activated representations: Adding a piece of evidence from a high-repetition design. Neuropsychologia, 51(10), 1989–1997. doi:10.1016/j.neuropsychologia.2013.06.006.
Shiffrin, R. M., & Schneider, W. (1977). Controlled and automatic human information processing. II. Perceptual learning, automatic attending and a general theory. Psychological Review, 84(2), 127–190.
Silva-Pereyra, J., Rivera-Gaxiola, M., Aubert, E., Bosch, J., Galan, L., & Salazar, A. (2003). N400 during lexical decision tasks: a current source localization study. Clinical Neurophysiology, 114(12), 2469–2486.
Smith, J. L., Johnstone, S. J., & Barry, R. J. (2006). Effects of pre-stimulus processing on subsequent events in a warned Go/NoGo paradigm: response preparation, execution and inhibition. International Journal of Psychophysiology, 61(2), 121–133. doi:10.1016/j.ijpsycho.2005.07.013.
Smith, J. L., Johnstone, S. J., & Barry, R. J. (2007). Response priming in the Go/NoGo task: the N2 reflects neither inhibition nor conflict. Clinical Neurophysiology, 118(2), 343–355. doi:10.1016/j.clinph.2006.09.027.
Smith, J. L., Johnstone, S. J., & Barry, R. J. (2008). Movement-related potentials in the Go/NoGo task: the P3 reflects both cognitive and motor inhibition. Clinical Neurophysiology, 119(3), 704–714. doi:10.1016/j.clinph.2007.11.042.
Smith, J. L., Smith, E. A., Provost, A. L., & Heathcote, A. (2010). Sequence effects support the conflict theory of N2 and P3 in the Go/NoGo task. International Journal of Psychophysiology, 75(3), 217–226. doi:10.1016/j.ijpsycho.2009.11.002.
Srinivasan, R. (2005). Event-related potentials: A methods handbook. In T. C. Handy (Ed.), High-resolution EEG: Theory and practice In (pp. 167–188). Cambridge: MIT Press.
Sternberg, S. (1966). High-speed scanning in human memory. Science, 153(3736), 652–654.
Strik, W. K., Fallgatter, A. J., Brandeis, D., & Pascual-Marqui, R. D. (1998). Three-dimensional tomography of event-related potentials during response inhibition: evidence for phasic frontal lobe activation. Electroencephalography and Clinical Neurophysiology, 108(4), 406–413. doi:10.1016/s0168-5597(98)00021-5.
Stroop, J. (1935). Studies of interference in serial verbal interactions. Journal of Experimental Psychology, 18, 643–662.
Tachibana, H., Aragane, K., & Sugita, M. (1996). Age-related changes in event-related potentials in visual discrimination tasks. Electroencephalography and Clinical Neurophysiology, 100(4), 299–309.
Tays, W. J., Dywan, J., Mathewson, K. J., & Segalowitz, S. J. (2008). Age differences in target detection and interference resolution in working memory: an event-related potential study. Journal of Cognitive Neuroscience, 20(12), 2250–2262. doi:10.1162/jocn.2008.20158.
Tays, W. J., Dywan, J., & Segalowitz, S. J. (2009). General proactive interference and the N450 response. Neuroscience Letters, 462(3), 239–243. doi:10.1016/j.neulet.2009.07.025.
Thomas, S. J., Johnstone, S. J., & Gonsalvez, C. J. (2007). Event-related potentials during an emotional stroop task. International Journal of Psychophysiology, 63(3), 221–231. doi:10.1016/j.ijpsycho.2006.10.002.
Thomas, S. J., Gonsalvez, C. J., & Johnstone, S. J. (2009). Sequence effects in the Go/NoGo task: inhibition and facilitation. International Journal of Psychophysiology, 74(3), 209–219. doi:10.1016/j.ijpsycho.2009.09.002.
Thomas, C., Vom Berg, I., Rupp, A., Seidl, U., Schroder, J., Roesch-Ely, D., et al. (2010). P50 gating deficit in Alzheimer dementia correlates to frontal neuropsychological function. Neurobiology of Aging, 31(3), 416–424. doi:10.1016/j.neurobiolaging.2008.05.002.
Tian, Y., & Yao, D. (2008). A study on the neural mechanism of inhibition of return by the event-related potential in the Go/NoGo task. Biological Psychology, 79(2), 171–178. doi:10.1016/j.biopsycho.2008.04.006.
Tillman, C. M., & Wiens, S. (2011). Behavioral and ERP indices of response conflict in stroop and flanker tasks. Psychophysiology, 48(10), 1405–1411. doi:10.1111/j.1469-8986.2011.01203.x.
Tipper, S. P. (1985). The negative priming effect: inhibitory priming by ignored objects. The Quarterly Journal of Experimental Psychology Section A Human Experimental Psychology, 37(4), 571–590.
Tun, P. A., O’Kane, G., & Wingfield, A. (2002). Distraction by competing speech in young and older adult listeners. Psychology and Aging, 17(3), 453–467.
Turner, G. R., & Spreng, R. N. (2012). Executive functions and neurocognitive aging: dissociable patterns of brain activity. Neurobiology of Aging, 33(4), 826 e821-813, doi:10.1016/j.neurobiolaging.2011.06.005
Vallesi, A. (2011). Targets and non-targets in the aging brain: a go/nogo event-related potential study. Neuroscience Letters, 487(3), 313–317. doi:10.1016/j.neulet.2010.10.046.
Vallesi, A., Stuss, D. T., McIntosh, A. R., & Picton, T. W. (2009). Age-related differences in processing irrelevant information: evidence from event-related potentials. Neuropsychologia, 47(2), 577–586. doi:10.1016/j.neuropsychologia.2008.10.018.
van Boxtel, G. J., van der Molen, M. W., Jennings, J. R., & Brunia, C. H. (2001). A psychophysiological analysis of inhibitory motor control in the stop-signal paradigm. Biological Psychology, 58(3), 229–262.
Van Veen, V., & Carter, C. S. (2002). The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience, 14(4), 593–602. doi:10.1162/08989290260045837.
Van’t Ent, D., & Apkarian, P. (1999). Motoric response inhibition in finger movement and saccadic eye movement: a comparative study. Clinical Neurophysiology, 110(6), 1058–1072.
Verbruggen, F., & Logan, G. D. (2008a). Automatic and controlled response inhibition: associative learning in the go/no-go and stop-signal paradigms. Journal of Experimental Psychology: General, 137(4), 649–672. doi:10.1037/a0013170.
Verbruggen, F., & Logan, G. D. (2008b). Long-term aftereffects of response inhibition: memory retrieval, task goals, and cognitive control. Journal of Experimental Psychology: Human Perception and Performance, 34(5), 1229–1235. doi:10.1037/0096-1523.34.5.1229.
Verhoef, K., Roelofs, A., & Chwilla, D. J. (2009). Role of inhibition in language switching: evidence from event-related brain potentials in overt picture naming. Cognition, 110(1), 84–99. doi:10.1016/j.cognition.2008.10.013.
Verona, E., Sprague, J., & Sadeh, N. (2012). Inhibitory control and negative emotional processing in psychopathy and antisocial personality disorder. Journal of Abnormal Psychology, 121(2), 498–510. doi:10.1037/a0025308.
Wascher, E., & Tipper, S. P. (2004). Revealing effects of noninformative spatial cues: an EEG study of inhibition of return. Psychophysiology, 41(5), 716–728. doi:10.1111/j.1469-8986.2004.00198.x.
Wascher, E., Schneider, D., Hoffmann, S., Beste, C., & Sänger, J. (2012). When compensation fails: attentional deficits in healthy ageing caused by visual distraction. Neuropsychologia, 50(14), 3185–3192. doi:10.1016/j.neuropsychologia.2012.09.033.
West, R., & Alain, C. (1999). Event-related neural activity associated with the stroop task. Cognitive Brain Research, 8(2), 157–164.
West, R., & Alain, C. (2000). Age-related decline in inhibitory control contributes to the increased stroop effect observed in older adults. Psychophysiology, 37(2), 179–189.
White, C. N., Ratcliff, R., & Starns, J. J. (2011). Diffusion models of the flanker task: discrete versus gradual attentional selection. Cognitive Psychology, 63(4), 210–238. doi:10.1016/j.cogpsych.2011.08.001.
Wild-Wall, N., Falkenstein, M., & Hohnsbein, J. (2008). Flanker interference in young and older participants as reflected in event-related potentials. Brain Research, 1211, 72–84. doi:10.1016/j.brainres.2008.03.025.
Wostmann, N. M., Aichert, D. S., Costa, A., Rubia, K., Moller, H. J., & Ettinger, U. (2013). Reliability and plasticity of response inhibition and interference control. Brain and Cognition, 81(1), 82–94. doi:10.1016/j.bandc.2012.09.010.
Yeung, N., Botvinick, M. M., & Cohen, J. D. (2004). The neural basis of error detection: conflict monitoring and the error-related negativity. Psychological Review, 111(4), 931–959. doi:10.1037/0033-295x.111.4.931.
Yi, Y., & Friedman, D. (2011). Event-related potential (ERP) measures reveal the timing of memory selection processes and proactive interference resolution in working memory. Brain Research, 1411, 41–56. doi:10.1016/j.brainres.2011.07.004.
Yi, Y., & Friedman, D. (2014). Age-related differences in working memory: ERPs reveal age-related delays in selection- and inhibition-related processes. Neuropsychology, Development, and Cognition Section B, Aging, Neuropsychology and Cognition, 21(4), 483–513. doi:10.1080/13825585.2013.833581.
Zhang, W., & Lu, J. (2012). Time course of automatic emotion regulation during a facial Go/Nogo task. Biological Psychology, 89(2), 444–449. doi:10.1016/j.biopsycho.2011.12.011.
Zhang, J. X., Wu, R., Kong, L., Weng, X., & Du, Y. (2010). Electrophysiological correlates of proactive interference in the ‘Recent Probes’ verbal working memory task. Neuropsychologia, 48(7), 2167–2173. doi:10.1016/j.neuropsychologia.2010.04.008.
Acknowledgments
This work was supported by a fellowship from the Portuguese national funding agency for science, research and technology [FCT; (SFRH/BD/70011/2010/Psicologia)].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pires, L., Leitão, J., Guerrini, C. et al. Event-Related Brain Potentials in the Study of Inhibition: Cognitive Control, Source Localization and Age-Related Modulations. Neuropsychol Rev 24, 461–490 (2014). https://doi.org/10.1007/s11065-014-9275-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11065-014-9275-4