Abstract
In this study, a new method based on electromembrane extraction (EME) followed by corona discharge ion mobility spectrometry (CD-IMS) was used for preconcentration and quantification of malachite green in water samples. In the EME procedure, the charged malachite green migrated into the supported liquid membrane (SLM) under an applied potential. The extraction efficiency of malachite green was assessed based on two phase EME under effective parameters including applied voltage, extraction time, pH of the sample solution, stirring rate, and salt addition in the sample solution. The analytical performance of the developed EME method was studied under the optimum extraction condition. The dynamic linear range and low limit of detection of the EME method were 5–250 ng mL−1 and 1.5 ng mL−1, respectively. The preconcentration factor of 150 and the RSD% of 3.8–7.6% were also achieved using EME method. Finally, the proposed method was successfully tested for the extraction and analysis of malachite green in different water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many physical and chemical contaminants infect the environment all around the world. The main pollution types include air pollution, light pollution, noise pollution, plastic pollution, soil contamination, radioactive contamination, thermal pollution, visual pollution, and water pollution [1,2,3,4,5]. Among these contaminants, water pollution has become a serious issue across the world because water is one of the most critical natural resources for life [1]. The causes of water pollution include a wide range of chemicals and pathogens as well as physical parameters. Pollutants may consist of organic and inorganic materials. A group of organic substances that contaminates water are triarylmethane dyes (malachite green (MG)) [2]. MG and MG derivatives dyes are synthetic organic compounds containing triphenylmethane backbones. As these compounds are intensely colored, they are industrially produced as dyes [3].
Moreover, MG is extensively used across the world because of its high efficacy for treatment of fungicide, parasiticide, and antiseptic, antibacterial properties in the aquaculture. Also, MG is used as a dye in food, textile, silk, leather, cotton, paper, and other industries for one or the other purposes [4,5,6,7]. MG, like other triphenylmethane compounds, may cause cancer and mutagenesis because of its high toxicity [8,9,10]. Hence, the usage of these compounds in aquaculture and various industries has created serious concerns. In recent decades, designing and development of methods to determine the synthetic dyes from the aqueous media, has covered a prominent area of the analytical chemistry researches.
Due to the low concentration of MG in wastewater and water samples, sample preparation and preconcentration step is necessary before direct analysis. For this purpose, various methods to determine MG from aqueous media, including molecularly imprinted solid-phase extraction (MISPE) [11,12,13], magnetic solid phase extraction (MSPE) [14], solid phase extraction (SPE) [15, 16], cloud point extraction (CPE) [8, 17, 18], solvent extraction [19], and dispersive liquid-liquid microextraction (DLLME) [20,21,22], covered the vast area of the analytical chemistry researches.
EME is a miniaturized liquid-liquid extraction technique developed for sample preparation of aqueous samples before the analysis by chromatography, electrophoresis, mass spectrometry, and other procedures in analytical chemistry [23]. The EME method usually involves the use of a small volume of organic solvent in the lumen of hollow fiber as a supported liquid membrane (SLM) and application of an electrical field across the SLM. This method has attracted remarkable attentions due to its desirable features such as utilization of reduced organic solvents, low extraction time, rapid processing, and small volumes of sample and reasonable analysis costs. Also, due to the fact that extraction procedure is accomplished under the effect of an electrical field, the extraction efficiency can be improved with controlling of the electrical field [24].
Several analytical systems such as UV-Visible spectrophotometry, and high-performance liquid chromatography (HPLC) has extensively applied for the determination of MG [25, 26]. It is worth to mention that HPLC is an expensive device with high analysis time. HPLC usually uses a large volume of organic solvents that causes environmental pollution and also increases the cost of the analysis. Also, the ion mobility spectrometer (IMS) system could identify the MG. IMS is one of the separation techniques used to identify and quantify the ionized molecules in the gas phase [27]. These ions are separated in the presence of carrier gas based on their mobility. The main advantages of IMS involves low detection limit, simplicity, portability, fast response, and relatively low cost [28]. Also, IMS does not use organic solvent unlike HPLC, so it’s an environmentally friendly device.
As far as we know, EME and IMS have not been used to identify MG yet. So, in this work, we aimed to develop EME/IMS technique for identification and determination MG as an important fungicide/ antibacterial for the first time. In the EME technique presence of driving force across the SLM, can leading to decrease the extraction time, and consequently increase preconcentration factor. Some of experimental conditions, including the organic solvent, applied voltage, extraction time, pH of the sample solution, stirring rate, and salt concentration, were optimized for the EME-IMS method. In the end, this presented method was successfully applied for the extraction and determination of MG in wastwater samples.
Experimental
Instruments and reagents
The used DC power supply was a PV-300 model (Mobtaker Aryaei J, Zanjan, Iran) with applied voltage in the range of 0–300 V, providing currents in the range of 0–1 mA. Platinum wires (diameter 0.2 mm) were applied as electrodes with an inter-electrode distance of 5 mm in the sample (donor) and acceptor solutions. A digital pH-meter (Cyberscan 2100, Eutech Instruments, Singapore) was applied for pH measurements.
MG, NaOH, HCl, and NaCl were purchased from Merck (Darmstadt, Germany). All organic solvents, including 2-nitrophenyl octyl ether (NPOE), 1-octanol, n-hexadecane, n-heptadecane, and nitrobenzene, were from Fluka (Buchs, Switzerland). HPLC grade water was obtained through a Milli-Q® system (Millipore, Milford, MA, USA) and was used to prepare all solutions. All chemicals used in analysis were analytical grade.
Standard solution and real samples
The stock solution of MG was prepared by weighing and dissolving an appropriate amount of MG in 10 mL HPLC-grade water to prepare a solution with concentration of 1000 mg L−1. The stock solution was kept in the amber glass and protected from the light at 4 °C in the refrigerator to be prevented from decomposition. All of the required working solutions were obtained by daily dilution of the stock solution. The pH of the sample solution was adjusted by addition of HCl solution.
The wastewater sample was collected from the Shahid Beheshti University (Tehran, Iran). Moreover, the river and sea water samples from shanderman and Caspian sea in the north of Iran. The fish farming waters were collected from the fish pools breeding in Jajroude. All of the water sample solutions were filtered through membrane filters (0.45 μm) immediately after collecting.
Ion mobility spectrometer conditions
Corona discharge Ion mobility spectrometer (CD-IMS) (model 1000) (Isfahan, TOF Tech. Pars) used in this work was manufactured at Isfahan University of Technology (Iran). The CD-IMS included an ionization region (including the corona discharge needle region) and a drift region (16 cm in length). All experiments were conducted in positive ionization mode, and nitrogen as the drift and carrier gases was used by flow rate of 1000 and 600 mL min−1, respectively. Other conditions of the IMS components can be found from the work reported previously [28]. All IMS spectra were recorded by Pico scope software, and each IMS spectrum was an average of 300 individual spectra. The peak area of the IMS spectrum was calculated by Vis-IMS software. The optimized experimental conditions in IMS for recording the spectra were as following: pressure 660 Torr, injection port temperature 250 °C, IMS cell temperature 200 °C, typical shutter grid pulse width 100, corona voltage 700 V, and the drift field of 437 V cm−1.
Procedure for EME
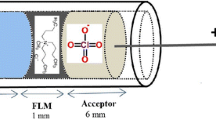
First, the polypropylene hollow fiber cut into 6 cm segments. Then, these pieces of polypropylene hollow fiber were inserted in the glass vial and dipped in the organic solvent serving as the SLM. After a few minutes, a piece of hollow fiber was taken and excess of solvent was removed with a medical wipe. Then, 20 μL of organic solution was introduced into the lumen of the hollow fiber using an HPLC syringe, and finally, the end of hollow fiber was closed by heat. Subsequently, the hollow fiber containing the acceptor solution was placed in the sample solution. Positive and negative platinum electrodes were placed in the donor and acceptor solutions, respectively and connected to a DC power supply. The cell prepared for EME was placed on a magnetic stirrer. Magnetic stirrer and power supply were simultaneously turned on, and the extraction was accomplished for a prescribed time. After the extraction was completed, the magnetic stirrer and the power supply were turned off. Then, the acceptor solution was collected with an HPLC syringe and transferred directly to a micro-vial. In the end, 1 μL of acceptor solution was injected to CD-IMS using a GC syringe. The schematic illustration of the MG electromembrane extraction (MG-EME) is shown in Fig. 1.
Results and discussion
Optimization of EME procedure
The performance of the proposed procedure was evaluated to achieve the highest extraction efficiency of MG. For this purpose, the effect of different factors, including organic solvent, applied voltage, extraction time, pH of the sample solution, stirring rate, and salt addition. In EME method was studied in detail.
Variation in SLM composition (organic solvent)
The nature of the solvent between the donor and acceptor solution plays a critical role in the EME efficiency. This solvent must have some characteristic including certain electrical conductivity for electrokinetic migration of ions across the SLM; on the other hand, SLM should have the lowest level of the electrical resistance for keeping the electrical current in the system. Also, low vapor pressure to prevent losing the solvent during the experiments is necessary. Moreover, it should be immiscible with water so that it can be easily interred the pores of polypropylene hollow fiber. Therefore, a suitable organic solvent can effectively increase the efficient extraction of the target analyte. As a result, a series of experiments were accomplished with different types of organic solvents, including 2-nitrophenyl octyl ether (NPOE), 1-octanol, n-hexadecane, n-heptadecane, and nitrobenzene. As can be seen in Fig. 2a high peak area demonstrat that the 1-octanol has the best extraction efficiency compar with the others. Therefore, 1-octanol was selected as the optimum organic solvent for the rest of the experiments.
a. Effect of the SLM solvent on the extraction efficiency. Extraction conditions: (100 ng mL−1 of MG, applied voltage: 50.0 V, sample solution pH 1.0, stirring rate 1000.0 rpm, extraction time of 30.0 min.) b. Effect of applied voltage on the extraction efficiency. Extraction conditions (100 ng mL−1 of MG, sample solution pH 1.0, stirring rate 1000.0 rpm, extraction time 30.0 min, SLM solvent: 1-octanol.) c. Effect of extraction time on the extraction efficiency. Extraction conditions: (100 ng mL−1 of MG, applied voltage 40.0 V, sample solution pH of 1.0, stirring rate 1000.0 rpm, SLM solvent: 1-octanol.) d. Effect of pH of sample solution on the extraction efficiency. Extraction conditions: (100 ng mL−1 of MG, applied voltage 40.0 V, stirring rate 1000.0 rpm, extraction time 20.0 min, SLM solvent: 1-octanol)
Effect of voltage in EME
In the EME process, voltage plays a pivotal role in providing a significant and efficient electrokinetic migration. Voltage as driving force causes transport of MG ions across the SLM [29, 30]. To find the optimum condition, a series of experiments were carried out in the various applied potentials in the range 0.0–70 V. As can be seen in Fig. 2b, when extraction process was carried out free of voltage, the extraction efficiency was very low. But, the results showed that increasing in the applied potential; analyte could effectively migrate from the donor solution (DP) towards the acceptor solution (AP). Also, by increasing the voltage up to 40.0 V, the peak areas increased. Further, at the voltages higher than the 40.0 V, the extraction efficiency decreased that can be due to electrolysis reaction in donor and acceptor solutions via the following reactions.
Thus, as voltage increases, the concentration of hydronium ions in acceptor solution decreases; consequently, pH in the acceptor solution gradually increases. Also, at the higher voltages, bubble formation in the surface of SLM, resulting in reduction in flux of analyte ions across the SLM [31]. Consequently, voltage of 40.0 V was selected as applied potential across the SLM in the next experiments.
Effect of extraction time
Since in the EME process, there is an equilibrium distribution between two sides of SLM, time as one of the most crucial parameter plays a significant effect on the extraction efficiency. In this work, effect of extraction time on the extraction of MG was investigated in the range from 5.0 to 30.0 min. The results are shown in Fig. 2c The extraction efficiency of MG increased by increase in time up to the 20.0 min. In longer times, the peak areas for MG decreased. It may be due to joule heating phenomenon, which can result in evaporation or dissolution of organic phase in the sample solution. These effects might be attributed to unstabilization of the transport of analytes or could result in back-extraction of target analytes to the SLM. Therefore, for the rest of this work, extraction time of 20.0 min was chosen as the best time in the EME process.
Effect of the pH sample solution
In the following step of optimization, the pH value of donor solution was considered to explore the efficiency of extraction of MG. In fact, the target analytes should be in the ionized forms to be easily transferred across the SLM. The pH of donor solution was studied in the range of 1.0–4.0 to determine the optimum amount of extraction (Fig. 2d). Since the pKa of MG is 4.08, the MG in this pH range exists in its ionic form; therefore the MG carries a positive charge to be able to move under the electrical field toward the cathode electrode. Therefore, the decrease in the pH of the donor solution enhanced the concentration of ionic form of analyte in the sample solution, resulting in an increase the extraction efficiency. Thus, the pH of 1.0 was selected for the subsequent experiments.
Effect of stirring rate
In the EME process, the agitation speed can enhance the transfer of ions across the SLM and decrease the time required to reach a thermodynamic equilibrium [32, 33]. In the other words, agitation in the sample solution reduces the nernst diffusion layer thickness near SLM [34, 35]. Therefore, the effect of stirring rate on the extraction efficiency was assessed within the range of 250.0–1250.0 rpm with 20.0 min as extraction time (Fig. 3a). The results showed that the peak areas increased by increasing the stirring rate from 250.0 to 1000.0 rpm, but the paek area of MG decreased at higher stirring rates. It may be due to violent convection in the sample solution that cause the unstability of SLM. Hence, stirring rate of 1000.0 rpm was chosen in the subsequent experiments.
a Effect of stirring rate on the extraction efficiency. Extraction conditions: (100 ng mL−1 of MG, applied voltage 40.0 V, sample solution pH 1.0, extraction time 20.0 min, SLM solvent: 1-octanol.) b. Effect of salt addition on the extraction efficiency. Extraction conditions: (100 ng mL−1 of MG, applied voltage 40.0 V, sample solution pH 1.0, extraction time 20.0 min, SLM solvent: 1-octanol, stirring rate 1000 rpm)
Effect of ionic strength
Ionic strength is another parameter that can affect the flux of the analyte ions across SLM. According to previous studies on the EME method, increase in total ionic concentration of donor solution impresses the ion balance and causes decrease in flux of ions of donor solution to the SLM. To conduct the effect of ionic strength on the extraction efficiency of basic analyte, various concentrations of sodium chloride (NaCl, 0.0–5.0%, w/v) in the donor solution were assessed (Fig. 3b). The results showed that with increase in the concentration of sodium chloride in the solution, the competition among target analytes and sodium cations increased and in turn, the peak area decreased. Thus, migration of analyte ions would be more efficient in the absence of salt, and further studies were performed under such conditions.
Method validation
Analytical parameters of the proposed EME method was evaluated under the optimized extraction condition; Calibration curve was drawn with a concentration range of 5.0–250.0 ng mL−1 for the MG in water samples. The linearity was considered by the least-squares regression method, correlation coefficients (R2) 0.9948, and limit of detection 1.5 ng mL−1was assessed with (S/N = 3). Intra-day accuracy or repeatability of this method was performed by injection of five replicate of sample analyte (100 ng mL−1), while inter-day accuracy was studied by measurement of the same concentration over five days in succession. The RSDs were obtained to be in the range of 3.8–7.6%. This proposed method showed the acceptable reproducibility for the determination of MG (Table 1). Also, five sample solutions with a concentration of 100 ng mL−1 for the determination of preconcentration factor (PF) of target analyte were prepared. The PF was calculated as the ratio of the final concentration of an analyte in the acceptor solution to an initial concentration of the analyte in the donor solution.
Comparison of the proposed method with LPME and the other reported methods
The comparative study between the obtained results in the proposed method with some of analytical methods that have been reported in the literature for the determination of MG (Table 2). As can be seen, this presented method provided the lower LOD and high preconcentration factor rather than that of conventional procedures using molecularly imprinted solid phase extraction [36], cloud point extraction [37, 38], dispersive liquid-liquid microextraction [39], micro-cloud point extraction [18], and dispersive solid-phase extraction [40]. In addition, the other reported method usually requires moderate to large amounts of high-purity organic solvents that are potentially toxic and expensive. But, the consumption of organic solvent in the EME technique is minimal, without the need for solvent evaporation after the extraction, and the required equipment is very simple and inexpensive. On the other hand, the hollow fiber can be discarded after each extraction to eliminate possible carry-over and cross-contamination problems as compared to the solid-phase microextraction. Also, the excellent clean up obtained implies a great advantage over other sample treatment procedures. Moreover, the IMS due to swift response compared to the other methods minimizes the analysis time.
Real sample analysis
Owing to importance of poisonous substances in the environment and threatening the lives of organisms on the sea water, river water, wastewater, and fish farming waters, the measurement of this pollutant is crucial. To study the matrix effect and the applicability of the proposed method, determination of trace concentration of MG in the water samples were studied. For this purpose, five environmental water samples including Caspian sea water, wastewater from the Shahid Beheshti university, Shanderman river water, Fish farming water 1, and Fish farming water 2 were assessed under the optimum condition. To evaluate the matrix effect of the target analyte, the real samples were spiked with the MG standard solution at a concentration level of 20.0, and 50.0 ng mL−1 and the relative recoveries of this target were determined to be 85–98% (Table 3). Figure 4 was shown the Spectrums of the nonspiked and spiked in water sample, with 20.0 and 50.0 ng mL−1 of MG after EME extraction under the optimum conditions.
Conclusion
The present work demonstrated that EME procedure coupled with CD-IMS could be used for determination of MG. The proposed method has many advantageous such as simplicity, minimal usage of organic solvents, cheap, rapidity without any stage of sample preparation or clean up, and environmentally friendly technique. Moreover, due to the low cost of the extraction device in EME technique, the hollow fibers that used in any extraction can be discarded and avoid any memory effect or cross contamination. Also, this proposed method has acceptable accuracy and good sensitivity for the separation, preconcentration, and determination of trace concentration of MG in environmental waters.
References
Vörösmarty CJ, Green P, Salisbury J, Lammers RB (2000) Global water resources: vulnerability from climate change and population growth. Science 289(5477):284–288
Nidheesh PV, Zhou M, Oturan MA (2018) An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 197:210–227
Yan Q, Schmidt BF, Perkins LA, Naganbabu M, Saurabh S, Andreko SK, Bruchez MP (2015) Near-instant surface-selective fluorogenic protein quantification using sulfonated triarylmethane dyes and fluorogen activating proteins. Org Biomol Chem 13(7):2078–2086
Tanyol M (2017) Rapid malachite green removal from aqueous solution by natural zeolite: process optimization by response surface methodology. Desalin Water Treat 65:294–303
Wang D, Liu L, Jiang X, Yu J, Chen X (2015) Adsorption and removal of malachite green from aqueous solution using magnetic β-cyclodextrin-graphene oxide nanocomposites as adsorbents. Colloids Surf A Physicochem Eng Asp 466:166–173
Yakout AA, Shaker MA (2016) Dodecyl sulphate functionalized magnetic graphene oxide nanosorbent for the investigation of fast and efficient removal of aqueous malachite green. J Taiwan Inst Chem Eng 63:81–88
Srivastav AK, Roy D (2018) Acute toxicity of malachite green (triarylmethane dye) and pyceze (bronopol) on carbohydrate metabolism in the freshwater fish Heteropneustes fossilis (Bloch.). Int J Fish Aquat Stud 6:27–30
Bahram M, Keshvari F, Najafi-Moghaddam P (2011) Development of cloud point extraction using pH-sensitive hydrogel for preconcentration and determination of malachite green. Talanta 85(2):891–896
Gholami M, Vardini MT, Mahdavinia GR (2016) Investigation of the effect of magnetic particles on the crystal violet adsorption onto a novel nanocomposite based on κ-carrageenan-g-poly (methacrylic acid). Carbohydr Polym 136:772–781
Michaels GB, Lewis DL (1985) Sorption and toxicity of azo and triphenylmethane dyes to aquatic microbial populations. Environ Toxicol Chem 4(1):45–50
Baggiani C, Anfossi L, Baravalle P, Giovannoli C, Giraudi G, Barolo C, Viscardi G (2009) Determination of banned Sudan dyes in food samples by molecularly imprinted solid phase extraction-high performance liquid chromatography. J Sep Sci 32(19):3292–3300
Zhao C, Zhao T, Liu X, Zhang H (2010) A novel molecularly imprinted polymer for simultaneous extraction and determination of Sudan dyes by on-line solid phase extraction and high performance liquid chromatography. J Chromatogr A 1217(45):6995–7002
Long C, Mai Z, Yang Y, Zhu B, Xu X, Lu L, Zou X (2009) Determination of multi-residue for malachite green, gentian violet and their metabolites in aquatic products by high-performance liquid chromatography coupled with molecularly imprinted solid-phase extraction. J Chromatogr A 1216(12):2275–2281
Zhao J, Wei D, Yang Y (2016) Magnetic solid-phase extraction for determination of the total malachite green, gentian violet and leucomalachite green, leucogentian violet in aquaculture water by high-performance liquid chromatography with fluorescence detection. J Sep Sci 39(12):2347–2355
Mirzajani R, Bagheban M (2016) Simultaneous preconcentration and determination of malachite green and fuchsine dyes in seafood and environmental water samples using nano-alumina-based molecular imprinted polymer solid-phase extraction. Int J Environ Anal Chem 96(6):576–594
Sun H, Wang L, Qin X, Ge X (2011) Simultaneous determination of malachite green, enrofloxacin and ciprofloxacin in fish farming water and fish feed by liquid chromatography with solid-phase extraction. Environ Monit Assess 179(1–4):421–429
Jin Y, Ma P, Liang F, Gao D, Wang X (2013) Determination of malachite green in environmental water using cloud point extraction coupled with surface-enhanced Raman scattering. Anal Methods 5(20):5609–5614
Ghasemi E, Kaykhaii M (2016) Application of micro-cloud point extraction for spectrophotometric determination of malachite green, crystal violet and Rhodamine B in aqueous samples. Spectrochim Acta A Mol Biomol Spectrosc 164:93–97
Tao Y, Chen D, Chao X, Yu H, Yuanhu P, Liu Z, Yuan Z (2011) Simultaneous determination of malachite green, gentian violet and their leuco-metabolites in shrimp and salmon by liquid chromatography–tandem mass spectrometry with accelerated solvent extraction and auto solid-phase clean-up. Food Control 22(8):1246–1252
Xu B, Song D, Wang Y, Gao Y, Cao B, Zhang H, Sun Y (2014) In situ ionic-liquid-dispersive liquid–liquid microextraction of Sudan dyes from liquid samples. J Sep Sci 37(15):1967–1973
Gao Z, Liu T, Yan X, Sun C, He H, Yang S (2013) Application of ionic liquid-based microwave-assisted extraction of malachite green and crystal violet from water samples. J Sep Sci 36(6):1112–1118
Biparva P, Ranjbari E, Hadjmohammadi MR (2010) Application of dispersive liquid–liquid microextraction and spectrophotometric detection to the rapid determination of rhodamine 6G in industrial effluents. Anal Chim Acta 674(2):206–210
Pedersen-Bjergaard S, Rasmussen KE (2006) Electrokinetic migration across artificial liquid membranes: new concept for rapid sample preparation of biological fluids. J Chromatogr A 1109(2):183–190
Gjelstad A, Pedersen-Bjergaard S (2014) Electromembrane extraction—three-phase electrophoresis for future preparative applications. Electrophoresis 35(17):2421–2428
Li L, Lin Z-z, Chen X-m, Zhang H-y, Lin Y-d, Lai Z-z, Huang Z-y (2015) Molecularly imprinted polymers for extraction of malachite green from fish samples prior to its determination by HPLC. Microchim Acta 182(9-10):1791–1796. https://doi.org/10.1007/s00604-015-1513-9
Li G, Zhang X, Zhang L, Xu S, Li C (2015) Salt-assisted graphene oxide dispersive solid phase microextraction for sensitive detection of malachite green and crystal violet by HPLC. Chromatographia 78(15–16):979–985
Asadi M, Valadbeigi Y, Tabrizchi M (2019) Thermionic sodium ion source versus corona discharge in detection of alkaloids using ion mobility spectrometry. Int J Ion Mobil Spectrom 22:51–5828
Mirzaei F, Fakhari AR, Hashemzadeh A, Amini MM (2019) Sensitive determination of ketamine, methylphenidate, and tramadol in urine and wastewater samples by porous aromatic Framework-48 assisted electromembrane extraction coupled with ion mobility spectrometer. Int J Ion Mobil Spectrom:1–9. https://doi.org/10.1007/s12127-019-00255-x
Atarodi A, Chamsaz M, Moghaddam AZ, Tabani H (2017) Introduction of fullerene as a new carrier in electromembrane extraction for the determination of ibuprofen and sodium diclofenac as model acidic drugs in real urine samples. Chromatographia 80(6):881–890
Gjelstad A, Rasmussen KE, Pedersen-Bjergaard S (2007) Simulation of flux during electro-membrane extraction based on the Nernst–Planck equation. J Chromatogr A 1174(1–2):104–111
Seidi S, Yamini Y, Heydari A, Moradi M, Esrafili A, Rezazadeh M (2011) Determination of thebaine in water samples, biological fluids, poppy capsule, and narcotic drugs, using electromembrane extraction followed by high-performance liquid chromatography analysis. Anal Chim Acta 701(2):181–188
Sarafraz-Yazdi A, Amiri AH, Es’haghi Z (2008) BTEX determination in water matrices using HF-LPME with gas chromatography–flame ionization detector. Chemosphere 71(4):671–676
Balchen M, Gjelstad A, Rasmussen KE, Pedersen-Bjergaard S (2007) Electrokinetic migration of acidic drugs across a supported liquid membrane. J Chromatogr A 1152(1–2):220–225
Rezazadeh M, Yamini Y, Seidi S, Ebrahimpour B (2013) Electromembrane surrounded solid phase microextraction: a novel approach for efficient extraction from complicated matrices. J Chromatogr A 1280:16–22
Nojavan S, Sirani M, Asadi S (2017) Investigation of the continuous flow of the sample solution on the performance of electromembrane extraction: comparison with conventional procedure. J Sep Sci 40(19):3889–3897
Li YH, Yang T, Qi XL, Qiao YW, Deng AP (2008) Development of a group selective molecularly imprinted polymers based solid phase extraction of malachite green from fish water and fish feed samples. Anal Chim Acta 624(2):317–325
An L, Deng J, Zhou L, Li H, Chen F, Wang H, Liu Y (2010) Simultaneous spectrophotometric determination of trace amount of malachite green and crystal violet in water after cloud point extraction using partial least squares regression. J Hazard Mater 175(1–3):883–888
Pourreza N, Elhami S (2007) Spectrophtometric determination of malachite green in fish farming water samples after cloud point extraction using nonionic surfactant triton X-100. Anal Chim Acta 596(1):62–65
Bidari A, Ganjali MR, Norouzi P (2011) Surfactant enhance DLLME/FO-LADS: assay of malachite Green level in aquatic environment of trout fish. CLEAN–Soil, Air, Water 39(1):83–87
Razi-Asrami M, Ghasemi JB, Amiri N, Sadeghi SJ (2017) Simultaneous spectrophotometric determination of crystal violet and malachite green in water samples using partial least squares regression and central composite design after preconcentration by dispersive solid-phase extraction. Environ Monit Assess 189(4):196
Acknowledgments
Financial support from the Research Affairs of Shahid Beheshti University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mirzaei, F., Mohammadi Nilash, M. & Fakhari, A.R. Development of a new electromembrane extraction combined with ion mobility spectrometry for the quantification of malachite green in water samples. Int. J. Ion Mobil. Spec. 23, 153–160 (2020). https://doi.org/10.1007/s12127-020-00259-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12127-020-00259-y