Abstract
This study describes application of free liquid membrane (FLM) in micro-electromembrane extraction (μ-EME) of Cr(VI) from wastewater samples. Amount of Cr(VI) was quantified by electrothermal atomic absorption spectrometry. The transportation of Cr(VI) across the FLM was explored by electrokinetic migration and ion-exchange process. FLM and acceptor solution types, pH of donor and acceptor solutions, applied electrical potential, as well as FLM thickness were optimized. Presence of an anion exchange carrier (methyl trialkyl-ammonium chloride, Aliquat 336) in FLM facilitated Cr(VI) transportation. The best performance was observed for 1-octanol (containing 5% Aliquat 336) with thickness of 1 mm used as FLM, under applied electrical potential of 75 V, when 0.5 M NaClO4 and 0.1 M HCl were used as the acceptor and donor phases, respectively; and the extraction time was set to 5 min. Linearity was obtained in the working range of 0.5–14.0 ng mL−1 Cr(VI) (R2 > 0.98). The calculated limit of detection was below 0.06 ng mL−1. Application of this method to wastewater samples showed that relative recoveries of the spiked Cr(VI) in the samples were in the range of 73.8–85.1%, based on the standard addition method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Despite recent improvements in the field of analytical instruments, due to complexity of matrices and low concentration of target analyte, most samples cannot be directly injected into analytical instruments, so that sample pretreatment is necessary prior to analyses (Kuban and Bocek 2014a). Liquid-liquid extraction (LLE) (Pedersen-Bjergaard et al. 2000) and solid-phase extraction (SPE) (Snow 2000) are known procedures for sample clean-up and preconcentration of analytes. During the past two decades, microextraction procedures have been shown to be suitable alternatives for clean-up of samples with complex matrices and preconcentration of target analytes (Mehdinia and Aziz-Zanjani 2013; Bojko and Pawliszyn 2014). The microextraction procedures have been focused firstly on improving the disadvantages of LLE and SPE. Solid-phase microextraction (SPME) (Arthur and Pawliszyn 1990) has been developed as a solvent-free micro-scale extraction technique in the past two decades (Mehdinia and Aziz-Zanjani 2013; Memarian et al. 2016). In comparison with large-scale liquid–liquid extraction (LLE), liquid-phase microextraction (LPME) has more advantageous as it requires very small volumes of organic solvents, simple equipment and setup, in addition to showing high preconcentration factors and automation capability (Sarafraz-Yazdi and Amiri 2010). LPME is available in different modes including single-drop microextraction (SDME) (Jeannot et al. 2010), dispersive liquid-liquid microextraction (DLLME) (Zgoła-Grzeskowiak and Grzeskowiak 2011), and hollow-fiber based liquid phase microextraction (HF-LPME) (Rasmussen and Pedersen-Bjergaard 2004; Ghambarian et al. 2012). In HF-LPME, the fiber is impregnated with suitable organic solvent before acceptor solution is filed into the lumen of fiber; then, the fiber is placed into the stirring donor solution. The supported liquid membrane (SLM) separates the two donor and acceptor solutions, and analytes will be transferred across the SLM depending on their SLM-aqueous solutions partition coefficients. Although the problem of organic solvent instability has been resolved by HF-LPME procedure, long extraction time is required by the extraction processes to achieve equilibrium (Lee et al. 2008; Pedersen-Bjergaard and Rasmussen 2008). In order to accelerate this extraction procedure, analyte transfer was done by application of an electric potential in a procedure named electromembrane extraction (EME) (Pedersen-Bjergaard and Rasmussen 2006; Gjelstad and Pedersen-Bjergaard 2011; Petersen et al. 2011; Marothu et al. 2013; Gjelstad and Pedersen-Bjergaard 2013). Although EME is a quick and suitable extraction method, some new methods have been recently proposed to address its problems. For instance, Nojavan and Asadi (2016) proposed EME using two separate cells for the simultaneous extraction of acidic and basic analytes, Davarani et al.’s (2016) study introduced a new EME system through a virtually rotating SLM, and Moazami et al. (2014) developed a new pulse EME system to improve EME efficiency. A typical EME extraction device is made up of an organic solvent-impregnated porous polypropylene (PP) material to form an SLM. The SLM is then placed between two aqueous solutions (donor and acceptor) and acts as a barrier for analyte transfer from one solution (donor) into another (acceptor) (Marothu et al. 2013; Gjelstad and Pedersen-Bjergaard 2013). SLMs normally offer efficient omission of major sample matrix interferences (e.g., inorganic ions and proteins in biological samples) (Kuban and Bocek 2012). However, despite their great properties, SLMs are associated with various difficulties. Precise thickness of the SLM, which is essential for basic studies on extraction processes, is difficult to measure. Variability of the membrane thickness is limited by a narrow range of commercially accessible hollow fibers (e.g., inner/outer diameters (ID/OD) and material thickness) and may vary for larger sets of extraction units since the material fabrication process is not absolutely precise, and visual observation of the analyte migration is not possible as the membranes are not transparent. In addition, application of electric field across SLMs distorts the organic phase in SLMs, resulting in the collapse of EME technique (Kuban and Bocek 2014b), especially for biological samples with complex matrices. The matrices, which contain lipids, proteins, and other macromolecules, have usually an emulsification effect on the organic solvent and partial depletions of the organic phase in SLMs have been reported (Gjelstad et al. 2009). Then, the electric current flows mainly across the places of lowest resistance (i.e., depletions in SLMs) where the current increases drastically, resulting in overheating, SLM penetration, and ultimately collapse of the extraction system (Gjelstad et al. 2009; Strieglerova et al. 2011a, b).
Free liquid membranes (FLMs) have been recently proposed as an alternative to SLMs in EMEs (Kuban and Bocek 2014c). A typical FLM is formed as a plug of organic solvent, which is placed between donor and acceptor solutions, and is principally used in similar operational regimes as the SLM. Several aspects are, however, different; FLMs (and also donor and acceptor solutions) are characterized by their exact volume and thickness, cross-sectional areas of FLMs can be simply altered and the microextraction system is transparent, providing online visual monitoring of the whole extraction process including the cross-membrane transfer of analytes and bubble creations. Micro-electromembrane extraction (μ-EME) using FLM has been applied to extract basic drugs from undiluted biological samples (Kuban and Bocek 2014c), and also to extract an anionic dye, 4,5-dihydroxy-3-(p-sulfophenylazo)-2,7-naphthalene disulfonic acid, trisodium salt (SPADNS), and a cationic dye, crystal violet (Kuban and Bocek 2014b), to demonstrate the effects of electrolysis on acceptor and donor solutions in EME (Kuban and Bocek 2014d) and to preconcentrate anionic and cationic dyes, SPADNS, and phenosafranine (Kuban and Bocek 2014a).
Although most of the metals are present in cationic form (Kuban et al. 2011), some of them can also have anionic forms (metal oxoanions), for example: arsenate ion (AsO43−), chromate ion (CrO42−), and selenate ion (SeO42−). There are some reports on EME of anionic form of metals. In a report, Safari et al. (2013) proposed a dual EME system for the speciation of chromium using 1-octanol as the SLM, with their system operating at 30 V for 9 min. In another report, Chanthasakda et al. (2016) proposed an electro-assisted system (electro-enhanced HF-LPME) for determination of chromium using 1-heptanol containing 5% Aliquat 336 as the SLM. The system was applied at 30 V for 5 min of extraction time. Recently, speciation of Cr(VI) and Cr(III) using EME procedure was reported by Tahmasebi and Davarani (2016). All of the reported methods were applied for extraction of Cr(VI) from simple matrices such as drinking and tap water samples. Also most of them used UV-Vis as a less-sensitive detection system.
In the present work, the μ-EME of Cr(VI) oxoanion across FLM was assisted by an ionic carrier. This heavy metal was chosen as the model in this study regarding its toxicity in the environment. Determination of heavy metals in real wastewater samples is very difficult due to the presence of many interferences. Thus a superior clean-up and a suitable pre-concentration factor are two valuable outcomes of the mentioned procedure, leading to an accurate and selective measurement of the low concentrations of Cr(VI) in the complex wastewater samples including those produced by plating, textile, and paint manufacturing industries. Also, electrothermal atomic absorption spectrometry (ETAAS) as a high sensitive detection system was used after extraction for quantification of trace level of Cr(VI).
2 Materials and Methods
2.1 Chemicals
Organic solvents for FLM, i.e., 1-nonanol, 1-octanol, 1-heptanol, 1-hexanol, and 2-ethyl hexanol, (ETH) were obtained from Fluka (Buchs, Switzerland). The ionic carrier, tetra alkyl ammonium chloride (Aliquat 336), potassium dichromate (K2Cr2O7), hydrochloric acid (HCl), sodium hydroxide (NaOH), sodium perchlorate (NaClO4), sodium hydrogen sulfate (NaHSO4), sodium carbonate (Na2CO3), sodium chloride (NaCl), sodium orthovanadate (Na3VO4), standard solution of 1000 mg L−1 calcium chloride (CaCl2), chromium chloride (CrCl3), and lead nitrate (Pb(NO3)2) were purchased from Merck (Darmstadt, Germany). Ammonium orthomolybdate ((NH4)2MoO4) was obtained from Sigma Aldrich (St. Louis, MO, USA). All used chemicals were of analytical reagent grades. Deionized water was obtained by a Millipore water purification system (Milford, MA, USA).
2.2 Standard Solutions and Real Samples
Stock solution containing 0.1 mg mL−1 of Cr(VI) was prepared in deionize water. Then, the required working standard solutions were freshly prepared by diluting appropriate amount of stock solution with HCl solution to achieve the desired concentration of 3.0 ng mL−1 of Cr(VI) before extraction.
Plating wastewater sample was obtained from Sepehr Afroz Shimi Company (Tehran, Iran). Textile wastewater sample was obtained from Takestan Nassaji Company (Takestan, Iran). Paint wastewater sample was obtained from Khob Company (Tehran, Iran). Wastewater samples were diluted at 1:10 ratios using deionized water. Spiked samples were prepared by addition of stock standard solution of the analyte into the samples.
2.3 Instrumentation
2.3.1 Electromembrane Extraction Across Free Liquid Membrane
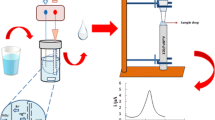
A schematic of μ-EME unit is shown in Fig. 1. The extraction device was made from a micro-hematocrit capillary tube (length 75 mm and ID 1.15 mm, volume 75 μL, Universal Medical Inc.), which is filled, successively, with acceptor solution, organic solvent (as the membrane), and finally with donor solution. All extractions were performed with the tubing height and volume of 7.5 cm and 75 μL, respectively. The volumes of acceptor solution, FLM, and donor solution were 6, 1, and 60 μL, respectively.
The μ-EME units were supplied by a EPS-600Z DC power supply (Paya Pajohesh Pars, Tehran, Iran) with a constant voltage in the range of 0–600 V, via platinum wires of 0.25 mm in diameter acting as electrodes. For μ-EME of Cr(VI), the positive electrode and negative electrode were placed into the acceptor and donor solutions, respectively, with the extraction voltage turned on. The extraction unit was positioned horizontally during the μ-EME. Extractions were performed at fully stagnant conditions, i.e., solutions in the units were not agitated/stirred. After the μ-EME, the acceptor phase was subjected to ETAAS.
2.3.2 Electrothermal Atomic Absorption Spectrometry
A Contor AA700 atomic absorption spectrophotometer (Jena, Germany) equipped with a graphite furnace atomizer GF 3000 and a continuous xenon lamp with a wavelength of 357.7 nm (as radiation source) was used to determine Cr(VI). The instrumental parameters and temperature program for the ETAAS are tabulated in Table 1. Used as sheath gas, argon (99.999% purity) was purchased from Roham Gas CO. (Tehran, Iran).
3 Results and Discussion
3.1 Free Liquid Membrane Composition
In electrically assisted extraction procedure, proper organic solvent selection as a membrane is very important (Yamini et al. 2014). The extraction efficiency of analytes has been shown to increase with decreasing viscosity of the organic solvent (Kuban and Bocek 2014b). In addition to organic solvent immiscibility in water, it must have an appropriate electrical resistance to minimize electrical current through the system even at high voltages (Balchen et al. 2009). In this experiment, extraction of Cr(VI) using five different organic solvents including 2-ethyl hexanol (ETH), 1-nonanol, 1-octanol, 1-heptanol, and 1-hexanol was investigated. Figure 2a shows the results of using mentioned organic solvents for the extraction. According to this, satisfactory results were obtained for 1-octanol when used as FLM. The better behavior of 1-octanol in comparison with 1-hexanol and 1-heptanol may be justified by its higher electrical resistance, which decreases the electrical current while increasing the system stability (Seidi et al. 2014). On the other hand, lower extraction recoveries were achieved when using 1-nonanol as the FLM. This was due to the higher viscosity of 1-nonanol which, in turn, decreased the corresponding diffusion coefficient (Middelthon-Bruer et al. 2008).
The effect of a organic solvent type, b carrier (Aliquat 336) concentration, and c membrane thickness on the extraction efficiency. Extraction conditions; voltage 100 V, donor solution pH 7 (deionized water containing 3.0 ng mL−1 Cr(VI)), acceptor solution NaClO4 (0.5 M, pH 7), extraction time 7.5 min, FLM thickness 1 μL (a, b), organic solvent (b, c): 1-octanol, carrier concentration (C): 5% w/v
3.2 Concentration of Aliquat 336 in 1-Octanol
Since Cr(VI) oxoanions are negatively charged, a cationic carrier was used for extraction. The ion exchange process between acceptor and donor phases by carrier is schematically described below:
where R3CH3N+ is the Aliquat 336, X− is the counter ion, and the subscripts (FLM), (DP), and (AP) indicate the location of the species in the FLM, donor solution, and acceptor solution, respectively.
In this experiment, the concentration of Aliquat 336 in 1-octanol was optimized. Investigating the amount of Aliquat 336 in the range of 0.25–10% (w/v), an increase in recovery was observed at the presence of the lowest amount of Aliquat 336. Figure 2b shows the recovery of μ-EME of Cr(VI) oxoanions across FLM for different values of Aliquat 336 in-1-octanol. The recovery increased even at low concentrations of Aliquat 336 (0.25%). The recovery gradually decreased as Aliquat 336 concentration exceeded 5%; i.e., an increase in the carrier concentration increased its viscosity; consequently, ionic migration might have been prohibited. Furthermore, increase in Aliquat 336 concentration would enhance the electrical conductivity of FLM, allowing for a rise in electrical current through the extraction system which would lead to bubble formation due to the occurrence of electrolysis at the electrodes (Chanthasakda et al. 2016). This may give rise to an unstable system making the results unreliable. For these reasons, 5% Aliquat 336 in 1-octanol was chosen as the optimum FLM.
3.3 Thickness of FLM
One of the most important advantages of this method (μ-EME) is that the thickness of three phases can be controlled with high precision by filling of different volumes of respective solutions into the microextraction device (Kuban and Bocek 2014b). Volume of an organic solvent aspirated into the μ-EME unit determines the thickness of FLM and thus the actual distance across which the analyte ions have to be transferred during μ-EME. In this experiment, the minimum FLM volume was set to 1.0 μL; two other volumes, at 0.5 μL increments, were also tested. The examined volumes of FLM (1.0, 1.5, and 2.0 μL) resulted in FLMs with thicknesses of ~ 1, 1.5, and 2 mm, respectively. The extraction efficiency increased with decrease of FLM thickness, where the maximum extraction recovery obtained with 1.0 μL of FLM. The magnitude of electrical resistance of organic layer as a membrane determines electrical current of the μ-EME system, so that significantly lower Cr(VI) transfer was obtained for thicker membranes. Figure 2c shows the recovery of μ-EME of Cr(VI) oxoanion across FLM for different FLM thicknesses. As the thickness of the organic layer between the acceptor and donor solutions is reduced, conductivity of the μ-EME system increases and, as a constant voltage was used, electrical filed experiences an increase, enhancing the extraction efficiency and vice versa (Kuban and Bocek 2014b).
3.4 Effect of Electrical Potentials
Based on the Nernst–Planck equation (Gjelstad et al. 2007), mass transfer of ions across the membrane is affected by applied voltage. μ-EME is based on the electromigration of charged analytes via an electric field. The flux of analytes through the membrane can be influenced by magnitude of applied voltage as strength of electric field depends on the applied voltage (Yamini et al. 2014; Gjelstad et al. 2007). To determine the optimal voltage, in this experiment, different electrical potentials (in the range of 25–125 V) were investigated. Figure 3 shows the effect of different voltages on the extractions efficiencies. Results showed that by increase of applied voltage up to 75 V, the analytical response increased; beyond this voltage, the recovery decreased. In other words, low voltages may decrease the extraction performance as it results in weak electric field; however, too high voltages (i.e., exceeding the optimum voltage) also decrease the extraction performance due to instability and bubble creation problems. Moreover, the magnitude of the electrical currents was observed in the range of 20–85 μA when the applied voltage varied in the range of 25–125 V. According to the results, the current linearly increased with the increase of the applied voltage, and a stable output was achieved when the constant voltage was in the range of 25–75 V. Higher voltages (> 75 V) led to unstable electrical currents, more electrolysis, and loss of acceptor solution. Thus, 75 V was chosen as the optimal potential voltage.
3.5 Acceptor Solution Composition
Composition of acceptor solution is an important factor affecting the extraction of analytes (Pedersen-Bjergaard and Rasmussen 2006). Since Cr(VI) oxoanions transfer across the membrane was not only driven by the electrokinetic migration, but also by an ion-exchange process, the acceptor solution should supply counter ions in order to enhance the ion exchange process described in Section 3.2.
In this work, 0.5 M NaCl and 0.5 M NaClO4 were investigated as supplies of Cl− and ClO4− counter ions, respectively. Figure 4a shows the effect of different types of acceptor solution on the extraction recovery. The results indicate that NaClO4 offered a higher preconcentration factor for the extraction of Cr(VI) than NaCl. The reason might be the structural similarity between ClO4− and HCrO4− which could result in more exchange. Different concentrations of NaClO4 in the acceptor solution (i.e., from 0.1 to 1.0 M) were studied in this study. Figure 4b shows the effect of different concentrations of NaClO4 on the extractions recoveries. The acceptor solution should have counter anions to be exchanged with the analyte. Results indicated the recovery increase by NaClO4 concentration enhancement in the acceptor solution. In addition, based on the Nernst–Planck equation (Gjelstad et al. 2007), the ion balance should be small, i.e., to obtain high flux, the sum of ions in the acceptor solution should be higher than that of donor solution. However, at NaClO4 concentrations greater than 0.5 M, the recovery dropped slightly. In fact, presence of higher concentrations of inorganic anions in acceptor solutions and their partial transfer across the FLM increased total μ-EME currents up to a certain concentration (Kuban and Bocek 2014c).
The effect of a salt type and b salt concentration on the extraction efficiency. Extraction conditions; voltage 75 V, donor solution pH 7 (deionized water containing 3.0 ng mL−1 Cr(VI)), acceptor solution NaClO4 (0.5 M, pH 7), extraction time 7.5 min, organic solvent 1-octanol, carrier concentration 5% w/v
3.6 Effect of Donor Solution pH
In an EME procedure, charged analytes are transferred from an aqueous sample solution into the acceptor solution through the organic membrane. In order to have ionic form of the target analytes, one can adjust pH value of the sample solution. In this work, the analyte-containing sample solution was diluted with different HCl and NaOH solutions to reach to pH values within the range of 1.0–13.0. Different pH values led to different extraction recoveries. Figure 5a shows the extraction recovery versus donor solution pH. Consequently, the highest extraction efficiency was achieved at pH = 1.0 (adjusted using HCl). According to ion exchange process described in Section 3.2, the chromium in HCrO4− form is suitable for this method as it interacts with the carrier. Thus, Cr(VI) is better to be in HCrO4− form to undertake the extraction through the FLM. On the other hand, considering different Cr(VI) structures at various pH values, entire deal of Cr(VI) in HCrO4− form at pH = 1, Fig. 5b shows a diagram of different structures of Cr(VI) in solutions at different pH values. Electric current (Rahmani et al. 2016) and bubble creation increase with acidity because of the corresponding reduction in H+ (2H+ + 2e = > H2). The high thickness of organic solvent between acceptor and donor solutions might be the cause for the decrease in electric current and bobble creation.
3.7 Effect of Acceptor Solution pH
It has been revealed that variation of pH value in the acceptor solution can influence the EME efficiency (Yamini et al. 2014; Seidi et al. 2014; Seidi et al. 2011). In this experiment, the acceptor solution pH was investigated in the range of 1.0–13.0. The obtained extraction recoveries are shown in Fig. 6a. The highest extraction recoveries were obtained at pH = 7.0. It seems that ion exchange process has less efficiency in acidic pH values and it increases with rise of pH in acceptor solution. Thus in this study, deionized water (pH ≈ 7) containing 0.5 M NaClO4 without any pH adjustment was used as an acceptor solution.
The effect of a pH of acceptor solution and b extraction time on the extraction efficiency. Extraction conditions; voltage 75 V, acceptor solution NaClO4 (0.5 M), donor solution pH 1.0 (containing 3.0 ng mL−1 Cr(VI)), organic solvent 1-octanol, carrier concentration 5% w/v, extraction time 7.5 min, acceptor solution pH (B) 7
3.8 Extraction Time
Extraction time is the main parameter in EME and determines the total amount of analytes extracted from sample solution (Strieglerova et al. 2011b). EME is known as a fast and higher efficiency procedure. In this experiment, in order to find the optimum extraction time, extractions were investigated with different extraction times (3–10 min). As shown in Fig. 6b, extraction efficiencies increased with increasing the extraction time, reaching maximum amount at first 5 min. In long extraction times, a contrary effect was seen on the extraction efficiency. In other words, at long extraction times (> 5 min), extraction recovery decreased, probably due to the electrolysis or back-extraction.
3.9 Validation of Proposed Method
In order to evaluate the practical applicability of the developed μ-EME technique for determination of chromium(VI), the optimized extraction conditions were used to determine the method detection limit, linearity, and precision. The performance of the method is summarized in Table 2. The linearity of the method was achieved in the range of 0.5–14 ng mL−1. Repeatability of the μ-EME-ETAAS measurements, reported as RSD values (n = 5) in instrument signals (absorbance), was 12.3% for 4.0 ng mL−1 of Cr(VI). External calibration curves were plotted and excellent linearity was achieved with correlation coefficient of 0.998 for Cr(VI). The enrichment factor (EF) and extraction recovery (ER) were calculated based on the following equations:
Where Ca,final is the final analyte concentration in the acceptor solution and Cd,initial denotes the initial analyte concentration within the sample solution. na,final shows the amount of analyte enriched in the acceptor solution while nd,initial is the total amount originally present in the sample solution. Va represents the volume of acceptor solution and Vd is the sample volume. EF of 9.1 was achieved which corresponds to recovery of 91.0%. The limit of detection (LOD) was experimentally calculated by considering the ETAAS signal to be distinctly discerned at a signal-to-noise (S/N) ratio of 3 at the final lowest concentration. Limit of detection (LOD) was found to be 0.06 μg L−1.
There are a number of reports on electrically assisted extraction methods used for the extraction of Cr(VI) ions from various samples. These methods were compared with μ-EME (Table 3). The comparison indicated that in terms of extraction time and recovery, μ-EME is highly efficient compared to previously reported methodologies. Also, the repeatability and LOD of the current method were comparable with other methods.
3.10 Effect of Interfering Ions
The influence of some cations and anions on the recovery of Cr(VI) using the proposed method was investigated. A variation on the extraction efficiency more than ± 10% was considered as interference for the extraction and determination of Cr(VI). Interferences were investigated in the presence of a constant concentration of analyte (10 ng mL−1) and different amounts of interfering ions. Table 4 shows the results for nine interfering species including VO43−, MnO42−, Ca2+, Pb2+, SO42−, PO43−, Cl−, Cr3+, and Mn2+. The recovery of Cr(VI) decreased with increasing the interfering ions in donor phase, so that a negative error was observed in electromembrane extraction of Cr(VI). This can be attributed to the interaction of HCrO4− with interfering cations that prevents the extraction of HCrO4− into the acceptor phase. Also, free anions from these salts will always be in donor solutions and as Cr(VI) is extracted as anion, co-extraction of these small anions will also play a significant role in Cr(VI) transport since Aliquat 336 is a non-selective anion-exchanger.
3.11 Analyses of Wastewater Samples
The matrix of the real sample can affect the extraction performance. Therefore, the standard addition procedure can be applied to obtain matrix-matched calibration curves for more accurate results (Chanthasakda et al. 2016). When using matrix-matched calibration curves, the recoveries of spiked Cr(VI) were excellent. To determine the amount of Cr(VI) in real samples, the real samples were first diluted, then filtered, and finally their pH values were adjusted to 1.0. By diluting the wastewater samples, the amount of produced electric current decreased and extraction system was stable. Also the repeatability of results increased. For plating manufacturing in three stages, 2, 4, and 6 ng mL−1 of Cr(VI) were added into the real sample. For textile manufacturing in three stages, 1, 2, and 4 ng mL−1 of Cr(VI) were added into the real sample. For paint manufacturing in three stages, 0.5, 1, and 2 ng mL−1 of Cr(VI) were added into real sample. The wastewater samples were analyzed according to the obtained optimum extraction conditions. Finally, the curve of absorbance unit in terms of added values of Cr(VI) was obtained for each sample. The results are shown in Table 5.
4 Conclusion
The development of analytical methods for speciation and determination of potentially toxic chemical elements has become of crucial importance, mainly when toxicological characteristics are dependent on the oxidation state, as can be seen for Cr(VI). As a simple, rapid, and environmentally friendly microextraction method with high sample clean-up, this study employed μ-EME across free liquid membrane for the selective extraction and preconcentration of Cr(VI) oxoanions from different wastewater samples. This method is based upon the electro-kinetic migration of ionized compounds, and by respective migration of Cr(VI) and Cr(III) ions to the positive and negative electrodes, Cr(VI) species (as carcinogenic agents) could be separated from the Cr(III) species in aqueous samples. The method yielded high recovery (> 90.0%). It was found that use of an ionic carrier such as Aliquat 336 could facilitate the transportation of ions across FLM. Providing the user with visual monitoring, this method allows observation of possible bubble creation and system collapse. In comparison to hollow fiber-based EME, μ-EME is a very simple microextraction procedure. In a way that filling the capillary tube with the organic and aqueous solvents, and the extraction process is comfortable and repeatable. The obtained results show that μ-EME is a promising choice for the selective extraction of the Cr(VI) species. Besides, it is environmentally friendly due to its low organic solvent consumption.
References
Arthur, C. L., & Pawliszyn, J. (1990). Solid phase microextraction with thermal desorption using fused silica optical fibers. Analytical Chemistry, 62, 2145–2148.
Balchen, M., Halvorsen, T. G., Reubsaet, L., & Pedersen-Bjergaard, S. (2009). Rapid isolation of angiotensin peptides from plasma by electromembrane extraction. Journal of Chromatography. A, 1216, 6900–6905.
Bojko, B., & Pawliszyn, J. (2014). In vivo and ex vivo SPME: A low invasive sampling and sample preparation tool in clinical bioanalysis. Bioanalysis, 6, 1227–1239.
Boutorabi, L., Rajabi, M., Bazregar, M., & Asghari, A. (2017). Selective determination of chromium (VI) ions using in-tube electro-membrane extraction followed by flame atomic absorption spectrometry. Microchemical Journal, 132, 378–384.
Chanthasakda, N., Nitiyanontakit, S., & Varanusupakul, P. (2016). Electro-enhanced hollow fiber membrane liquid phase microextraction of Cr(VI) oxoanions in drinking water samples. Talanta, 148, 680–685.
Davarani, S. S. H., Moazami, H. R., Memarian, E., & Nojavan, S. (2016). Electromembrane extraction through a virtually rotating supported liquid membrane. Electrophoresis, 37, 339–346.
Ghambarian, M., Yamini, Y., & Esrafili, A. (2012). Developments in hollow fiber based liquid-phase microextraction: principles and applications. Microchimica Acta, 177, 271–294.
Gjelstad, A., & Pedersen-Bjergaard, S. (2011). Electromembrane extraction: a new technique for accelerating bioanalytical sample preparation. Bioanalysis, 3, 787–797.
Gjelstad, A., & Pedersen-Bjergaard, S. (2013). Recent developments in electromembrane extraction. Analytical Methods, 5, 4549–4557.
Gjelstad, A., Rasmussen, K. E., & Pedersen-Bjergaard, S. (2007). Simulation of flux during electro-membrane extraction based on the Nernst–Planck equation. Journal of Chromatography. A, 1174, 104–111.
Gjelstad, A., Rasmussen, K. E., & Pedersen-Bjergaard, S. (2009). Electromembrane extraction of basic drugs from untreated human plasma and whole blood under physiological pH conditions. Analytical and Bioanalytical Chemistry, 393, 921–928.
Jeannot, M. A., Przyjazny, A., & Kokosa, J. M. (2010). Single drop microextraction—development, applications and future trends. Journal of Chromatography. A, 1217, 2326–2336.
Kuban, P., & Bocek, P. (2012). Capillary electrophoresis with capacitively coupled contactless conductivity detection: a universal tool for the determination of supported liquid membrane selectivity in electromembrane extraction of complex samples. Journal of Chromatography. A, 1267, 96–101.
Kuban, P., & Bocek, P. (2014a). Preconcentration in micro-electromembrane extraction across free liquid membranes. Analytica Chimica Acta, 848, 43–50.
Kuban, P., & Bocek, P. (2014b). Micro-electromembrane extraction across free liquid membranes. Instrumentation and basic principles. Journal of Chromatography. A, 1346, 25–33.
Kuban, P., & Bocek, P. (2014c). Micro-electromembrane extraction across free liquid membranes. Extractions of basic drugs from undiluted biological samples. Journal of Chromatography. A, 1337, 32–39.
Kuban, P., & Bocek, P. (2014d). The effects of electrolysis on operational solutions in electromembrane extraction: the role of acceptor solution. Journal of Chromatography. A, 1398, 11–19.
Kuban, P., Strieglerova, L., Gebauer, P., & Bocek, P. (2011). Electromembrane extraction of heavy metal cations followed by capillary electrophoresis with capacitively coupled contactless conductivity detection. Electrophoresis, 32, 1025–1032.
Lee, J. Y., Lee, H. K., Rasmussen, K. E., & Pedersen-Bjergaard, S. (2008). Environmental and bioanalytical applications of hollow fiber membrane liquid-phase microextraction: a review. Analytica Chimica Acta, 624, 253–268.
Marothu, V. K., Gorrepati, M., & Vusa, R. (2013). Electromembrane extraction—a novel extraction technique for pharmaceutical, chemical, clinical and environmental analysis. Journal of Chromatographic Science, 51, 619–631.
Mehdinia, A., & Aziz-Zanjani, M. O. (2013). Advances for sensitive, rapid and selective extraction in different configurations of solid-phase microextraction. Trends in Analytical Chemistry, 51, 13–22.
Memarian, E., Davarani, S. S. H., Nojavan, S., & Kazemi-Movahed, S. (2016). Direct synthesis of nitrogen-doped graphene on platinum wire as a new fiber coating method for the solid-phase microextraction of BXes in water samples: comparison of headspace and cold-fiber headspace modes. Analytica Chimica Acta, 935, 151–160.
Middelthon-Bruer, T. M., Gjelstad, A., Rasmussen, K. E., & Pedersen-Bjergaard, S. (2008). Parameters affecting electro membrane extraction of basic drugs. Journal of Separation Science, 31, 753–759.
Moazami, H. R., Nojavan, S., Zahdi, P., & Davarani, S. S. H. (2014). Electronic simulation of the supported liquid membrane in electromembrane extraction systems: improvement of the extraction by precise periodical reversing of the field polarity. Analytica Chimica Acta, 841, 24–32.
Nojavan, S., & Asadi, S. (2016). Electromembrane extraction using two separate cells: a new design for simultaneous extraction of acidic and basic compounds. Electrophoresis, 37, 587–594.
Pedersen-Bjergaard, S., & Rasmussen, K. E. (2006). Electrokinetic migration across artificial liquid membranes: new concept for rapid sample preparation of biological fluids. Journal of Chromatography. A, 1109, 183–190.
Pedersen-Bjergaard, S., & Rasmussen, K. E. (2008). Liquid-phase microextraction with porous hollow fibers, a miniaturized and highly flexible format for liquid–liquid extraction. Journal of Chromatography. A, 1184, 132–142.
Pedersen-Bjergaard, S., Rasmussen, K. E., & Halvorsen, T. G. (2000). Liquid–liquid extraction procedures for sample enrichment in capillary zone electrophoresis. Journal of Chromatography. A, 902, 91–105.
Petersen, N. J., Rasmussen, K. E., Pedersen-Bjergaard, S., & Gjelstad, A. (2011). Electromembrane extraction from biological fluids. Analytical Sciences, 27, 965–972.
Rahmani, T., Rahimi, A., & Nojavan, S. (2016). Study on electrical current variations in electromembrane extraction process: relation between extraction recovery and magnitude of electrical current. Analytica Chimica Acta, 903, 81–90.
Rasmussen, K. E., & Pedersen-Bjergaard, S. (2004). Developments in hollow fibre-based, liquid-phase microextraction. Trends in Analytical Chemistry, 23, 1–10.
Safari, M., Nojavan, S., Davarani, S. S. H., & Morteza-Najarian, A. (2013). Speciation of chromium in environmental samples by dual electromembrane extraction system followed by high performance liquid chromatography. Analytica Chimica Acta, 789, 58–64.
Sarafraz-Yazdi, A., & Amiri, A. (2010). Liquid-phase microextraction. Trends in Analytical Chemistry, 29, 1–14.
Seidi, S., Yamini, Y., Heydari, A., Moradi, M., Esrafili, A., & Rezazadeh, M. (2011). Determination of thebaine in water samples, biological fluids, poppy capsule, and narcotic drugs, using electromembrane extraction followed by high-performance liquid chromatography analysis. Analytica Chimica Acta, 701, 181–188.
Seidi, S., Rezazadeh, M., Yamini, Y., Zamani, N., & Esmaili, S. (2014). Low voltage electrically stimulated lab-on-a-chip device followed by red-green-blue analysis: a simple and efficient design for complicated matrices. Analyst, 139, 5531–5537.
Snow, N. H. (2000). Solid-phase micro-extraction of drugs from biological matrice. Journal of Chromatography. A, 885, 445–455.
Strieglerova, L., Kuban, P., & Bocek, P. (2011a). Rapid and simple pretreatment of human body fluids using electromembrane extraction across supported liquid membrane for capillary electrophoretic determination of lithium. Electrophoresis, 32, 1182–1189.
Strieglerova, L., Kuban, P., & Bocek, P. (2011b). Electromembrane extraction of amino acids from body fluids followed by capillary electrophoresis with capacitively coupled contactless conductivity detection. Journal of Chromatography. A, 1218, 6248–6255.
Tahmasebi, Z., & Davarani, S. S. H. (2016). Selective and sensitive speciation analysis of Cr(VI) and Cr(III), at sub-μg/L levels in water samples by electrothermal atomic absorption spectrometry after electromembrane extraction. Talanta, 161, 640–646.
Yamini, Y., Seidi, S., & Rezazadeh, M. (2014). Electrical field-induced extraction and separation techniques: promising trends in analytical chemistry—a review. Analytica Chimica Acta, 814, 1–22.
Zgoła-Grzeskowiak, A., & Grzeskowiak, T. (2011). Dispersive liquid-liquid microextraction. Trends in Analytical Chemistry, 30, 1382–1399.
Acknowledgements
Financial support from the Research Affairs of Shahid Beheshti University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nojavan, S., Rahmani, T. & Mansouri, S. Selective Determination of Chromium(VI) in Industrial Wastewater Samples by Micro-Electromembrane Extraction Combined with Electrothermal Atomic Absorption Spectrometry. Water Air Soil Pollut 229, 89 (2018). https://doi.org/10.1007/s11270-018-3744-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-018-3744-y