Abstract
Background
Keratoameloblastoma (KA) is an uncommon and controversial variant of ameloblastoma exhibiting central keratinisation. Due to their rarity, there is limited information in the literature on their clinical, radiologic and histologic features. This study adds seven additional cases of KA to the literature, and reviews the current published literature on this rare entity.
Methods
KAs were retrospectively reviewed over a 20-year period from three Oral and Maxillofacial Pathology Laboratories. Included cases were examined and the diagnosis confirmed under conventional microscopy. Immunohistochemistry with the use of a monoclonal antibody against calretinin was performed on included cases. The clinical, radiologic and histologic features of the seven new cases of KA were analysed and compared to existing cases in the literature.
Results
KAs presented at a mean age of 40 years with a nearly equal gender distribution and a mandibular predilection (65%). The majority (92%) of cases presented with localised swelling with associated pain in 32% of cases. Mixed density or internal calcifications were noted in 40% of cases. All tumours presented with bony expansion, with cortical destruction noted in 62% of cases. Histologically, all tumours consisted of solid and cystic follicles with surface parakeratinisation and lamellated accumulations of central keratin. In areas the cystic follicles had an epithelial lining suggestive of an OKC. There were focal luminal areas of loosely arranged polygonal cells reminiscent of the stellate reticulum. The basal cells consisted of columnar cells with evidence of palisading and prominent subnuclear vacuolisation. Of the cases treated via tumour resection, 27% presented with tumour recurrence.
Conclusion
This case series reports seven additional cases of KA, taking the total to 26 reported cases. The identification of subtle histologic features, including focal stellate reticulum-like central areas, subnuclear vacuolisation and lamellated-type central keratinisation, are key in diagnosing KA. The radiologic features will often indicate signs of aggressiveness such as cortical destruction, differentiating KA from OKC. All cases were completely negative for calretinin IHC, limiting its use in distinguishing KA from OKC. Further large series are needed to expand the current understanding of this rare variant of ameloblastoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ameloblastoma is a benign epithelial odontogenic neoplasm with aggressive biological behaviour and a tendency for local recurrence if incompletely excised [1,2,3]. In the 2017 World Health Organization (WHO) classification of benign odontogenic tumours, ameloblastomas are subdivided to include ameloblastoma (often termed conventional ameloblastoma), unicystic ameloblastoma and extraosseous/peripheral ameloblastoma [3,4,5]. Several histologic variants have been described, with two or more variations often occurring in the same tumour [3, 6]. More recently, the rare variant adenoid ameloblastoma (with dentinoid), has been better characterised, and shown to lack the characteristic molecular mutations often seen in conventional ameloblastomas [7, 8]. These variants generally do not influence tumour behaviour or surgical management [6].
Histologic variants of ameloblastoma that exhibit keratinisation include acanthomatous ameloblastoma, keratoameloblastoma (KA), and papilliferous keratoameloblastoma (PKA) [6, 9, 10]. Of these, intraosseous KA and PKA are extremely rare tumours with 19 cases of KA (Table 1) [6, 9,10,11,12,13,14,15,16,17,18,19,20,21,22] and only six cases of PKA (Table 2) [16, 23,24,25,26,27,28] reported in English-language literature. These figures may however not be entirely accurate as many cases may be undiagnosed or incorrectly interpreted as other entities due to the considerable histologic overlap. A single case of KA involving the palate, without bone involvement (peripheral KA), has been reported in a 32-year-old female [29].
The term “keratoameloblastoma” was first coined by Pindborg in 1970 [28], with the first confirmed case reported by Altini et al. in 1976 [11]. In 1992, the WHO loosely defined KA as an ameloblastoma with extensive keratinization [30]. Siar and Ng better defined the entity with a series of four cases in 1993 [12]. Subsequently, controversy emerged regarding the classification of KA as a separate subtype of ameloblastoma [9]. Norval et al. suggested that keratoameloblastoma be considered a variant of acanthomatous ameloblastoma [13]. Siar and Ng refuted this claim, noting that acanthomatous changes in ameloblastomas are relatively common, whereas keratinisation is rare, supporting the term KA [12]. The 2017 WHO classification of benign odontogenic tumours makes no mention of KA, reflecting an editorial decision to limit the classification to well-defined entities [3].
Keratoameloblastomas have been diagnosed in patients between 21 and 76 years of age, with a mean age of 44 years [9, 16, 17]. Initial studies revealed a distinct male predilection, with a male-to-female ratio of 3:1. However, an up-to-date literature review revealed a total of 10 cases in males and 9 cases in females, illustrating an almost equal gender distribution [6, 16, 17, 22, 31]. Overall, the most commonly affected location is the posterior mandible, with most cases presenting as painful swellings. KAs present radiologically as unilocular or multilocular radiolucencies with isolated cases exhibiting radiopacities within the lesion [16, 17]. Lee et al. noted that the presence of scattered hard tissue attenuation was a characteristic feature of KAs [9]. They postulated that this feature represented dystrophic calcification of the extensive keratin and necrotic debris produced by the tumour [9]. Displacement and root resorption of the adjacent teeth are common findings. KAs typically display bony expansion and perforation of the cortical plates [9, 16].
Since its initial description, lesions reported as KA have included a broad spectrum of histopathologic appearances [16]. The most thorough discussion regarding the histologic description of this tumour was undertaken by Whitt et al. [16], who subclassified it into 4 main groups: (1) papilliferous; (2) simple; (3) simple with odontogenic keratocyst-like features, and (4) complex. Overall, KAs typically show the following histologic features:
-
1.
Scattered cystic and solid epithelial follicles embedded in a mature fibrous connective tissue stroma consisting of loosely arranged polygonal or angular cells resembling the stellate reticulum of the enamel organ, and palisaded tall, columnar, ameloblast-like cells at the periphery;
-
2.
Epithelial follicles show extensive surface parakeratinisation with lamellated, “Pacinian-like” accumulations of central keratin;
-
3.
Some of these cystic follicles may have an epithelial lining suggestive of an odontogenic keratocyst (OKC);
-
4.
Prominent hard tissue formation showing a woven bone- or cementum-like appearance [6, 12, 13, 16, 20].
Given the non-encapsulated and locally infiltrative growth pattern of KA, wide excision accompanied by close clinical follow-up is regarded as the most appropriate treatment [16, 20]. The biological behaviour and prognosis of these rare tumours compared with other histologic variants of ameloblastoma is inconclusive due to limited reported cases and inadequate follow-up data [22].
Due to their rarity, there is limited information in the literature on the clinical, radiologic and histologic features of KA. This study aims to add an additional seven cases of KA to the literature, as well as review the current published literature on this rare entity. In addition, the key histologic features of these rare tumours are emphasised, which may serve as a useful reference in distinguishing KA from other histologic mimics.
Materials and Methods
The present retrospective descriptive study included seven cases of keratoameloblastoma retrieved from the archives of three Oral and Maxillofacial Pathology Laboratories as follows: four cases from South Africa (University of Pretoria); two cases from Brazil (Federal University of Rio de Janeiro); and one case from the United Kingdom (University of Sheffield). Demographic, clinical and radiographic data were reviewed for the period between 2000 and 2020 (20-years). All cases were examined under conventional microscopy using 4 um hematoxylin and eosin (H&E)-stained sections. The H&E slides were then scanned into high-resolution images using the Aperio Scanscope Slide Scanner (Leica Microsystems, Wetzlar, Germany), obtaining images in a “.svs” format and visualised using the ImageScope software (Leica microsystems). The final diagnosis of keratoameloblastoma was agreed upon by all authors based on previous histopathologic features of reported cases and as best described by Whitt et al. [16].
Calretinin has been proposed as a useful immunohistochemical marker in distinguishing ameloblastoma from other odontogenic entities [32,33,34,35]. Therefore, immunohistochemistry with the use of a monoclonal antibody against calretinin, was performed on all cases. Heat antigen retrieval in TRIS/EDTA high pH buffer was performed on 3 µm FFPE tissue block sections using a Dako PT Link retrieval unit (Agilent Technologies). After treatment with hydrogen peroxidase, sections were incubated in a 1:20 dilution of monoclonal mouse anti-human lyophilized calretinin antibody (Clone CAL6, Novocastra, LeicaBiosysytems, UK) for 60 min at room temperature and rinsed thereafter. Detection was performed with Novolink™ Polymer Detection System (Novocastra, Leica Biosystems) where after the sections were counterstained with Haematoxylin and mounted with a permanent mounting media. Appropriate positive controls were included in all cases.
The clinical, radiologic and histologic features of the seven additional cases of KA were analysed and compared to existing cases in the literature. All data was extracted from the reported cases and collated in a table format. For the radiologic features, either the in-text description was reviewed or included radiographs analysed.
The study was conducted following approval by the Faculty of Health Sciences Research Ethics Committee, University of Pretoria (33/2021). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Results
The detailed clinical and radiologic data of the seven cases of keratoameloblastoma included in this study are summarised in Table 3. The seven cases included five females and two males, with a mean age of 40 years (range 29–48 years). Three tumours affected the maxilla and four occurred within the mandible. The posterior regions of the jaws were more commonly involved, with three cases extending to involve the anterior regions. The main clinical presentation for all cases was an asymptomatic swelling in the affected region. The reported duration of symptoms was available in five cases, with a mean period of 12 months.
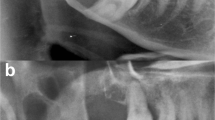
Radiographic examinations were available in all cases. Tumours were mainly described as well-circumscribed radiolucent lesions that often exhibited a loss of demarcation involving one of the tumour margins. Three cases presented as unilocular lesions and four as multilocular lesions. Five cases showed evidence of internal calcifications (Fig. 1). Root resorption and teeth displacement by the tumour was seen in four and three cases respectively. Cortical perforation was apparent in six cases, whereas two maxillary cases showed evidence of sinus encroachment (Fig. 2).
Microscopically, all cases showed similar histologic features, demonstrating solid and cystic tumour follicles in a fibrous connective tissue stroma with varying degrees of chronic inflammation (Fig. 3A). The follicles showed extensive surface parakeratinisation with lamellated accumulations of central keratin (Fig. 3B). In areas the cystic follicles had an epithelial lining suggestive of an OKC (Fig. 3C). Importantly, all cases showed at least focal luminal areas of loosely arranged polygonal cells reminiscent of the stellate reticulum of the enamel organ (Fig. 3D). The basal cells were comprised of tall, columnar cells with evidence of palisading and prominent subnuclear vacuolisation (Fig. 3E). Four cases showed scattered dystrophic calcifications with an associated foreign body giant cell response (Fig. 3F). Immunohistochemical (IHC) staining for calretinin was performed on all cases, with a complete absence of staining in all tumours.
Histopathologic features of keratoameloblastomas. A Solid and cystic tumour follicles in a fibrous connective tissue stroma (original magnification × 20). B Follicles containing abundant lamellated central keratin (original magnification × 40). C Epithelial lining suggestive of an OKC (original magnification × 100). D Other areas more reminiscent of the stellate reticulum (original magnification × 200). E Basal columnar cells with nuclear palisading and subnuclear vacuolisation (original magnification × 200). F Scattered dystrophic calcifications with a foreign body giant cell response (original magnification × 100)
Data regarding treatment was available for six cases. One patient only underwent an incision biopsy of the tumour and was subsequently lost to follow-up. All remaining patients were treated via surgical resection. Follow-up data was available in six cases, with recurrence only noted in one patient 6 years after tumour resection.
The addition of seven new cases with those reported in the literature takes the total to 26 cases of KA, which were available for further analysis. Overall, KA presented at a mean age of 40 years (range 21–76). The male to female ratio was 1:1.2, indicating a nearly equal gender distribution. The reported clinical duration of tumour presentation (data was only available for 18 patients) was 19 months with a range of 2 weeks to more than 84 months. Symptoms were reported in 25 patients, with the majority (92%) presenting with localised swelling. Associated pain was reported in 32% of cases and was often described as dull or intermittent. Less frequent reported symptoms included tooth mobility (12%), with paraesthesia and a mass lesion extruding from an extraction socket being reported in one case each. The posterior regions of both jaws were involved in 62% of cases and the anterior region in 27% of cases. Three cases (12%) in the current series extended to involve both the anterior and posterior segments of the jaw. KA had a predilection for the mandible (65%) over the maxilla (35%). The involved subsites in decreasing frequency were: posterior mandible (50%), anterior mandible and posterior maxilla (both 23%), and lastly the anterior maxilla (15%) with some cases extending to involve more than one subsite.
The radiologic borders of the tumour were analysed in 20 cases, with well-defined borders seen in 45% of cases, poorly-defined borders in 20% of cases, and a focal loss of border definition in 35% of cases. Tumour locularity was analysed in 24 cases, with 54% of cases appearing multilocular and the remainder having a unilocular appearance. Unilocular cases had a nearly equal mandibular and maxillary distribution, but 77% of multilocular cases occurred in the mandible. Radiologic tumour density was described in 25 cases, with purely radiolucent lesions noted in 60% of cases. This figure may not be entirely accurate due to the lack of advanced imaging in a number of cases. Internal calcifications were noted in 32% of cases, with either a mixed density or ground glass appearance noted in 8% of cases. All lesions resulted in bony expansion, with cortical destruction noted in 62% of cases. Tooth displacement (23%) was more common than root resorption (15%). Encroachment of the maxillary sinus or nasal cavity was noted in 33% of maxillary cases.
The treatment of patients with KA was reported in 24 cases, with 19 cases (79%) receiving resection and 5 cases (21%) receiving conservative treatment consisting of either enucleation or curettage. One patient only underwent an incision biopsy during the course of this study. Follow-up information was available for 15 patients. Of the cases treated via tumour resection, 3 cases (27%) had recurrence occurring at 7 months, 5 and 6 years respectively. Eight cases (73%) treated via resection had no evidence of recurrence at the time of the respective studies. For tumours treated conservatively, two cases had recurrence (50%), one with six recurrences, and two had no evidence of recurrence (50%) after a maximum follow-up period of 1 year.
Discussion
Keratoameloblastoma is a rare variant of ameloblastoma exhibiting surface parakeratinisation and central keratinisation in a so-called lamellated pattern [6, 9, 10]. The term KA was first coined by Pindborg in 1970 [28]. However, it was not until 1976 that the first case of KA was described by Altini et al. [11]. The 1992 WHO classification first loosely defined the entity as a variant of ameloblastomas with extensive keratinization [30]. Since then, several small case series and isolated case reports have attempted to better describe this rare variant of ameloblastoma.
According to the literature, KA has a predilection for male patients and affects a wide age range [9, 16, 17]. In the current review, patients with KA presented at a similar mean age as conventional ameloblastomas with a peak incidence in the 3rd to 5th decades of life [36]. The nearly equal male to female ratio of KA also corresponded to that of conventional ameloblastomas. The reported clinical duration is slightly longer than that of conventional ameloblastomas, with cases with associated pain presenting earlier [37]. The reported symptoms of pain and swelling also correspond to the current literature [37]. Although a mandibular predominance was found in the current study (1.9:1), the predilection for the mandible was not as dominant as larger reports on conventional ameloblastomas (10:1) [36]. The posterior mandible is the most common subsite for KA, corresponding to that of conventional ameloblastomas [36, 37].
Keratoameloblastoma usually presents as either unilocular or multilocular expansile radiolucencies with areas of cortical perforation and evidence of central hard tissue attenuation [16, 17]. The radiologic borders of KA tend to have higher frequencies of loss of demarcation than conventional ameloblastomas, although the locularity corresponds to that of conventional ameloblastomas [36, 37]. Internal calcifications or a mixed radiologic appearance have been reported in conventional ameloblastomas, but at lower frequencies than that of KA (37%) [36, 38]. An exception is the desmoplastic variant of ameloblastoma, which often presents as a mixed density lesion [39]. Dystrophic calcifications could explain the mixed radiologic appearance seen in some ameloblastomas, particularly the acanthomatous variant [40]. In KA, the mixed radiologic appearance is due to the extensive keratin production undergoing dystrophic calcification. The effects on surrounding structures, such as cortical perforation, is also not unlike conventional ameloblastomas. However, root resorption in KA appears to be less frequent compared to conventional ameloblastomas [38]. Unfortunately, some radiologic features, such as the frequency of cortical perforation, root resorption and internal calcifications, may have been underreported due to a lack of advanced imaging in many cases.
Whitt et al. proposed a subclassification system for KAs following an extensive review of the histologic features of previously reported cases. The current authors consider this subclassification to be overly complicated and unnecessary. Furthermore, in contrast to Whitt et al. [16], we recognise KA and PKA as separate entities, diagnosed as such based on their unique histologic features.
Keratoameloblastoma is characterised by cystic and solid epithelial follicles in a fibrous stroma. The follicles consist of discohesive polygonal or angular cells resembling the stellate reticulum. In the current series, the stellate reticulum-like central areas were minimal, possibly due to extensive central keratinisation. The peripheral aspect of the follicles shows tall, columnar cells with reverse nuclear polarity and subnuclear vacuolisation [6, 12, 13, 16]. The current authors believe that this subnuclear vacuolisation is particularly important in distinguishing KA from OKC. All cases in the current series showed prominent subnuclear vacuolisation. These follicles often have an epithelial lining resembling an OKC, but contain extensive central parakeratinisation giving a characteristic lamellated appearance, another key feature in the diagnosis of KA. This feature was seen in all cases in the current study. The keratinisation often undergoes dystrophic calcification resulting in the characteristic luminal hard tissue attenuation seen on radiographic examination [9]. Central dystrophic calcifications were present in 4 of the current cases, 2 of which showed evidence of radiologic signs on advanced imaging.
The so-called solid variant of OKC (SOKC) and OKCs with multiple daughter cysts may show a significant clinical and histologic overlap with KA [20, 31, 41,42,43]. This overlap has resulted in diagnostic difficulties in distinguishing these histologically similar, but separate entities. To date, only nine reported cases of SOKC have been described in the English literature [31]. Moreover, some authors consider SOKC and KA to be histogenetically related, with some postulating ameloblastomatous transformation of an existing OKC [22, 42,43,44]. Additionally, a correspondence letter from Ide et al. reports a case of unicystic ameloblastoma showing late recurrence as a mixture of follicular ameloblastoma and KA, further raising additional diagnostic and classification difficulties [44]. The current authors believe that the term “solid” OKC is a misnomer, as the entity actually consists of variably sized cysts and solid islands embedded in a fibrous stroma. The histologic distinction between KA and SOKC is important, as it has been suggested that KA may have a higher recurrence rate than SOKC [31]. Geng et al. identified two histologic criteria that may useful in distinguishing these entities. The prominent histologic pattern should determine the final diagnosis. This paired with the location of keratin formation, which in a KA presents as luminal lamellated keratinisation, may also be a helpful distinguishing feature [43]. When available, molecular testing may also be useful in distinguishing these rare entities. Zhang et al. recently screened nine cases of SOKC for BRAF V600E and PTCH1 mutations, finding only a single missense mutation for PTCH1. Of note, all nine cases were negative for BRAF V600E mutations, a mutation found in up to 90% of ameloblastomas [31]. These results should however be viewed with caution as no clear criteria were defined to distinguish SOKC from KA. Carcinoma cuniculatum (CC) also represents an important entity in the differential diagnosis of KA [45]. Although this entity more commonly involves the plantar surface of the foot, oral manifestations are well reported in the literature. CC is a locally aggressive malignancy, often presenting with bone destruction. The histologic appearance of keratin-filled channels and cores lined by well-differentiated squamous epithelium gives rise to the so-called “rabbit-burrow” appearance. Neutrophilic microabscesses are a common feature of this entity [45, 46]. Overall, these features may result in the misdiagnosis of an infected keratin-forming cyst [45]. Primary intraosseous carcinoma (PIOC) is another keratinising malignant tumour of the jawbones that arises from odontogenic epithelium [3]. These may arise de novo, from odontogenic cysts or in rare instances from odontogenic tumours. PIOC typically resembles keratinising squamous cell carcinoma with varying degrees of dysplasia, allowing distinction from SOKC and KA [3, 22]. Moreover, the proportions of p53 and Ki-67 positivity are significantly higher in PIOC, emphasising the utility of these immunohistochemical stains in the differential diagnosis of KA [31]. A good representative biopsy is key to avoid misdiagnosis by missing the subtle but important histologic features of KA, as described earlier.
Several studies have highlighted the use of calretinin in distinguishing ameloblastoma from other odontogenic entities, in particular OKC [32,33,34,35]. Altini et al. demonstrated calretinin positivity in the epithelium of both unicystic and conventional ameloblastomas [32]. In both subtypes, calretinin expression occurred almost exclusively in the cells of the stellate reticulum-like epithelium [32]. DeVilliers et al. found that all cases of ameloblastoma showed positive calretinin staining, whereas none of the OKC cases showed positive staining [33]. In contrast, none of the current cases showed evidence of calretinin staining in any of the tumour components. It is postulated that this may be due to the extensive central keratinisation at the expense of stellate reticulum-like areas, where calretinin expression is expected. Further studies are necessary to determine the expression pattern of calretinin in KAs.
Keratoameloblastomas have been treated with resection accompanied by close clinical follow-up [16]. In the current review, 19 cases were treated by surgical resection and 5 cases with conservative treatment. Recurrence was noted in 27% of resected cases and 50% of conservatively treated cases with follow-up information. Unfortunately, due to low absolute numbers and a lack of adequate follow up accurate conclusions on recurrence cannot be drawn. Due to limited data, the biological behaviour and prognosis of KAs are not well known [22]. However, it is well established that recurrence rate in conventional ameloblastomas is lower in cases treated via resection compared to conservative treatment [47].
Limitations of the current study includes its retrospective nature, resulting in limited clinical information for final analysis. This highlights the necessity for detailed recording of clinical information during the examination of a patient. The short follow-up periods of the current series and previously published cases makes assessment of the biological behavior and treatment outcomes difficult. Additionally, due to limited resources, no molecular testing was performed on any of the included cases. Future studies are necessary to better characterise the molecular aberrations in these rare odontogenic tumours.
Conclusion
This case series reports seven additional cases of KA, taking the total to 26 reported cases. The majority of cases presented as asymptomatic swellings with either a unilocular or multilocular radiolucent appearance. The identification of subtle histologic features, including focal stellate reticulum-like central areas, subnuclear vacuolisation and lamellated-type central keratinisation, are key in diagnosing KA. Additionally, the radiologic signs will often indicate signs of aggressiveness such as cortical destruction, therefore differentiating from OKC. All cases were completely negative for calretinin IHC, limiting its use in distinguishing KA from OKC. Further large series are needed to expand on the current understanding of this rare variant of ameloblastoma.
References
Brierley DJ, Hunter KD. Odontogenic tumours. Diagn Histopathol. 2015;21(9):370–9.
Seethala RR. Update from the 4th Edition of the World Health Organization classification of head and neck tumours: preface. Head Neck Pathol. 2017;11(1):1–2.
El-Naggar AK, Chan JKC, Rubin Grandis J, Takata T, Slootweg PJ. WHO classification of head and neck tumours. World Health Organization Classification of tumours. 4th ed. Lyon: International Agency for Research on Cancer; 2017.
Speight PM, Takata T. New tumour entities in the 4th edition of the World Health Organization Classification of head and neck tumours: odontogenic and maxillofacial bone tumours. Virchows Arch. 2018;472(3):331–9.
Wright JM, Vered M. Update from the 4th edition of the World Health Organization classification of head and neck tumours: odontogenic and maxillofacial bone tumors. Head Neck Pathol. 2017;11(1):68–77.
Takeda Y, Satoh M, Nakamura S, Ohya T. Keratoameloblastoma with unique histological architecture: an undescribed variation of ameloblastoma. Virch Arch. 2001;439(4):593–6.
Loyola AM, Cardoso SV, de Faria PR, Servato JP, Eisenberg AL, Dias FL, et al. Adenoid ameloblastoma: clinicopathologic description of five cases and systematic review of the current knowledge. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(3):368–77.
Coura BP, Dos Santos JN, Fonseca FP, Bernardes VF, de Aquino SN, Jorge Junior J, et al. Adenoid ameloblastoma with dentinoid is molecularly different from ameloblastomas and adenomatoid odontogenic tumors. J Oral Pathol Med. 2021;50(10):1067–71.
Lee C, Park BJ, Yi WJ, Heo MS, Lee SS, Huh KH. Keratoameloblastoma: a case report and a review of the literature on its radiologic features. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(5):e219–25.
Sisto JM, Olsen GG. Keratoameloblastoma: complex histologic variant of ameloblastoma. J Oral Maxillofac Surg. 2012;70(4):860–4.
Altini M, Lurie R, Shear M. A case report of keratoameloblastoma. Int J Oral Surg. 1976;5(5):245–9.
Siar CH, Ng KH. Combined ameloblastoma and odontogenic keratocyst’ or ’keratinising ameloblastoma. Br J Oral Maxillofac Surg. 1993;31(3):183–6.
Norval EJ, Thompson IO, van Wyk CW. An unusual variant of keratoameloblastoma. J Oral Pathol Med. 1994;23(10):465–7.
Said-al-Naief NA, Lumerman H, Ramer M, Kopp W, Kringstein GJ, Persenchino F, et al. Keratoameloblastoma of the maxilla. A case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84(5):535–9.
Kaku T, Ohuchi T, Hattori Y, Nishimura M, Nakade O, Abiko Y, et al. Keratoameloblastoma of the mandible. J Oral Pathol Med. 2000;29(7):812.
Whitt JC, Dunlap CL, Sheets JL, Thompson ML. Keratoameloblastoma: a tumor sui generis or a chimera? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(3):368–76.
Adeyemi B, Adisa A, Fasola A, Akang E. Keratoameloblastoma of the mandible. J Oral Maxillofac Pathol. 2010;14(2):77–9.
Ketabi MA, Dehghani N, Sadeghi HM, Shams MG, Mohajerani H, Azarsina M, et al. Keratoameloblastoma, a very rare variant of ameloblastoma. J Craniofac Surg. 2013;24(6):2182–6.
Raj V, Chandra S, Bedi RS, Dwivedi R. Keratoameloblastoma: report of a rare variant with review of literature. Dent Res J (Isfahan). 2014;11(5):610–4.
Bedi RS, Sah K, Singh A, Chandra S, Raj V. Keratoameloblastoma or kerato-odontoameloblastoma: report of its soft tissue recurrence with literature review. Quant Imaging Med Surg. 2015;5(6):898–908.
Palaskar SJ, Pawar RB, Nagpal DD, Patil SS, Kathuriya PT. Keratoameloblastoma a rare entity: a case report. J Clin Diagn Res. 2015;9(3):ZD05-7.
Prabhakar M, Sivapathasundharam B, Logeswari J, Manikandhan R. Keratoameloblastoma of oral cavity: report of two cases. Medico Legal Update. 2020;20(4):2248–54.
Kuberappa PH, Anuradha A, Kiresur MA, Bagalad BS. Papilliferous keratoameloblastoma—a rare entity: a case report with a review of literature. J Oral Maxillofac Pathol. 2020;24(Suppl 1):S2–6.
Konda P, Bavle RM, Muniswamappa S, Makarla S, Venugopal R. Papilliferous keratoameloblastoma of the mandible—a rare case report. J Clin Diagn Res. 2016;10(8):ZD08-11.
Collini P, Zucchini N, Vessecchia G, Guzzo M. Papilliferous keratoameloblastoma of mandible: a papillary ameloblastic carcinoma: report of a case with a 6-year follow-up and review of the literature. Int J Surg Pathol. 2002;10(2):149–55.
Mohanty N, Rastogi V, Misra SR, Mohanty S. Papilliferous keratoameloblastoma: an extremely rare case report. Case Rep Dent. 2013;2013: 706128.
Altini M, Slabbert HD, Johnston T. Papilliferous keratoameloblastoma. J Oral Pathol Med. 1991;20(1):46–8.
Pindborg JJ. Pathology of the dental hard tissues. Philadelphia: Saunders; 1970.
Parikh N, Nandini C, Jain S, Mansata AV. Peripheral keratoameloblastoma: a novel case report. J Oral Maxillofac Pathol. 2018;22(2):249–53.
Kramer I, Pindborg JJ, Shear M. WHO international histological classification of tumours: histological typing of odontogenic tumours. 2nd ed. New York: Springer; 1992.
Zhang R, Yang J, Zhang J, Hong Y, Xie X, Li T. Should the solid variant of odontogenic keratocyst and keratoameloblastoma be classified as the same entity? A clinicopathological analysis of nine cases and a review of the literature. Pathology. 2021;53(4):478–86.
Coleman H, Altini M, Ali H, Doglioni C, Favia G, Maiorano E. Use of calretinin in the differential diagnosis of unicystic ameloblastomas. Histopathology. 2001;38(4):312–7.
DeVilliers P, Liu H, Suggs C, Simmons D, Daly B, Zhang S, et al. Calretinin expression in the differential diagnosis of human ameloblastoma and keratocystic odontogenic tumor. Am J Surg Pathol. 2008;32(2):256–60.
Rudraraju A, Venigalla A, Babburi S, Soujanya P, Subramanyam RV, Lakshmi KR. Calretinin expression in odontogenic cysts and odontogenic tumors and the possible role of calretinin in pathogenesis of ameloblastoma. J Oral Maxillofac Pathol. 2019;23(3):349–55.
D’Silva S, Sumathi MK, Balaji N, Shetty NK, Pramod KM, Cheeramelil J. Evaluation of calretinin expression in ameloblastoma and non-neoplastic odontogenic cysts—an immunohistochemical study. J Int Oral Health. 2013;5(6):42–8.
Dhanuthai K, Chantarangsu S, Rojanawatsirivej S, Phattarataratip E, Darling M, Jackson-Boeters L, et al. Ameloblastoma: a multicentric study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(6):782–8.
MacDonald-Jankowski DS, Yeung R, Lee KM, Li TK. Ameloblastoma in the Hong Kong Chinese. Part 1: systematic review and clinical presentation. Dentomaxillofac Radiol. 2004;33(2):71–82.
MacDonald-Jankowski DS, Yeung R, Lee KM, Li TK. Ameloblastoma in the Hong Kong Chinese. Part 2: systematic review and radiological presentation. Dentomaxillofac Radiol. 2004;33(3):141–51.
Waldron CA, El-Mofty SK. A histopathologic study of 116 ameloblastomas with special reference to the desmoplastic variant. Oral Surg Oral Med Oral Pathol. 1987;63(4):441–51.
Kang BC, Lee JS, Yoon SJ, Kim Y. Ameloblastoma with dystrophic calcification: a case report with 3-dimensional cone-beam computed tomographic images of calcification. Imaging Sci Dent. 2020;50(4):373–6.
Ide F, Mishima K, Saito I. Solid-cystic tumor variant of odontogenic keratocyst: an aggressive but benign lesion simulating keratoameloblastoma. Virchows Arch. 2003;442(5):501–3.
Ide F, Ito Y, Muramatsu T, Saito I, Abiko Y. Histogenetic relations between keratoameloblastoma and solid variant of odontogenic keratocyst. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(6):812–3.
Geng N, Lv D, Chen QM, Zhu ZY, Wu RQ, He ZX, et al. Solid variant of keratocystic odontogenic tumor with ameloblastomatous transformation: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(2):223–9.
Ide F, Ito Y, Nishimura M, Ogawa I, Kikuchi K. Keratoameloblastomatous transformation of a recurrent unicystic ameloblastoma: a novel case raising diagnostic and classification difficulties. Pathology. 2022;54(3):386–8.
Fonseca FP, Pontes HA, Pontes FS, de Carvalho PL, Sena-Filho M, Jorge J, et al. Oral carcinoma cuniculatum: two cases illustrative of a diagnostic challenge. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(4):457–63.
van Heerden WFP, van Zyl AW, Bunn BK. Surgical pathology of oral cancer. Diagn Histopathol. 2017;23(6):235–42.
Laborde A, Nicot R, Wojcik T, Ferri J, Raoul G. Ameloblastoma of the jaws: management and recurrence rate. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134(1):7–11.
Funding
This research did not receive any specific Grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors read and approved the final manuscript. Conceptualisation: LR, WVH; Methodology: LR, CS; Formal analysis and investigation: LR, CS, WVH; Writing—original draft preparation: LR, CS; Writing—review and editing: LR, CS, FPF, ACA, MJR, SAK, KDH, PMS, WVH; Supervision: WVH, PMS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This study was approved by the University of Pretoria, Faculty of Health Sciences Research Ethics Committee (Reference No.: 33/2021). All procedures followed the ethical standards of the Helsinki Declaration of 1975, as revised in 2008.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Robinson, L., Smit, C., Fonseca, F.P. et al. Keratoameloblastoma: A Report of Seven New Cases and Review of Literature. Head and Neck Pathol 16, 1103–1113 (2022). https://doi.org/10.1007/s12105-022-01470-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-022-01470-5