Abstract
The archetypal solitary fibrous tumor (SFT) features fibroblastic cells with varying cellularity without any particular architectural arrangement in a collagenous matrix, with staghorn vessels, CD34 and STAT6 expression, and NAB2::STAT6. To date, this fusion is thought to be specific for SFT. With more routine use of fusion gene panels, the histologic diversity of NAB2::STAT6-positive tumors is increasingly appreciated. Here we present four head and neck tumors harboring NAB2::STAT6 but exhibiting remarkably unusual morphologic features for SFT. All cases were pulled from the authors’ consultation files. Immunohistochemistry was performed, along with targeted RNA sequencing in all cases, plus DNA next-generation sequencing on two. The cases arose in the nasal cavity (n = 2), retromolar trigone (n = 1) and parapharynx (n = 1), in patients ranging from 39 to 54 (mean, 44). Both nasal cases were biphasic, with a variably cellular collagenized stroma that resembled SFT but also interspersed malignant epithelial and neuroepithelial nests. One of the nasal cases also exhibited overt rhabdomyoblastic differentiation within both components. The two non-nasal cases were comprised of plump, epithelioid cells that were diffusely positive for pan-cytokeratin. One of these cases had prominent cystic change lined by overtly squamous epithelium. STAT6 immunostaining was positive in all cases, although the epithelial/neuroepithelial nests in the sinonasal cases were negative. All cases were confirmed to harbor NAB2::STAT6 by RNA sequencing. The two sinonasal cases were also found to harbor oncogenic mutations. The presented cases highlight a much broader histologic diversity than previously known for neoplasms with NAB2::STAT6. The biphasic nasal cases closely resemble teratocarcinosarcoma, while the epithelioid, cytokeratin-positive cases could be conceptualized as “adamantinoma-like,” to borrow terminology already in use for Ewing sarcomas with complex epithelial differentiation. To identify similar cases, pathologists should have a low threshold for using STAT6 immunohistochemistry on any difficult-to-characterize head and neck tumor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increased use of next-generation molecular techniques has not only identified new disease entities, it has also led to the expansion of the histologic spectra of well-established neoplasms. Examples of the former include biphenotypic sinonasal sarcoma and microsecretory adenocarcinoma [1, 2], and of the latter include tumors which harbor the canonical Ewing sarcoma-associated EWSR1::FLI1 fusion but also show complex epithelial differentiation (adamantinoma-like Ewing sarcoma) [3].
The diagnosis of solitary fibrous tumor (SFT) has been recently revolutionized by the discovery of a recurrent NAB2::STAT6 fusion [4] which led to the development of STAT6 immunohistochemistry [5]. These tools have facilitated a definitive diagnosis of solitary fibrous tumor as NAB2::STAT6 is, to date, regarded as pathognomonic for SFT. Rare examples of solitary fibrous tumors with unusual histologic features such as squamous and neuroendocrine differentiation have recently been described [6], suggesting its histologic and immunophenotypic spectra may be much broader than previously believed. Moreover, increasing molecular testing has revealed that some fusions are not as specific as previously believed.
Herein we report 4 unusual head and neck neoplasms that harbored NAB2::STAT6 gene fusions with STAT6 immunoexpression but that showed unusual features that departed dramatically from those of classic SFT.
Methods
All cases were pulled from the authors’ surgical pathology and consultation files. All were previously unpublished. The cases were reviewed, with various histologic features tabulated. Routine diagnostic immunohistochemistry was performed clinically, with appropriate controls, on 4-μm whole-slide sections using standardized automated protocols on Ventana BenchMark Ultra autostainers (Ventana). RNA sequencing (RNA-Seq) was done on all 4 cases and DNA next-generation sequencing (NGS) was performed on 2 cases as described in detail elsewhere [7]. In brief, both RNA and DNA were isolated from 10 um whole-slide tissue sections using Qiagen AllPrep kits (Qiagen, Germantown, MD). A modified TruSight RNA Pan-Cancer kit (Illumina, San Diego, CA) was utilized to make a sequencing library which contained all exons from 1425 cancer-related genes. Sequencing was then performed on the NextSeq 550 (Illumina) with a minimum of 6,000,000 mapped reads. All fusions and variants were reviewed in the Integrated Genomics Viewer (Broad Institute, Cambridge, MA). The Star-Fusion algorithm was used to call fusions, and somatic variants were identified using databases including dbSNP and gnomAD.
Results
Teratocarcinosarcoma-Like Cases (Table 1)
Case 1
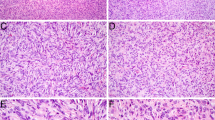
A 30-year-old male presented with a 5.8 cm right-sided nasal mass. Imaging studies showed an expansile, well-circumscribed, lobulated, hypervascular, heterogeneously enhancing mass that occluded the middle and posterior right nasal cavity and extended through the posterior choanae to fill the nasopharynx. There was no intracranial extension. Histologic sections demonstrated a tumor expanding underneath the sinonasal mucosa composed of several different epithelial and mesenchymal components (Fig. 1). A primitive, round cell component with neuroepithelial features including nuclear molding, evenly distributed chromatin, and pseudorosettes with production of neurofibrillary-like matrix was scattered throughout the tumor. In areas these cells appeared more epithelial with foci of overt squamous differentiation, while in other areas they showed a morphologic transition to rhabdomyoblasts. This neuroepithelial component was positive for chromogranin and synaptophysin, the squamous components were positive for pancytokeratin, and the rhabdomyoblastic cells were positive for desmin and MYOD1. The epithelial and neuroepithelial nests were set in a moderately hypercellular and collagenous stroma made up of fibroblastic cells along with many thin-walled vascular channels as well as staghorn-type vessels. Foci of overtly rhabdomyoblastic differentiation with desmin and MYOD1 expression was also noted within the spindled stromal cells in addition to the round cell component. There were associated complex cysts lined by bland columnar respiratory-type epithelium; whether these components were part of the neoplasm or entrapped sinonasal elements remains uncertain.
Case 1 consisted of nests of round cells, set in a fibrous stroma with a few dilated vessels (A). Some of the tumor nests were squamous and showed nuclear beta-catenin immunostaining (inset) (B), but most of them were made up of primitive cells with high nuclear:cytoplasmic ratios and foci of glio-fibrillary stroma (C). Scattered rhabdomyoblasts (arrows) that were desmin positive (inset) were noted within the round cell and spindle cell tumor components (D). There were numerous benign-appearing invaginations of surface epithelium, many of which were cystic. It was unclear whether these were entrapped elements, or part of the tumor itself (E). STAT6 immunostain was diffusely positive, but within the spindle cell component only (F)
A diagnosis of sinonasal teratocarcinosarcoma was made. RNA-Seq and DNA NGS revealed a NAB2::STAT6 fusion, along with mutations in CCND1, CTNNB1 and PRKCB. This unexpected finding prompted STAT6 immunohistochemistry which revealed diffuse nuclear STAT6 expression in the spindled fibroblastic cells with absence of staining in the neuroepithelial and epithelial components. Beta-catenin immunostain was strongly expressed in the nuclei of the squamoid elements but showed membranous expression in all other areas. Twenty-eight right neck and twenty-one left neck nodes were negative. He was treated with adjuvant radiation. Twenty-seven months after initial surgery, he had no radiographic evidence of recurrent or metastatic disease.
Case 2
A 54-year-old female had a 12-year history of a left nasal cavity mass that was found incidentally on imaging studies and was presumed to be benign. This mass had previously given rise to periodic nasal obstruction and epistaxis. Over the last year the mass increased in size with worsening obstructive symptoms. By imaging there was a 7.3 cm heterogeneously enhancing mass with peripheral cystic change, involving the right nasal cavity, left maxillary sinus, and nasopharynx. A biopsy was performed and showed scant fragments of a spindle cell neoplasm suspicious for SFT that was positive for CD34 and STAT6, and negative for AE1/AE3, S100 and desmin. Three months later the patient underwent tumor debulking.
Pathologic exam of this material unexpectedly showed features of sinonasal teratocarcinosarcoma (Fig. 2). This tumor showed malignant neuroepithelial elements with molded, hyperchromatic nuclei and prominent neurofibrillary matrix. It also demonstrated glandular structures of various sizes composed of bland cuboidal to columnar cells with a moderate amount of eosinophilic cytoplasm and prominent mucin production. Squamoid cells with focal ghost cells were also present. The stroma was modestly cellular stroma with areas of collagen deposition and myxoid change and occasional dilated blood vessels. Pancytokeratin (AE1/AE3) was positive in the epithelium and weakly positive in the neuroepithelium; synaptophysin and INSM1 were positive in the neuroepithelium; CD34 was weakly expressed in the stroma; STAT6 was diffusely positive in the stroma but not in the epithelial or neuroepithelial components. Beta-catenin showed only membranous expression in all components and SMARCA4 was intact. RNA-Seq and DNA NGS revealed NAB2::STAT6, along with mutations in BRCA2, BUB1B, LTBP1, and NF1. Three months postoperatively the patient was undergoing radiation therapy with no evidence of recurrent disease.
Case 2 was similar to case 1, with cellular basophilic nests scattered within a modestly cellular stroma (A). The nests were predominantly neural, with pseudorosettes and abundant neuropil-like stroma (B), but there was focal evidence of epithelial differentiation, here in the form of mucinous cells (C). There were additional dilated gland-like structures that could have been part of the tumor or entrapped elements (D). Focal ghost cells were noted (E). The tumor was diffusely positive for STAT6 within only the spindle cells of the tumor (F)
Adamantinoma-Like Cases (Table 2)
Case 3
A 39-year-old female presented with a nodule in the left oral cavity for 4 months with progressive, painless growth. Imaging of the neck showed a nodule between the left anterior tonsillar pillar and the retromolar trigone measuring approximately 2.5 to 3 cm. A fine needle aspiration of the mass at an outside hospital reportedly showed possible moderately differentiated squamous cell carcinoma. An excisional biopsy was performed, which revealed, within the oral submucosa, a well circumscribed mostly solid and partially cystic mass showing bands of fibrosis, slit-like vessels, and hemorrhage (Fig. 3). It was vaguely multinodular to plexiform. Tumor cells were uniform, epithelioid to spindled with scant eosinophilic to clear cytoplasm and uniform oval nuclei, arranged as solid sheets and vague fascicles of uniform cellularity. There was little pleomorphism. The mitotic rate was 12/10 high power fields, with no atypical mitotic forms. The solid areas did not display specific differentiating light microscopic features but the cystic zone was lined with overtly squamous epithelium. There was no ropy collagen or prominent nested growth pattern. Minor salivary glands were noted which were believed to represent entrapped elements. Tumor cells were also diffusely positive for p40 and p63 in both the solid and cystic foci, with rare tumor cells showing S100 protein expression along with variably intense, diffuse expression of pancytokeratin (AE1/AE3) and CD56. CD34, CD99, SOX10, SMA, Desmin, and GFAP were negative and there was no nuclear beta-catenin accumulation. Ki-67 showed an approximately 50% proliferation rate.
Case 3 was a large mass made up of solid nodules with foci of entrapped minor salivary glands (top) (A) and foci of cystic change lined by squamous epithelium (inset) (B). The tumor cells were epithelioid to vaguely spindled, with uniform oval nuclei and pale eosinophilic cytoplasm (C). By immunohistochemistry, the solid and cystic elements (insets) were positive for pan-cytokeratin (D), p40 (E), and STAT6 (F)
A diagnosis of GLI1-altered neoplasm was strongly suspected. Since STAT6 overexpression can be seen in GLI1-amplified neoplasms [8], STAT6 immunohistochemistry was performed and found to be diffusely positive in both the solid and cystic epithelial foci. However, MDM2 and CDK4, markers which are typically co-amplified when the nearby GLI1 gene is amplified [8] were negative, and FISH studies were negative for any GLI1 abnormalities. RNA-seq revealed a NAB2::STAT6 fusion. No additional therapy was performed, and 6 months postoperatively there was no evidence of disease.
Case 4
A 53-year-old female presented with a 6.5 cm parapharyngeal mass that was excised. Histologic sections showed a well-circumscribed, hypercellular tumor growing in sheets and trabeculae composed of uniform, oval to epithelioid cells with scant eosinophilic to clear cytoplasm (Fig. 4). There were thick anastomosing sheets of collagen deposition. Trabecular areas also showed production of basement membrane-type material set in a loose stroma. Staghorn-type vessels were present. S100 protein, pancytokeratin (AE1/AE3) and desmin were diffusely and strongly positive. Tumor cells were negative for CD34, SMA, SOX10, MYOD1, synaptophysin, and CD56.
Case 4 grew as a solid mass with scattered dilated vessels, with a circumscribed edge in the squamous submucosa (A). The tumor was trabecular to nested, with areas of stromal hyalinization (B). The tumor cells were epithelioid with uniform oval nuclei and abundant eosinophilic cytoplasm (C). By immunohistochemistry the tumor was diffusely positive for pan-cytokeratin (D), S100 protein (E), and STAT6 (F)
The S100 positivity, along with the nested growth and epithelioid tumor cells, again prompted strong consideration of a GLI1-altered neoplasm. STAT6 was performed and found to be diffusely positive in tumor nuclei, but immunohistochemistry for MDM2 was negative and a GLI1 FISH study was negative for alterations. RNA-Seq revealed NAB2::STAT6 fusion. No patient follow-up was available.
Discussion
Classically, SFT features bland fibroblastic cells arranged without any particular architectural pattern (so-called “patternless pattern”) in a collagenized stroma with characteristic staghorn-type vessels, CD34 and STAT6 expression and NAB2::STAT6 gene fusion [4]. SFT may affect any body site. Although overtly malignant SFTs exist, conventional SFT is regarded as a tumor of indeterminate malignant potential, i.e., usually indolent but occasionally behaving unexpectedly. In addition, dedifferentiated examples have been described where conventional areas of SFT are associated with areas lacking typical SFT features along with necrosis, nuclear pleomorphism with heterologous rhabdomyosarcomatous, chondrosarcomatous or osteosarcomatous differentiation [9]. In addition, molecularly-confirmed SFT showing squamous and neuroendocrine differentiation [6], epithelioid cell features and keratin expression [10], adipocytic differentiation [11] have been described, along with myxoid, epithelioid, and giant cell rich examples [12]. Herein we report four NAB2::STAT6 fusion-containing neoplasms arising in the head and neck which departed dramatically from the currently known spectrum of SFT.
Both cases 1 and 2 were biphasic tumors containing a spindle cell neoplasm with admixed primitive neuroepithelial and carcinomatous elements. Case 1 also featured rhabdomyoblastic differentiation. Thus cases 1 and 2 met diagnostic criteria for sinonasal teratocarcinosarcoma, a rare but well established sinonasal tumor defined by the presence of carcinomatous, neuroendocrine, and sarcomatous differentiation. However, for both cases STAT6 was expressed in the stromal elements and RNA-seq demonstrated NAB2::STAT6 fusion. The combination of these elements in two different cases is perplexing and difficult to explain. They could represent “collision tumors” or tumor-to-tumor metastases of SFT with another sinonasal tumor type. The fact that the epithelial/neuroepithelial elements were negative for STAT6 lends some support to that hypothesis. Moreover, the presence of oncogenic mutations in addition to NAB2::STAT6 fusion suggests different molecular events driving the different components. After all, nuclear beta-catenin expression was seen only in the squamous epithelial nests in case 1 which harbored CTNNB1 mutation. On the other hand, the mere existence of two very similar cases argues against a highly improbable coincidence of two such rare tumors. More to that point, it is not clear what the SFT would be colliding with, as the non-spindled components do not have an independent place in current sinonasal tumor classification schemes by histology, immunohistochemistry, or mutational profile. While these admixed elements fit well as common components of teratocarcinosarcoma, they do not meet criteria for olfactory neuroblastoma, neuroendocrine carcinoma, squamous cell carcinoma, or any other well-described sinonasal tumor out of this context. Furthermore, all components were intimately admixed throughout in both cases, in contrast to what one would expect with two distinct tumors “colliding” with one another. Indeed, in the case with rhabdomyoblastic differentiation, the rhabdomyoblasts of the neuroepithelial component appeared to blend with those of the stromal component. These cases could represent a SFT with divergent neuroepithelial and carcinomatous differentiation, but the absence of STAT6 expression in the epithelial/neuroepithelial component is difficult to explain in such a scenario. Another possibility is that one tumor is somehow inducing development of the other. While not a perfect correlate, these cases may be similar to the handful of peculiar cases reported as pituitary adenoma intimately admixed with rhabdomyosarcoma [13,14,15]. Finally, perhaps these are truly sinonasal teratocarcinosarcomas in which the stromal component somehow acquired a NAB2::STAT6 fusion and differentiated towards SFT, while the neuroepithelial and carcinomatous components remained without it. We are not, however, aware of any other multiphenotypic neoplasms including a spindle cell component that harbored a fusion characteristic of a different tumor type. Rooper et al. demonstrated that most teratocarcinosarcomas are SMARCA4-deficient tumors [16], but SMARCA4-intact examples are not yet well understood. SMARCA4-intact teratocarcinosarcoma may represent a heterogeneous group of neoplasms, some of which could be genetically SFT.
Cases 3 and 4 both featured uniform oval to epithelioid tumor cells and expressed pancytokeratin. Case 3 diffusely expressed p40 and p63 with overt squamous differentiation, and case 4 was strongly S100-positive. Both were negative for CD34. For both epithelioid cases, a diagnosis of GLI1-altered neoplasm was strongly considered as that tumor is made up of nests of epithelioid, monotonous cells, often with staghorn vessels and frequent S100 positivity. The strong STAT6 positivity supported that notion given its positivity in some GLI1-amplified tumors (the STAT6 gene is located adjacent to the GLI1 gene on 12q and is therefore often coamplified along with GLI1) [8]. Despite their notoriously heterogeneous immunoprofiles, GLI1-amplified tumors are not usually so strongly positive for pancytokeratin, and overt squamous differentiation has not yet been described in this tumor. Moreover, when STAT6 is overexpressed in a GLI1-amplified tumor, MDM2 and CDK4 are also usually overexpressed reflecting amplification of those nearby genes. Another diagnostic consideration for both epithelioid cases was a myoepithelial tumor, either of salivary or soft tissue origin. In the head and neck region a tumor made up of uniform epithelioid cells with expression of pancytokeratin, p63, p40 and/or S100 is certainly suggestive of a myoepithelial neoplasm. STAT6 expression, on the other hand, would be entirely unexpected for a myoepithelioma or myoepithelial carcinoma. Finally, confirming the NAB2::STAT6 fusion by RNA-seq or fluorescence in situ hybridization excludes mimickers like GLI1-altered neoplasms, myoepithelial tumors, and all other considerations. The overtly epithelial nature of these cases may represent divergent, complex epithelial differentiation. An excellent, emerging example of that phenomenon is the so-called adamantinoma-like Ewing sarcoma which occurs most often in the head and neck, especially salivary glands, sinonasal tract and thyroid gland. Beyond this rare entity, there are numerous examples of divergent epithelial differentiation in mesenchymal neoplasms, and it is possible that this phenomenon is simply underrecognized for SFT. Indeed, epithelial expression has even been reported in SFT, though those cases were different because they were in the setting of de-differentiation and overtly malignant features [6, 10].
Finally, it must also be considered that these peculiar neoplasms are not actually SFT at all, but rather distinct, as-yet undefined, novel neoplasms that happen to harbor NAB2::STAT6. This is a particularly intriguing notion for cases 3 and 4 which bore essentially no resemblance to SFT at all. There are numerous examples of markedly dissimilar neoplasms that share identical fusions, for example hyalinizing clear cell carcinoma, clear cell sarcoma, and angiomatoid fibrous histiocytoma with EWSR1::CREB1/ATF1 [17]. Increased molecular testing is continuing to reveal that even fusions once thought to be very specific for a particular entity, e.g., EWSR1::WT1 in desmoplastic small round cell tumor, may be seen in other tumor types [18]. Determining whether these tumors are distinct will clearly require more cases. Liberal use of STAT6 immunohistochemistry in working up unusual, difficult-to-characterize epithelioid tumors will be very helpful in identifying them.
To summarize, the spectrum of head and neck harboring NAB2::STAT6 includes cases that strongly resemble teratocarcinosarcoma and markedly epithelial cases resembling GLI1-altered neoplasms and myoepithelial tumors. These tumors may represent ends of a much broader histologic and immunohistochemical spectrum than previously recognized for SFT. On the other hand, these tumors may represent entirely novel, heretofore unrecognized neoplasms that simply share SFT’s molecular signature. Further investigation into these unusual NAB2::STAT6-containing neoplasms will be needed to determine what their behavior is and how it differs from its closest mimickers. To identify additional cases and further clarify the precise nature of these tumors, pathologists should consider including STAT6 routinely in the diagnostic evaluation of any head and neck tumor that is difficult to place into a diagnostic category.
Data Availability
Possible upon reasonable request, deidentified for maintenance of anonymity and compliance with IRB approval.
Code Availability
Not applicable.
References
Fritchie KJ, Jin L, Wang X, Graham RP, Torbenson MS, Lewis JE, et al. Fusion gene profile of biphenotypic sinonasal sarcoma: an analysis of 44 cases. Histopathology. 2016;69(6):930–6.
Bishop JA, Weinreb I, Swanson D, Westra WH, Qureshi HS, Sciubba J, et al. Microsecretory adenocarcinoma: a novel salivary gland tumor characterized by a recurrent MEF2C-SS18 fusion. Am J Surg Pathol. 2019;43(8):1023–32.
Rooper LM, Bishop JA. Soft tissue special issue: adamantinoma-like ewing sarcoma of the head and neck: a practical review of a challenging emerging entity. Head Neck Pathol. 2020;14(1):59–69.
Chmielecki J, Crago AM, Rosenberg M, O’Connor R, Walker SR, Ambrogio L, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet. 2013;45(2):131–2.
Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2014;27(3):390–5.
Lu C, Alex D, Benayed R, Rosenblum M, Hameed M. Solitary fibrous tumor with neuroendocrine and squamous dedifferentiation: a potential diagnostic pitfall. Hum Pathol. 2018;77:175–80.
Bishop JA, Gagan J, Baumhoer D, McLean-Holden AL, Oliai BR, Couce M, et al. Sclerosing polycystic “adenosis” of salivary glands: a neoplasm characterized by PI3K pathway alterations more correctly named sclerosing polycystic adenoma. Head Neck Pathol. 2020;14(3):630–6.
Agaram NP, Zhang L, Sung YS, Singer S, Stevens T, Prieto-Granada CN, et al. GLI1-amplifications expand the spectrum of soft tissue neoplasms defined by GLI1 gene fusions. Mod Pathol. 2019;32(11):1617–26.
Olson NJ, Linos K. Dedifferentiated solitary fibrous tumor: a concise review. Arch Pathol Lab Med. 2018;142(6):761–6.
Creytens D, Ferdinande L, Van Dorpe J. Multifocal cytokeratin expression in a dedifferentiated solitary fibrous tumor with heterologous rhabdomyosarcomatous differentiation: a challenging diagnosis! Int J Surg Pathol. 2018;26(5):423–7.
Lee JC, Fletcher CD. Malignant fat-forming solitary fibrous tumor (so-called “lipomatous hemangiopericytoma”): clinicopathologic analysis of 14 cases. Am J Surg Pathol. 2011;35(8):1177–85.
Baranov E, Hornick JL. Soft tissue special issue: fibroblastic and myofibroblastic neoplasms of the head and neck. Head Neck Pathol. 2020;14(1):43–58.
Lu J, Chen L. Molecular profile of a pituitary rhabdomyosarcoma arising from a pituitary macroadenoma: a case report. Front Endocrinol (Lausanne). 2021;12:752361.
Stein TD, Chae YS, Won N, Lee JH, Hedley-Whyte ET. A 34-year-old man with bitemporal hemianopsia. Brain Pathol. 2014;24(1):107–10.
Duncan VE, Nabors LB, Warren PP, Conry RM, Willey CD, Perry A, et al. Primary sellar rhabdomyosarcoma arising in association with a pituitary adenoma. Int J Surg Pathol. 2016;24(8):753–6.
Rooper LM, Uddin N, Gagan J, Brosens LAA, Magliocca KR, Edgar MA, et al. Recurrent loss of SMARCA4 in sinonasal teratocarcinosarcoma. Am J Surg Pathol. 2020;44(10):1331–9.
Thway K, Fisher C. Tumors with EWSR1-CREB1 and EWSR1-ATF1 fusions: the current status. Am J Surg Pathol. 2012;36(7):e1–11.
Schoolmeester JK, Folpe AL, Nair AA, Halling K, Sutton BC, Landers E, et al. EWSR1-WT1 gene fusions in neoplasms other than desmoplastic small round cell tumor: a report of three unusual tumors involving the female genital tract and review of the literature. Mod Pathol. 2021;34(10):1912–20.
Funding
This study was funded by the Jane B. and Edwin P. Jenevein M.D Endowment for Pathology at UT Southwestern Medical Center. No external funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
All authors confirm they have meaningfully contributed to the research and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board (IRB 112017-073), which did not require informed consent.
Informed Consent
The IRB-approved study was classified as exempt, which does not require informed consent.
Consent for Publication
Consent for publication was obtained from all individual participants for whom identifying information is uniquely included in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stevens, T.M., Rooper, L.M., Bacchi, C.E. et al. Teratocarcinosarcoma-Like and Adamantinoma-Like Head and Neck Neoplasms Harboring NAB2::STAT6: Unusual Variants of Solitary Fibrous Tumor or Novel Tumor Entities?. Head and Neck Pathol 16, 746–754 (2022). https://doi.org/10.1007/s12105-022-01444-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-022-01444-7