Abstract
The oral lymphoepithelial cyst (OLC) is an uncommon lesion whose pathogenesis remains poorly understood. The aim of this study was to report the clinicopathologic features of the OLCs and to verify a possible association between OLCs and subgemmal neurogenous plaque (SNP) in the posterior lateral region of the tongue. A retrospective descriptive cross-sectional study was carried out. A total of 106,282 biopsy records of oral and maxillofacial lesions from six oral pathology services in Brazil were analyzed. All cases of OLCs were reviewed, and clinical and histopathological data were collected. Immunohistochemical reactions for S-100 protein were performed to confirm the diagnosis of SNP. Among all lesions, there were 132 (0.11%) cases of OLCs. The series comprised 83 females (62.9%) and 49 males (37.1%), with a 1.7:1 female-to-male ratio and a mean age of 45.8 ± 17.7 years. Most cases involved the tongue (n = 80; 62.0%) and presented clinically as asymptomatic papules or nodules with a yellow or whitish color. Microscopically, most of the cysts were entirely lined by parakeratinized stratified epithelium (n = 89; 67.4%) and filled with desquamated cells, keratin debris, amorphous eosinophilic material, and inflammatory cells in varying amounts. Connection with the epithelium of oral mucosa was observed in 18 cases (13.6%). SNP was found in 9/80 (11.2%) cases involving the tongue. The clinical and demographic features of OLCs were similar to those described in previous studies. Overall, this lesion has a predilection for the posterior region of the tongue of female adults. Clinicians must include the OLC in the differential diagnosis of yellow/white papules and nodules of the oral cavity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The lymphoepithelial cyst (LC) is an uncommon lesion that corresponds to less than 1% of all lesions affecting the oral cavity [1, 2]. Most oral lesions occur in female adults and clinically present as a small, yellowish submucosal nodule on the tongue or floor of the mouth [2, 3]. Although most oral lymphoepithelial cysts (OLCs) are usually painless lesions, some rare cases may exhibit discomfort, pain, and a burning sensation [4]. It is essential to have in mind that the association of OLCs and subgemmal neurogenous plaques (SNP), structures recently described and characterized by the presence of nerve plexus associated with taste buds in the posterolateral region of the tongue [5], may occur and the SNP component may be the responsible for the pain [4].
Microscopically, OLCs are covered by stratified squamous epithelium with a dense lymphocytic infiltrate in the fibrous capsule, often containing lymphoid follicles [1, 2, 4, 6]. Despite the indolent clinical behavior, with no tendency to recurrence after conservative surgical excision, OLCs are frequently misdiagnosed clinically due to clinical appearance similar to several oral lesions [7]. Therefore, an accurate diagnosis requires careful morphological evaluation.
Several theories have been proposed about the pathogenesis of OLCs [8,9,10,11]; however, there is still no consensus as to whether these lesions represent true cysts that develop from ectopic glandular epithelium trapped in the normal lymphoid tissue of the oral cavity during embryogenesis (Waldeyer’s ring) [8, 9] or pseudocysts that arise due to obstruction of a tonsillar crypt [10].
Since the first description in the oral cavity made by Gold et al. [6], few well-documented large series of OLCs have been reported in the English-language literature [1, 2, 4, 7, 8, 11,12,13,14], some addressing only clinical characteristics [7, 14]. Thus, the aim of this multicenter study was to contribute to an additional large series of OLCs, with emphasis on unusual clinical and morphological findings. Additionally, we characterize the histomorphometric features of SNPs using digital analyses and immunohistochemistry to better understand their relationship to OLCs of the tongue. To the best of our knowledge, this study is the largest series of OLCs to date. Also, we provided a critical discussion emphasizing the etiopathogenesis, clinical and histopathological characteristics of these uncommon oral lesions.
Materials and Methods
Study Design and Sample
Cases diagnosed as OLCs were retrieved from the archives of six Brazilian Oral and Maxillofacial Pathology services (Table 1). Patient age, sex, ethnicity, symptoms, anatomical location, size, color, consistency, surface texture, treatment performed, recurrence, HIV infection status (when available), and the main clinical diagnosis were obtained from clinical records and evaluated. The recurrence was determined by a new histopathological diagnosis of OLC in the same anatomical location and same patient.
Morphological Evaluation
Histopathological analysis was performed under a light microscope (Olympus CX31, Olympus Japan Co., Tokyo, Japan). Five-micrometer hematoxylin and eosin-stained sections were obtained from each case. Two oral pathologists re-evaluated the histopathological features of the lesions. Briefly, the morphological features studied were: type of the lining epithelium (parakeratinized stratified squamous epithelium, non-keratinized stratified squamous epithelium and/or ciliated pseudostratified columnar), cystic content (desquamated epithelial cells, amorphous eosinophilic material and/or polymorphonuclear leukocytes, lymphocytes and plasma cells), the pattern of lymphoid tissue (lymphoid tissue surrounding the cystic cavity totally or partially, presence of follicular pattern with prominent germinal centers), continuity of cystic epithelium with overlying oral epithelium or glandular ductal epithelium, and adjacent anatomic structures to the cyst (acini or ducts of minor salivary glands, epithelial/epimyoepithelial islands). For the OLCs involving the tongue, the association with SNP, described as neurofibroma-like spindle cell proliferation, and an overlying epithelium containing taste buds was analyzed [1, 5]. Immunohistochemical reactions for S-100 protein (polyclonal, dilution 1:10,000, Dako) were performed with 3‐µm tissue sections on silanized slides, using standard protocols to confirm the diagnosis SNP. Periodic acid‐Schiff (PAS; PAS Stain Kit, Abcam, ab150680) was used to demonstrate the presence of goblet cells.
Histomorphometric Study of SNPs
Additionally, histomorphometric analysis of SNPs according to the methodology proposed by Pellicioli et al. was carried out to characterize better and establish a possible relationship with OLCs [15]. Briefly, H&E slides and immunohistochemical slides were scanned using an Aperio ScanScope CS scanner (Aperio Technologies, Inc., Vista, CA). The mean distance between the SNPs and OLCs was assessed by drawing a straight line from the periphery of the neural structures to the edge of the lymphoid tissue of the cystic capsule. SNPs in close contact with the oral epithelium or lymphoid tissues were considered to be juxtaposed to these structures. The mean distance between the SNPs and the oral epithelium was assessed by drawing a straight line from the periphery of the SNP to the basement membrane of the nearest oral epithelial ridge. The mean area of the SNPs was calculated by drawing a line surrounding the nerve fibers of the SNPs. All analyzes were performed using Aperio ImageScope software (Aperio Technologies, Inc., Vista, CA).
Data Analysis
Descriptive and quantitative data analyses were performed using the Statistical Package for the Social Sciences (SPSS) for Windows 20.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables were expressed as mean, median, and standard deviation values. Categorical variables were expressed as the absolute number of cases and percentage values. Chi-square test and Fisher’s exact test were used to evaluate the association between clinical and demographic characteristics, adopting a P‐value of ≤ 0.05 and 95% confidence interval.
Results
Clinical Features
A total of 106,282 surgical specimens were received at the centers studied; of these, 132 (0.11%) were OLCs. The allocation of OLC cases by the center is presented in Table 1. The demographic and clinical data are summarized in Table 2. Eighty-three (62.9%) cases occurred in females and 49 (37.1%) in males, with a female-to-male ratio of 1.7:1. The mean age of the patients was 45.8 ± 17.7 years (ranging: 04–88 years). Patients in the fifth (n = 24, 18.2%) and sixth (n = 24, 18.2%) decades of life were most affected, and the majority were Caucasian (n = 60, 65.9%). Only three lesions (2.4%) occurred in children (age < 10 years). The tongue was the most affected site (n = 80; 62.0%), followed by the floor of the mouth (n = 32; 24.8%). Other anatomical sites included buccal mucosa, soft and hard palate, lower lip, and anterior tonsillar pillar. Also, there was one rare case of bilateral OLC affecting the tongue and a synchronous presentation with squamous cell carcinoma (SCC) in the tongue.
Clinically, OLCs often presented as a well-circumscribed sessile or pedunculated papules or nodules, measuring from 0.4 to 2.0 cm (0.45 ± 0.28) and firm (n = 46; 54.1%) to soft (n = 35; 41.2%) in consistency. Most of the cases presented a yellow (n = 64; 54.7%) or whitish (n = 26; 22.2%) coloration (Fig. 1). Most of them were asymptomatic (n = 93, 91.2%); however, some patients (n = 9; 8.8%) reported burn sensation and slight pain. The evolution time varied from 2 weeks to 6 years (mean: 11.9 ± 17.4 months). Regarding the clinical diagnosis, about 52.5% of cases have been diagnosed as OLCs (n = 64; 52.5%). Other presumptive diagnoses included mucocele, fibrous hyperplasia, lipoma, squamous papilloma, sialolithiasis, lymphoid aggregates, dermoid and epidermoid cysts, and SCC. Treatment of all cases (n = 132; 100%) consisted of conservative surgical excision (excisional biopsy), and none case exhibited recurrence. There is no significant association between clinical and demographic characteristics (P > 0.05).
Clinical aspects of oral lymphoepithelial cysts. A and B Yellow–white mass along the lateral border of the tongue. C Slightly raised, normochromic nodule arising in the floor of the mouth (arrowhead). D Whitish papule located in the ventral surface of the tongue, on the left side of the glossodesmus, measuring approximately 0.4 cm. E Firm, yellow dome-shaped mass in the floor of the mouth mimicking a calcified “stone” within the submandibular gland excretory duct (sialolithiasis). F Occlusal radiograph excluded the possibility of calcified mass
Pathologic Features
On gross examination, most cases were described as a small, well-demarcated cystic cavity ranging in color from gray to brown, usually filled with a yellowish keratin-like material and some hemorrhagic brownish regions at the periphery of the specimen. The histopathological features are summarized in Table 3 and illustrated in Figs. 2, 3, and 4.
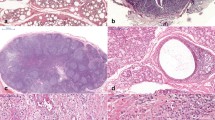
Classical histopathological features of the oral lymphoepithelial cysts. A and B Low and intermediated power showing cystic cavity lined by parakeratinized squamous epithelium and a fibrous capsule containing dense lymphocytic infiltrate with the formation of lymphoid follicles totally encircle the cyst. C Detail of the cystic lumen filed by numerous desquamated epithelial cells, cell debris, and keratin. D Note the flat epithelial–conjunctive interface, areas of epithelial atrophy (asterisk), and exocytosis. E and F Oral lymphoepithelial cysts showing lymphocytic inflammatory infiltrate partially surrounding the cystic cavity. Note the cystic epithelium connected to overlying oral mucosa (arrow) (hematoxylin and eosin stain)
Types of the epithelial lining of oral lymphoepithelial cysts. A and B Oral lymphoepithelial cyst showing non-keratinized epithelial lining and cystic lumen filled by eosinophilic amorphous material. C OLC covered by parakeratinized squamous epithelium. D Cystic epithelium showing hyperplasia of the parakeratin layer. E and F Oral lymphoepithelial cyst on the posterior border of the tongue lined by ciliated columnar epithelium (respiratory epithelium) containing goblet cells and a diffuse lymphocytic infiltrate in the capsule (hematoxylin and eosin stain)
Lymphoepithelial cyst of the tongue and subgemmal neurogenous plaques. A Low-power magnification showing a lymphoepithelial cyst, aggregates of lymphoid cells, and two subgemmal neurogenous plaques adjacent to lesion. B and C Detail of subgemmal neurogenous plaques showing neural spindle cells located in the lamina propria. D Note the cystic epithelium connected to the ductal epithelium (arrow) and acini and excretory ducts of minor salivary glands in close relationship with lymphoid tissue. E The immunohistochemistry showed neural spindle cells positive for S-100 protein (A–D hematoxylin and eosin stain, E immunohistochemistry)
Microscopically, most OLCs (n = 89; 67.4%) were lined entirely by parakeratinized stratified squamous epithelium, followed by non-keratinized squamous epithelium (n = 26; 19.7%). Thirteen cases (9.8%) show a combination of parakeratinized and non-keratinized areas in the same lesion. Three cysts (2.3%) exhibiting focal areas of respiratory metaplasia, and 1 (0.8%) was lined entirely by ciliated pseudostratified cylindrical epithelium containing mucous/goblet cells. Goblet cells were PAS-positive. Areas of epithelial hyperplasia (n = 85; 64.4%) and atrophy (n = 32; 24.3%) were frequent findings. Mucous/goblet cells were present only in lesions lined by ciliated pseudostratified columnar epithelium (n = 4; 3.0%). One case (0.8%) exhibited numerous macrophages with abundant foamy cytoplasm (CD68+) in the epithelial component. A connection between the cystic lining and the oral epithelium or ductal epithelium of minor salivary glands has been observed in 18 (13.6%) and 2 (1.5%) cases, respectively.
Desquamated epithelial cells and keratin debris (n = 43; 32.6%), amorphous eosinophilic material (n = 31; 23.5%), and inflammatory cells (n = 27; 20.5%) in varying amounts were present in the lumen of some cysts. In one lesion (0.8%) located on the floor of the mouth, several colonies resembling Actinomyces were observed.
Lymphoid tissue partially surrounded the cyst in most cases (n = 83; 62.9%). Germinal centers were not present in most lesions (n = 79; 59.8%). In these cases, only a diffuse, dense lymphocytic infiltrate was observed in the cyst capsule. However, 53 cases (40.2%) exhibited well-formed germinal centers, some (n = 6, 11.3%) exhibiting marked phagocytic activity.
Minor salivary glands (n = 31; 23.5%) and epithelial/epimyoepithelial islands (n = 11; 8.3%) were observed in the vicinity of some cysts in close relationship with the lymphoid tissue. SNPs were identified in nine patients, corresponding to about 11.3% of the tongue’s cases (n = 80). SNPs showed positivity for S-100 protein. The mean size of the SNPs cases was 0.191 mm2 (ranging: 0.006328–0.614231 mm2). In all patients (n = 9; 100%), SNPs were observed juxtaposed to the oral epithelium. The mean distance between OLCs and SNPs was 0.7812 mm (range 0.0452–0.1178 mm). Only one case showed neural structures in close relationship to lymphoid tissue of the cyst. In addition, most SNPs (n = 6; 66.7%) lacked the presence of lymphoid infiltrate. Ganglion cells were not observed in the samples. A significant association was found between the occurrence of SNPs and symptoms such as pain and burning in tongue lesions (P < 0.05; Fisher’s exact test).
Discussion
Herein we report the clinicopathological features of 132 additional cases of OLCs, the largest case series to date. OLCs are uncommon lesions, accounting for about 0.06 to 0.18% of all lesions diagnosed in the referred pathology services in the present investigation (Table 1), similar to previous reports [1, 2].
Our results show that OLCs tend to occur in female adults between the fifth and the sixth decades of life (mean age 45.8 years), being observed with a low frequency (8.0%) in pediatric patients, similar to other large series [1, 4, 7]. However, some studies have shown a predilection for men [8, 11]. Clinically, most oral lesions often present as a small, painless movable nodule, generally smaller than 1 cm in diameter, smooth surface, and colors ranging from yellow to white on the tongue or floor of the mouth [1, 2, 4, 7], similar to the present study. Although any area of the oral cavity may be affected, occurrence in the labial mucosa, as observed in our series, is rare, with only a few cases published in the literature [4, 14]. The duration varies from a few weeks to 10 years [7]. However, due to the small size and the lack of symptoms, most lesions are usually discovered during routine dental exams. Therefore, it is difficult to accurately assess the duration due to the patient’s lack of knowledge about the lesion.
Interestingly, there was an unusual case of bilateral OLC on the lateral posterior borders of the tongue of a 61-year-old female (previously published) [16]. This finding is relevant because bilateral lymphoepithelial cysts (LCs) of the parotid gland have been associated with human immunodeficiency virus (HIV) infection [17, 18]. Although our patient had no other oral manifestations associated with HIV, serological tests were requested, and the results were negative. We believe it is only an occasional finding since bilateral LCs in other locations, such as the thyroid gland and the cervical region, appear unrelated to HIV serological status [19,20,21]. However, in the present study, it was not possible to accurately assess a possible association between OLCs and HIV infection because, in most cases, information on HIV infection status was not available.
Due to its low prevalence and similarity with several yellow-white conditions of the oral cavity, OLCs are often confused in clinical practice [1, 2, 4, 7]. The differential diagnosis includes neoplasms (lipoma or a granular cell tumor), developmental lesions (Fordyce granules, dermoid and epidermoid cysts), calcified masses (sialolith and tonsillolith), reactive/inflammatory conditions, and oral manifestations of systemic diseases [22]. Recently, Yang et al. reported a series of 120 intraoral cases. In none of them, an OLC was mentioned as a provisional clinical diagnosis [7]. In the present study, only 52.5% of cases (n = 64) were clinically suspected as OLCs. These findings still demonstrate an unfamiliarity of these lesions among clinicians and emphasize the need to expand differential diagnoses of yellow oral mucosal entities and to include OLCs.
Histologically, most OLCs are lined by thin stratified squamous epithelium without rete ridges, which may be non-keratinized or keratinized [1, 2, 4, 11]. Less often, some cysts are lined by ciliated or nonciliated pseudostratified columnar epithelium, containing mucous/goblet cells. The cystic lumen exhibits a variable amount of desquamated epithelial cells, keratin debris, inflammatory cells (lymphocytes or polymorphonuclear leukocytes), and amorphous eosinophilic material [1, 2, 4]. The fibrous capsule shows an intense lymphocytic infiltrate that can totally or partially encircle the cyst [1, 2, 4]. In general, the lymphoid tissue exhibits a follicular pattern with well-formed germinal centers [1, 2]. Most of these characteristics were observed in the present study; however, in most cases (n = 79; 59.8%), only a diffuse dense lymphocytic infiltrate without lymphoid follicles was observed.
The association of OLCs located on the lateral region of the tongue with SNPs has been reported in the literature [1, 4, 15]. In the present study, nine SNPs were found in association with LCs of the tongue. Interestingly, most symptomatic cases (n = 6; 66.7%) were associated with SNPs. Although the simultaneous occurrence of SNPs and OLCs does not appear to be a true association between the two entities but rather an anatomical coincidence [1], the burning sensation and mild pain reported by these patients may be due to this association [4].
In addition, the synchronous occurrence of OLC and SCC in the oral cavity, similar to the current series, is extremely rare, with only one previous case reported in the English-language literature [23]. Since both lesions have a predilection for the tongue and floor of the mouth, they probably also represent coincidental events rather than a manifestation of common genetic or environmental risk [23, 24].
Over the years, several theories were suggested on the pathogenesis of these lesions in the oral cavity [10, 11]. According to Knapp, OLCs are pseudocysts that originate from tonsillar crypts’ obstruction due to accumulating desquamated epithelial cells and/or keratin [10]. The predilection of OLCs by areas of the oral cavity that have lymphoid aggregates and continuity of the cystic lining with the surface epithelium observed in some cases support this hypothesis [2]. However, other studies have not demonstrated this continuity even with the implementation of histological serial section [12]. Also, OLCs may arise in other oral locations devoid of oral tonsils [4]. Therefore, it is not always possible to explain the pathogenesis of the OLCs with this theory.
It has also been suggested that OLCs may arise from epithelial inclusions or epithelial remnants derived from salivary glands that may be entrapped on the oral lymphoid tissue cavity (Waldeyer’s ring) during embryogenesis [8]. Vickers and von Der Muhll (1966) studied the theory of enclavement experimentally. They concluded that the implantation of autogenous salivary gland epithelium in submandibular lymph nodes could originate LCs in hamsters [9]. The presence of OLCs lined by ciliated columnar epithelium with goblet cells in the present study suggests the participation of ductal epithelium in the pathogenesis of these lesions and adds support to the enclavement theory. In fact, metaplasia of the ductal epithelium is common and may be responsible for the stratified squamous epithelial lining that is so often seen in these cysts [9]. However, further studies focusing on the histogenesis of the cystic epithelium (glandular or surface epithelium) are necessary and an interesting approach to confirm this hypothesis.
However, this theory also fails to explain the occurrence, even rare, of LCs in areas of the oral cavity devoid of normal lymphoid tissue such as the labial mucosa, as observed in the present series. The reason why these lesions develop in sites devoid of lymphoid aggregates is even more interesting. Perhaps, the cyst can also originate from the excretory ducts of minor salivary glands or traumatic implantation of epithelial remnants of the oral mucosa in the connective tissue since epithelial islands and duct-like structures are often identified in the lymphoid tissue or near it [1, 2, 4], as observed in the present study. The continuity of the cystic epithelium with the ducts of minor salivary glands observed in some cases favors the last hypothesis [1]. Despite great discussion about the pathogenesis of OLCs, the lesions share similar morphological characteristics, which suggests that different mechanisms may play a role in developing OLCs.
Conservative surgical excision is the best treatment approach [1, 2, 4, 7]. The prognosis of OLC is excellent, with no reports of recurrence or malignant transformation [1, 2, 4, 7, 16]. In our study, there were no recurrences, which is expected for this lesion.
In summary, to the best of our knowledge, this is the largest case series of OLCs with detailed clinicopathologic analysis. Our findings are mostly consistent with those reported in the literature. Approximately half of the cases were clinically suspected as OLCs, demonstrating the unfamiliarity of OLCs among clinicians. This study illustrates the need to expand differential diagnoses of yellow oral mucosal entities and to include OLCs. In some cases of OLCs on the tongue, the biopsy specimen may be accompanied by an SNP in association with the cyst or as an adjacent independent structure.
Data Availability
Not applicable.
Code Availability
Not applicable.
Consent for Publication
Not applicable.
References
da Silva KD, Coelho LV, do Couto AM, de Aguiar MCF, Tarquínio SBC, Gomes APN, et al. Clinicopathological and immunohistochemical features of the oral lymphoepithelial cyst: a multicenter study. J Oral Pathol Med. 2020;49(3):219–26.
Sykara M, Ntovas P, Kalogirou EM, Tosios KI, Sklavounou A. Oral lymphoepithelial cyst: a clinicopathological study of 26 cases and review of the literature. J Clin Exp Dent. 2017;9(8):e1035–43.
Pinheiro JC, da Silva Barros CC, Rolim LSA, Pereira Pinto L, de Souza LB, de Andrade Santos PP. Oral lymphoid lesions: a 47-year clinicopathological study in a Brazilian population. Med Mol Morphol. 2019;52(3):123–34.
Custódio M, Tobouti PL, Matuck B, de Sousa SCOM. Incidental finding of subgemmal neurogenous plaque upon retrospective evaluation of oral lymphoepithelial cysts. Oral Maxillofac Surg. 2018;22(4):429–33.
McDaniel RK. Subepithelial nerve plexus (with ganglion cells) associated with taste buds. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87(5):605–9.
Gold C, Levittown NJ. Branchial cleft cyst located in the floor of the mouth. Oral Surg Oral Med Oral Pathol. 1962;15(9):1118–20.
Yang X, Ow A, Zhang CP, Wang LZ, Yang WJ, Hu YJ, et al. Clinical analysis of 120 cases of intraoral lymphoepithelial cyst. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(4):448–52.
Bhaskar SN. Lymphoepithelial cysts of the oral cavity. Report of twenty-four cases. Oral Surg Oral Med Oral Pathol. 1966;21(1):120–8.
Vickers RA, Gorlin RJ, Smart EA. Lymphoepithelial lesions of the oral cavity. Oral Surg Oral Med Oral Pathol. 1963;16(10):1214–22.
Knapp MJ. Pathology of oral tonsils. Oral Surg Oral Med Oral Pathol. 1970;29(2):295–304. https://doi.org/10.1016/0030-4220(70)90101-5.
Buchner A, Hansen LS. Lymphoepithelial cysts of the oral cavity. A clinicopathologic study of thirty-eight cases. Oral Surg Oral Med Oral Pathol. 1980;50(5):441–9.
Giunta J, Cataldo E. Lymphoepithelial cysts of the oral mucosa. Oral Surg Oral Med Oral Pathol. 1973;35(1):77–84.
Chaudhry AP, Yamane GM, Scharlock SE, SunderRaj M, Jain R. A clinico-pathological study of intraoral lymphoepithelial cysts. J Oral Med. 1984;39(2):79–84.
Uchoa-Vasconcelos AC, Filizola-de Oliveira DJ, Roman-Martelli SJ, Etges A, Neutzling-Gomes AP, Chaves-Tarquínio SB. Demographic profile of oral nonodontogenic cysts in a Brazilian population. Med Oral Patol Oral Cir Bucal. 2014;19(4):e308-12.
Pellicioli ACA, Fonseca FP, Silva RN, Gueiros LAM, de Almeida OP, Vargas PA, et al. Histomorphometric characterization of subgemmal neurogenous plaques. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123(4):477–81.
Silva IH, Romanach MJ, Carvalho AT, de Almeida OP, Leao JC, Gueiros LA. Bilateral lymphoepithelial cyst of the tongue: a case report. Gen Dent. 2013;61(6):32–4.
Favia G, Capodiferro S, Scivetti M, Lacaita MG, Filosa A, Lo Muzio L. Multiple parotid lymphoepithelial cysts in patients with HIV-infection: report of two cases. Oral Dis. 2004;10(3):151–4.
Mourad WF, Patel S, Young R, Khorsandi AS, Concert C, Shourbaji RA, et al. Management algorithm for HIV-associated parotid lymphoepithelial cysts. Eur Arch Otorhinolaryngol. 2016;273(10):3355–62.
Harkness MK, Biswas CK. Bilateral neck swelling in an elderly man. J R Soc Med. 2002;95(10):503–5. https://doi.org/10.1258/jrsm.95.10.503.
Cassarino DS, Milas M, Folpe AL. Bilateral intrathyroidal lymphoepithelial cysts. Arch Pathol Lab Med. 2003;127(2):251–2.
Choi CJ, Choi SW, Cho JG, Woo JS. Bilateral lymphoepithelial cysts of the thyroid gland. Thyroid. 2010;20(1):111–3.
Schafer DR, Glass SH. A guide to yellow oral mucosal entities: etiology and pathology. Head Neck Pathol. 2019;13(1):33–46.
Costa FW, Pereira KM, Viana TS, Cavalcante RB, Nogueira AS. Simultaneous occurrence of a rare lymphoepithelial cyst and squamous cell carcinoma in the oral cavity. Braz J Otorhinolaryngol. 2011;77(2):270.
Cunha JLS, Déda Júnior WG, Sanchéz-Romero C, Bezerra BT, de Albuquerque-Júnior RLC. Gingival squamous cell carcinoma mimicking a non-neoplastic proliferative lesion in an older patient. Gerodontology. 2020;37(3):303–6.
Acknowledgements
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The author John Lennon Silva Cunha received a PhD Scholarship from CNPq.
Funding
No funding sources to disclose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest were declared concerning the publication of this article.
Ethical Approval
The study was approved by the Ethics Committee of the School of Dentistry of Piracicaba (Protocol nº 10723019.0.1001.514).
Informed Consent
The patient provided an informed consent declaration to permit the use of images and medical information and the manuscript is in accordance with the Institutional Ethics Committee.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cunha, J.L.S., Roza, A.L.O.C., Cruz, V.M.S. et al. Oral Lymphoepithelial Cyst: A Collaborative Clinicopathologic Study of 132 Cases from Brazil. Head and Neck Pathol 16, 268–277 (2022). https://doi.org/10.1007/s12105-021-01352-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-021-01352-2