Abstract
Peripheral localisation of papillary thyroid microcarcinoma (PTMC), in comparison with intraparenchymal PTMC (i-PTMC) is related to some clinicopathological features related with biological aggressiveness, including lymph node metastasis (LNM). The expression of PD-L1 in tumour cell has been associated with increased tumour survival, progression, and potentially an aggressive clinical course. This study evaluates the relation between clinicopathological features of PTMC, including tumour localisation, with PD-L1 immunoexpression. The study included 99 patients with the histological diagnosis of PTMC (≥ 5 mm). PD-L1 protein expression was assessed by immunohistochemistry. PTMCs were divided into the four following groups: G1– peripherally localised PTMC (p-PTMC) with PD-L1 expression; G2–p-PTMC without PD-L1 expression; G3–i-PTMC with PD-L1 expression and G4–i-PTMC without PD-L1 expression. G1 was the most frequent (n = 46; 46.5%), followed by G4 (n = 25; 25.3%) and similar distribution of G3 (n = 15; 15.2%) and G2 (n = 13; 13.1%). In comparison with other groups, G1 was significantly associated with classical morphology, invasive growth, lymphatic invasion (LI), vascular invasion (VI), psammoma bodies, intratumoral fibrosis, PD-L1 positive tumour-infiltrating lymphocytes, and multinuclear giant cells (MGCs). G4 more commonly exhibited follicular morphology, expansive/circumscribed growth, and absence of the following: intratumoural fibrosis, LI, VI, psammoma bodies, PD-L1 positive tumour-infiltrating lymphocytes, and MGCs. LNMs were significantly more frequent in G1 in comparison with the other groups (p = 0.000). In conclusion, morphology and tumour microenvironment of p-PTMC with PD-L1 expression is different from i-PTMC without PD-L1 expression. The differences between these two groups of PTMC include clinicopathological features related with biological aggressiveness such as the occurrence of LNM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Papillary thyroid microcarcinoma (PTMC) is a size defined variant of papillary thyroid carcinoma (PTC) with the largest dimension less or equal to 10 mm [1]. PTMC is the most common variant of PTC and accounts for almost half of the newly diagnosed PTCs [2, 3]. Most of PTMCs are diagnosed incidentally after surgery for benign thyroid diseases with an average prevalence of 71% [4]. PTMC has an excellent prognosis and disease-specific mortality is reported in less than 1% of patients [3, 5]. Despite the favourable prognosis, PTMC may give rise to lymph node metastases (LNM) in 2 to 61.4% of cases, loco-regional recurrences (LRR) may occur in 2.4% to 8% of cases, whereas distant metastases were reported in less than 1% of the patients [3,4,5,6].
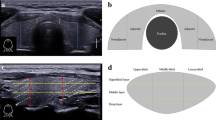
PTMC is often described in “subcapsular” or peripheral location within the thyroid gland [7]. PTMC with peripheral location has been associated with specific clinicopathological features such as LRR, LNM, extra-thyroid extension (ETE), presence of tall cells, infiltrative growth pattern, significant intratumoural fibrosis (ITF), and V600EBRAF mutation [8,9,10,11].
Programmed death ligand-1 (PD-L1), also referred as B7-H1 or CD274, belongs to the family of B7 transmembrane proteins or immunoregulatory ligands [12, 13] expressed on activated T and B lymphocytes, dendritic cells, and macrophages as well as on healthy cells of different organs and tissues [13, 14]. Binding of PD-L1 to a programmed cell death protein 1 receptor (PD-1; CD279) is necessary for the functional activation of the PD-1/PD-L1 signalling pathway, which leads to dysfunction, exhaustion, anergy, and finally apoptosis of autoreactive and/or reactive T lymphocytes [15]. Under physiological conditions PD-1/PD-L1 signalling pathway enables the control of the immune response and the maintenance of immune homeostasis by preventing cell or tissue damage caused by a prolonged and/or intense inflammation or autoimmune reaction [13,14,15]. As a result of constant activation of oncogenic signalling pathways, genetic alteration and inflammatory signalling, tumour cells tend to express PD-L1 [16]. PD-L1 expression on tumour cells has been associated with inhibition of T cell-mediated anti-tumour immune response leading to tumour progression and aggressive clinical course of malignancies [13, 15]. Expression of PD-L1 has been identified in several types of human malignant tumours enabling the use of immunetherapy targeting the PD-1/PD-L1 pathway [13,14,15].
PD-L1 expression has been reported in 6.1% to 82.5% of PTCs [16, 17] but its meaning is still to be defined. Most studies conclude that PD-L1 expression in PTC is related to a higher risk of disease recurrence. Other associations of PD-L1 expression in PTCs have been reported with the presence of concurrent chronic lymphocytic thyroiditis (CLT) and V600EBRAF mutation [18, 19].
Only a few studies have explored the expression of PD-L1 in tumour and immune cells of PTMCs. Chowdhury et al. reported membranous and cytoplasmic PD-L1 immunoexpression in tumour cells in 52.2% and 61.6% of PTMCs, respectively [20]. In the study by Aghajani MJ et al., a series of 75 PTCs including 39 PTMCs, reported cytoplasmic and/or membranous expression of PD-L1 in 66.7% of cases [21]. In a series of 10 PTCs coexistent with CLT, 2 cases were PTMCs and both of them disclosed PD-L1 membranous expression [22].
This study aimed to evaluate the relation between clinicopathological features of PTMC, including tumour location with the expression of PD-L1.
Material and Methods
Patients Selection
Patient selection was done retrospectively from the archive of the Institute of Pathology and Forensic Medicine of the Military Medical Academy, Belgrade, Serbia, from January 2013 till December 2018. From the total number of consecutively diagnosed PTMCs (n = 446), PTMCs with a minimum size of 5 mm were diagnosed in 116 cases (26%). Finally, the selected group of PTMCs consisted in 99 patients submitted to total or nearly total thyroidectomy with or without lymphadenectomy. The study did not include 17 patients with PTMC diagnosed after lobectomy or patients with tumours with a significant loss of tissue representation in paraffin blocks. None of the 99 patients from the selected group had a concomitant PTC larger than 10 mm although some had multifocal disease. None of the patients were previously treated with immunosuppressive therapy. The study was approved by the Military Medical Academy ethics committee.
Clinicopathological Features and Histological Examination of PTMC
General clinical data such as age, gender, type of surgery, presence of local and/or distant metastasis were obtained from the medical records of the patients. Haematoxylin and eosin-stained (H&E) slides of all cases were reviewed by two pathologists (BK, SC) and histological parameters were analysed according to the classification of the World Health Organization Classification of Tumours of Endocrine Organs [1]. The staging of disease was done according to the 8th Edition of the AJCC/TNM Staging System for PTC [23].
The following features were evaluated in each case: tumour size, presence of LNM (cases were considered negative for LNM when its presence was excluded after histological and/or image studies and clinical examination), presence of distant metastases (evaluated according to clinical data from patients records), extra-thyroid extension (ETE) (absent, minimal/microscopic or gross/macroscopic), predominant architectural pattern of the tumour (papillary, follicular, solid, etc.), growth pattern (infiltrative or expansive/circumscribed), lymphatics invasion (LI), vascular invasion (VI), intratumoural fibrosis (ITF) (absent/mild or moderate/extended), multifocality/intraglandular spread (MFT/IGS), stromal calcification and psammoma bodies (PBs). Additionally, all PTMCs were analysed for the presence of tumour-infiltrating lymphocytes (TILs) and multinucleated giant cells (MGCs). LI was defined as a direct permeation of tumour cells or the presence of viable tumour emboli or PB within endothelial-lined spaces of peritumoural thyroid tissue or peri-thyroid fibro-adipose tissue [24]. VI was defined as a direct permeation of tumour cells through the wall of vessels with smooth muscle wall and/or with intraluminal erythrocytes as described previously by Mete and Asa [25]. MFT/IGS was assessed according to methodology reported by Niemeier et al. [8] Stromal calcification and PBs were evaluated according to criteria from the study of Bai et al. [26]. The presence of intratumoral TILs was graded based on the methodology and recommendations used in the assessment of TILs in breast cancer as well as applied in the study of PTCs [27]. According to these criteria, all tumours were divided into two groups of TILs rich and TILs poor PTMC. In cases when TILs occupied 10% or more of the tumour stroma, case was considered as TILs-rich PTMC, otherwise, it was considered as TILs-poor PTMC [27]. The number of MGCs was determined semi-quantitatively after analysis of the whole sections of the tumour as previously published [28]. PTMCs were divided into tumours without MGCs, tumours with less than 3 MGCs, and PTMC with 3 or more MGCs per tumour section [28]. Location of PTMC was classified according to position of PMTC in relation with the periphery of the thyroid as peripheral PTMC (p-PTMC) or intraparenchymal PTMC (i-PTMC). p-PTMCs were defined as tumours localised just below the peri-thyroid fibro-adipose tissue, without benign thyroid tissue between the tumour and the peri-thyroid soft tissue in at least 20% of the tumour circumference. i-PTMC was defined as PTMC completely surrounded by benign thyroid tissue [8, 9]. Presence of CLT in thyroid gland was classified according to Mizukami’s criteria for CLT and applied in the study of PD-L1 expression in PTCs [27, 29].
Immunohistochemical Analysis of PD-L1 Expression
Formalin-fixed and paraffin-embedded blocks were cut into 4-μm-thick sections, deparaffinized in xylene, and rehydrated. PD-L1 staining was performed with an anti-human PD-L1 rabbit monoclonal antibody (Code M3653; Clone 22C3; Dako; Glostrup, Denmark) in a recommended dilution (1:50). Antigen retrieval was performed using Envision FLEX Target Retrieval Solution, Low pH (Code K8005, Dako, Glostrup, Denmark). Antibody binding was visualized using an EnVision™ FLEX+, HRP visualization system (Code K8012; Dako, Glostrup, Denmark) according to the manufacturer’s instructions. Human placental tissue was used as immunohistochemistry-positive control, whereas PD-L1 expression on tumour-associated macrophages and TILs were used as an internal positive control.
Immunohistochemical scoring of PD-L1 expression was performed based on the percentage of stained tumour cells and staining intensity on tumour cells in a whole section of the tumour as described previously in the studies of Aghajani et al. and Bastman et al. [21, 30].
The immunohistochemical expression of PD-L1 was evaluated according to the membranous and/or cytoplasmic intensity of reaction on tumour cells, semi-quantitatively as a 0-negative reaction, 1+ weak reaction, 2+ moderate reaction, and 3+ intense reaction. According to the percentage of positive tumour cells, the results were evaluated through the five following groups: (1) (< 1% of cells); (2) (1–10% of cells); (3) (11–33% of cells); (4) (34–66% of cells); (5) (67–100% of cells). Histiocytes with PD-L1 staining were not included in the evaluation of PD-L1 expression. Tumour stratification according to the level of expression PD-L1 was performed using the Allred score (AS). AS was derived from summing up the percentages of positive cells and the intensity of positively stained cells. Tumours with AS ≤ 2 were considered negative, tumours with AS 3 and 4 were considered focally/moderate positive, and tumours with AS > 4 were considered PTMCs with a diffuse expression of PD-L1 [21, 30]. PD-L1 expression was also evaluated in adjacent apparently normal thyroid cells, TILs, MGCs, and tumour histiocytes.
PTMCs were divided into four groups: group 1 (G1) included p-PTMCs with PD-L1 expression of any type (focal/moderate and diffuse). Group 2 (G2) included p-PTMCs without PD-L1 expression on tumours cells. Group 3 (G3) encompassed i-PTMCs with any type of PD-L1 expression, and Group 4 (G4) included i-PTMCs without PD-L1 expression on tumour cells.
Statistical Analysis
Complete statistical analysis of data was done with the statistical software package, SPSS Statistics 18.
Most of the variables were presented as frequency of certain categories, while statistical significance of differences was tested with the Chi square test (F test in case of expected frequencies < 5).
In case of continuous data, variables were presented as mean value ± standard deviation (SD), minimal and maximal values. Spearman correlation analysis was used to establish the relationship between parameters. All the analyses were estimated at minimal p < 0.05 level of statistical significance.
Results
Clinicopathological features of PTMCs
In the selected series of 99 PMTCs, the patients had a mean age of 49 ± 14.4 years (ranging from 19 to 85 years). There were 22 males (22.2%) and 77 females (77.8%). The mean tumour size was 7.6 ± 1.5 mm. Cervical LNMs were present in 30 cases (30.3%), including 15 cases with LNMs in the central neck compartment and 15 cases with lateral cervical LNMs, whereas in 69 cases (69.7%) there was no histological, ultrasonographic or clinical evidence of cervical LNM. None of the cases had documented distant metastases. Gross ETE was detected in 3 cases (3%), microscopic ETE was present in 46 cases (46.5%), whereas ETE was not detected in 50 cases (50.5%). LI was detected in 64 cases (64.6%), whereas in 35 cases (35.4%) LI was not found. Invasion of the lymphatic vessel with only viable tumour emboli was wound in 43 cases (67.2%), PB within intraparenchymal lymphatics was found in 8 cases (12.5%) and 13 cases (20.3%) disclosed both of these morphological features. VI was present in 15 cases (15.2%), whereas in 84 cases (84.8%) was not found. Most of the cases (n = 9; 60.0%) disclosed one focus of angioinvasion, 5 cases had two foci (33.3%) and one case had three foci (6.7%) of angioinvasion. The relation between clinicopathological features of PTMC and tumour location are summarised in Table 1. Association between LI, VI and presence of LNM in PTMC with distinct locations is described in Table 2.
Association of Clinicopathological Features of PTMCs with PD-L1 Expression
The focal expression of PD-L1 was observed in 32 cases (32.3%), diffuse expression was observed in 29 cases (29.3%) (total number of PTMCs with PD-L1 expression = 61.6%) and PD-L1 expression was not detected in 38 cases (38%). The mean value of the AS in analysed PTMC was 3.03 ± 2.04. Figure 1 demonstrated the expression of PD-L1 in PTMC tumour cells, as well as in immune cells.
The PD-L1 immunoexpression in tumour and immune cells of PTMC. a PTMC without expression for PD-L1 and multinuclear giant cells with PD-L1 expression (positive internal control). b The focus of PTMC cells with weak membrane and/or cytoplasmic expression of PD-L1. c PTMC with a focal weak to moderate, membrane and/or cytoplasmic expression of PD-L1. In the papillary stroma is noticeable presence of PD-L1 positive lymphocytes. d PTMC with diffuse membranous and/or cytoplasmic expression of PD-L1 with weak to moderate intensity. e and f The area of tumour cells with an intensive expression of PD-L1 (membrane and cytoplasmic reaction). a–e PD-L1 immunohistochemical staining, scanned at magnification × 400
PD-L1 expression in tumour cells was significantly associated with PTMC staging (p = 0.011), presence of LNM (p = 0.002), classical architectural pattern (p = 0.031), invasive growth (p = 0.000), LI (p = 0.001), stromal calcification (p = 0.049) and p-PTMC localisation (p = 0.000). The relation between clinicopathological features of PTMC and PD-L1 expression is presented in Table 3.
Expression of PD-L1 was also observed in ≥ 3 TILs per high power field in 78 cases (78.8%). The PD-L1 positive TILs occurred more often in TIL-rich PTMCs than in TIL-poor PTMCs (ρ = 0.288; p = 0.004) and in PTMCs with more than 3 MGCs in comparison with PTMCs with less than 3 MGCs (ρ = 0.272; p = 0.007). Besides tumour cells and TILs, PD-L1 expression was consistently expressed in tumour histiocytes as well as in MGCs.
PD-L1 expression in PTMC tumour cells significantly correlated with the presence of CLT (ρ = 0.210; p = 0.037). In 5 patients (5.05%) with CLT, PD-L1 expression was observed in benign thyrocytes with weak to moderate membranous and/or cytoplasmic expression. PD-L1 expression was not disclosed in benign cells in 58 cases (58.58%). In 36 cases (36.36%), the expression of PD-L1 in non-neoplastic cells was weak, nonspecific, and was detected on individual cells or small groups of thyrocytes. This nonspecific reaction was always detected in thyrocytes in close proximity to lymphocytes (regardless of CLT presence) near to the infiltrative edge of the tumour or away from PTMC.
Relation Between the Clinicopathological Features of PTMC, Tumour Location and PD-L1 Expression
PD-L1 was more often expressed in p-PTMCs in comparison with i-PTMCs (p = 0.000).Therefore, we hypothesized that p-PTMCs with PD-L1 expression are morphologically different from i-PTMCs without PD-L1 expression. This hypothesis was tested after dividing PTMC into four groups as abovementioned. It was observed that G1 group (PD-L1 positive p-PTMC) was the most frequent (n = 46; 46.5%), followed by G4 group (PD-L1 negative i-PTMC) (n = 25; 25.3%) and, similar frequencies were observed for groups G3 (PD-L1 positive i-PTMC) (n = 15; 15.2%) and G2 (PD-L1 negative p-PTMC) (n = 13; 13.1%). G1 group is characterised by classical architectural pattern (p = 0.000), invasive growth (p = 0.000), presence of LI (p = 0.000), presence of VI (p = 0.002), presence of PBs (p = 0.002), moderate/extended ITF (p = 0.002), presence of PD-L1 positive TILs (p = 0.019), and a larger number of MGCs (p = 0.024). Most importantly, G1 group exhibited a statistically significant association with the presence of LNM (p = 0.000). Unlike G1 group, G4 group more commonly exhibited follicular architectural pattern, expansive/circumscribe growth, absent/mild ITF, absence of LI, absence of VI, as well as PBs, PD-L1 positive TILs, and MGCs. None of the PTMC cases from G4 group showed LNMs. Table 4 summarises clinicopathological features of different PTMC groups.
Discussion
This study demonstrates that peripheral location of PTMCs is related to specifical clinicopathological features, some of those associated previously with biological aggressiveness, which is in line with the previous reports [8,9,10,11]. Recently, Tallini et al. [11] reported that p-PTMCs with ≥ 5 mm size are morphologically different from i-PTMC, more frequently give rise to LNM, disclosed classical architectural pattern, invasive growth, PBs, and ITF which are data consistent with the results of the present study. In the same study from Tallini et al. [11], PTMCs with the ≥ 5 mm size were more frequently localised in the "subcapsular" region, suggesting that peripheral location may be modulating tumour growth and survival. Features associated with peripheral PTMC location partially overlap those of the classic type PTC whereas those related with the i-PTMC approach those of the encapsulated/circumscribed follicular variant PTC or even the NIFT-P.
In the present study, p-PTMC disclosed more often both gross and microscopic ETE, something that may be due only because of proximity to extra-thyroid tissues. The unusual finding of one i-PTMC case with gross ETE was the result of the applied methodology. This case was 10 mm in size with elongated shape and intrathyroidal position that makes "subcapsular" part of it occupied less than 20% of tumour circumference which classified it as i-PTMC.
Further, due to lymphatic vessel density and size at the thyroid periphery and in peri-thyroid soft tissue, p-PTMCs with infiltrative growth also have the possibility of having early access to lymphatics that is associated with lymphogenic IGS and LNM [8, 24, 31, 32]. The presence of LI and VI was reported in a wide range of PTMCs, from 2.5% to 67% [9,10,11, 33,34,35,36], probably as the result of the difficulty to identify lymphatic invasion, differences in applied criteria for the assessment of LI and VI, and different inclusion of either i-PMTC or p-PTMC in the study series. In the present series, LI and VI was more frequent in p-PTMCs than in i-PTMCs, in accordance with previous results [10, 11]. Additionally, the presence of LI in p-PTMCs was related with the presence of LNM. Although the results of some studies suggest an association between LI of PTMC and PTC and the LNM [24, 34,35,36,37], there is general agreement that only the blood vessel invasion has a prognostic value [38]. Vascular and lymphatic endothelial cells immunohistochemical markers were not used in this study to prevent misinterpretation of LI from its histological mimics [36, 39] constituting a limitation.
In this series, a higher percentage of MTF/IGS was found in PTMCs than the one previously reported by several groups [4,5,6, 10, 11]. The possible causes of these differences are related to the methodology, as similar results were obtained in the series of aggressive PTMCs reported by Niemeier et al. [8]. The selected cases included in this study consisted of PTMCs of larger size that could influence the frequency of multiple thyroid involvement and a separation of multicentricity from IGS was not performed. As Tallini et al. [11] reported, the present series also disclose that p-PTMC had a higher frequency of MTF/IGS than i-PTMCs within the group of PTMCs with size ≥ 5 mm.
Because of the benign biological behaviour of PTMC [1,2,3,4,5,6,7], most of the earlier studies of PD-L1 expression in PTC did not include or did not specify a group of PTMC in their analysis [16, 17, 27, 30, 40,41,42,43]. It is generally accepted that a wide variability of reported PD-L1 expression in PTC is caused by different applied methodologies [16]. Using the previously described methodology [21] herein is reported PD-L1 expression in 61.6% of PTMC which is in accordance with the results of earlier studies [20, 21]. As reported by other authors, this work reports an association of PD-L1 expression in PTMC with invasive tumour growth [21, 41], LI [21], ETE [21, 42], LNM [42], and higher disease stage [20]. However, these findings have not been confirmed in meta-analyses [18, 19]. The current findings establishing an association between PD-L1 expression in PTMC and the presence of LNM are different from earlier published results [16, 17, 20,21,22, 27, 40,41,42,43]. However, Aghajami et al. reported, significant increase in the incidence of LNM in PD-L1 positive PTC with low CD8 + T cells infiltration [21], whereas Bastman et al. reported that PD-L1 expression by tumour cells and associated immune cells was higher in patients with LNM [30]. In addition, Ulisse et al. reported a statistically significant association between high levels of PD-L1 mRNA and LNM, ETE, and disease-free survival [44]. By all means, the metastatic potential of PTMC is multifactorial and linked to different molecular, immunological, and clinicopathological features [2, 8, 21, 26], which in our study partially correlate with the p-PTMC phenotype.
The association of PD-L1 expression in PTC with disease recurrence has been confirmed by two recent meta-analyses [18, 19]. In this series, the significance of PD-L1 expression in disease recurrence was not evaluated due to short follow-up of the patients and/or unavailability of clinical information, which is the main limitation of the study. Being that LRR of PTMCs has shown to be related to LNM and ETE [3,4,5] it may be assumed that PD-L1 expression in PTMC can influence the clinical course of the disease.
According to the herein reported findings and those reported by other authors, CLT is well recognised as an immunological/inflammatory microenvironment that has an influence on PD-L1 expression, not only in tumour cell but also in benign thyroid tissue and immune cells [17, 19,20,21,22, 27, 41].
In the present study, p-PTMC expressed PD-L1 more often when compared to i-PTMC, supporting the idea that different microenvironment are developing for each tumour depending on their location. This observation is supported by the concentration of features related with biological aggressiveness in p-PTMC with PD-L1 expression (G1 group). In addition, G1 group revealed significant differences in the number of PD-L1 positive TILs which can also modulate the anti-tumour immune response by activating PD-1/PD-L1 pathway [45]. Furthermore, G1 group had a significantly higher number of MGCs whose pro-tumorigenic role in PTC has been already demonstrated [28, 46]. The significant ITF also contributed to the different microenvironment of this p-PTMC group. The cancer-associated fibroblasts (CAF), as one of the main cells of the tumour microenvironment, display diverse immunomodulating functions. By current consensus, CAFs have predominant immuno-suppressive effects by reducing anti-tumoural T cells function [47]. The presence of ITF in PTMC is related to the larger tumour diameter, invasive growth, and the development of LNM [33, 48], suggesting active participation of ITF in PTMC progression. Recently, Inoue C et al. have detected the potential of CAF to induce PD-L1 expression on lung adenocarcinomas cells [49]. Additionally, the potential of PTMC to express PD-L1 probably enables tumour survival and long-standing evolution. Thus, it provides the necessary time for the development of ITF and PB, more commonly observed in PD-L1 positive p-PTMC. Morphological features of p-PTMCs in the current study are consistent with the previously described morphology of V600EBRAF mutated PTMC [9, 11, 39] that also overlaps with the classic morphology of PTC as stated above. Being that PD-L1 expression is significantly associated with V600EBRAF mutation status in PTC [20, 21, 48], here it is assumed that this association also applies to PTMC. Future studies are necessary for clarifying the association of V600EBRAF mutation and immunosuppressive microenvironment of PTMC, as well as to define the impact of PD-L1 expression in the clinical course of PTMC.
In conclusion, p-PTMCs are morphologically different from i-PTMC and more commonly related to clinicopathological features previously associated with biological aggressiveness. Apart from morphology, p-PTMCs display different tumour microenvironment including the higher potential of tumour cells to express PD-L1, which might enable tumour survival and progression including the development of LNM.
Data Availability
The data set generated and/or analyzed during the current study are available from the corresponding author.
References
Lloyd RV, Osamura RY, Klöpel G, Rosai J, editors. World Health Organization classification of tumours of endocrine organs. Lyon: IARC Press; 2017.
Soares P, Celestino R, da Rocha AG, Sobrinho-Simões M. Papillary thyroid microcarcinoma: how to diagnose and manage this epidemic? Int J Surg Pathol. 2014;22(2):113–9. https://doi.org/10.1177/1066896913517394.
Noguchi S, Yamashita H, Uchino S, Watanabe S. Papillary microcarcinoma. World J Surg. 2008;32(5):747–53. https://doi.org/10.1007/s00268-007-9453-0.
Roti E, degli-Uberti EC, Bondanelli M, Braverman LE. Thyroid papillary microcarcinoma: a descriptive and meta-analysis study. Eur J Endocrinol. 2008;159(6):659–73. https://doi.org/10.1530/EJE-07-0896.
Hay ID, Hutchinson ME, Gonzalez-Losada T, McIver B, Reinalda ME, Grant CS et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008;144(6):980–7; discussion 987–8. doi: https://doi.org/10.1016/j.surg.2008.08.035.
Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003;237(3):399–407. https://doi.org/10.1097/01.SLA.0000055273.58908.19.
DeLellis RA, Lloyd RV, Heitz PU, Eng C. World Health Organization classification of tumors. Pathology and genetics of tumors of endocrine organs. Lyon: IARC Press; 2004.
Niemeier LA, Kuffner Akatsu H, Song C, Carty SE, Hodak SP, Yip L, et al. A combined molecular-pathologic score improves risk stratification of thyroid papillary microcarcinoma. Cancer. 2012; 118: 2069–2077; 4: 2185. doi: https://doi.org/10.1002/cncr.26425.
Virk RK, Van Dyke AL, Finkelstein A, Prasad A, Gibson J, Hui P, et al. BRAFV600E mutation in papillary thyroid microcarcinoma: a genotype-phenotype correlation. Mod Pathol. 2013;26(1):62–70. https://doi.org/10.1038/modpathol.2012.152.
Ciobanu Apostol D, Giuşcă SE, Căruntu ID, Lozneanu L, Andriescu EC, Moscalu M. Relationships between clinicopathological prognostic factors in papillary thyroid microcarcinoma: a refined analysis based on 428 cases. Int J Clin Exp Pathol. 2017;10(8):8944–56.
Tallini G, De Leo A, Repaci A, de Biase D, Bacchi Reggiani ML, Di Nanni D, et al. Does the site of origin of the microcarcinoma with respect to the thyroid surface matter? A multicenter pathologic and clinical study for risk stratification. Cancers (Basel). 2020;12(1):246. https://doi.org/10.3390/cancers12010246.
Collins M, Ling V, Carreno BM. The B7 family of immune-regulatory ligands. Genome Biol. 2005;6(6):223. https://doi.org/10.1186/gb-2005-6-6-223.
Guan J, Lim KS, Mekhail T, Chang CC. Programmed death ligand-1 (PD-L1) expression in the programmed death receptor-1 (PD-1)/PD-L1 blockade: a key player against various cancers. Arch Pathol Lab Med. 2017;141(6):851–61. https://doi.org/10.5858/arpa.2016-0361-RA.
Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48(3):434–52. https://doi.org/10.1016/j.immuni.2018.03.014.
Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384–91. https://doi.org/10.1172/JCI80011.
Ahn S, Kim TH, Kim SW, Ki CS, Jang HW, Kim JS, Kim JH, Choe JH, Shin JH, Hahn SY, Oh YL, Chung JH. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr Relat Cancer. 2017;24(2):97–106. https://doi.org/10.1530/ERC-16-0421.
Cunha LL, Marcello MA, Morari EC, Nonogaki S, Conte FF, Gerhard R, Soares FA, Vassallo J, Ward LS. Differentiated thyroid carcinomas may elude the immune system by B7H1 upregulation. Endocr Relat Cancer. 2013;20(1):103–10. https://doi.org/10.1530/ERC-12-0313.
Aghajani M, Graham S, McCafferty C, Shaheed CA, Roberts T, DeSouza P, et al. Clinicopathologic and prognostic significance of programmed cell death ligand 1 expression in patients with non-medullary thyroid cancer: A systematic review and meta-analysis. Thyroid. 2018;28(3):349–361. https://doi.org/10.1089/thy.2017.0441.
Girolami I, Pantanowitz L, Mete O, Brunelli M, Marletta S, Colato C, et al. Programmed death-ligand 1 (PD-L1) is a potential biomarker of disease-free survival in papillary thyroid carcinoma: a systematic review and meta-analysis of PD-L1 immunoexpression in follicular epithelial derived thyroid carcinoma. Endocr Pathol. 2020;31(3):291–300. https://doi.org/10.1007/s12022-020-09630-5.
Chowdhury S, Veyhl J, Jessa F, Polyakova O, Alenzi A, MacMillan C, et al. Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget. 2016;7(22):32318–28. https://doi.org/10.18632/oncotarget.8698.
Aghajani MJ, Yang T, McCafferty CE, Graham S, Wu X, Niles N. Predictive relevance of programmed cell death protein 1 and tumor-infiltrating lymphocyte expression in papillary thyroid cancer. Surgery. 2018;163(1):130–6. https://doi.org/10.1016/j.surg.2017.04.033.
Lubin D, Baraban E, Lisby A, Jalali-Farahani S, Zhang P, Livolsi V. Papillary thyroid carcinoma emerging from hashimoto thyroiditis demonstrates increased PD-L1 expression, which persists with metastasis. Endocr Pathol. 2018;29(4):317–23. https://doi.org/10.1007/s12022-018-9540-9.
Amin MB, Edge S, Greene FL, Schilsky RL, Byrd DR, Gaspar LE, Washington MK, Gershenwald JE, Compton CC, Hess KR, editors. AJCC cancer staging manual. 8th ed. Cham, Switzerland: Springer International Publishing AG; 2016.
Cheng SP, Lee JJ, Chien MN, Kuo CY, Jhuang JY, Liu CL. Lymphovascular invasion of papillary thyroid carcinoma revisited in the era of active surveillance. Eur J Surg Oncol. 2020;46(10 Pt A):1814–9. https://doi.org/10.1016/j.ejso.2020.06.044.
Mete O, Asa SL. Pathological definition and clinical significance of vascular invasion in thyroid carcinomas of follicular epithelial derivation. Mod Pathol. 2011;24(12):1545–52. https://doi.org/10.1038/modpathol.2011.119.
Bai Y, Zhou G, Nakamura M, Ozaki T, Mori I, Taniguchi E, et al. Survival impact of psammoma body, stromal calcification, and bone formation in papillary thyroid carcinoma. Mod Pathol. 2009;22(7):887–94. https://doi.org/10.1038/modpathol.2009.38.
Bai Y, Niu D, Huang X, Jia L, Kang Q, Dou F, et al. PD-L1 and PD-1 expression are correlated with distinctive clinicopathological features in papillary thyroid carcinoma. Diagn Pathol. 2017;12(1):72. https://doi.org/10.1186/s13000-017-0662-z.
Brooks E, Simmons-Arnold L, Naud S, Evans MF, Elhosseiny A. Multinucleated giant cells’ incidence, immune markers, and significance: a study of 172 cases of papillary thyroid carcinoma. Head Neck Pathol. 2009;3(2):95–9. https://doi.org/10.1007/s12105-009-0110-9.
Mizukami Y, Michigishi T, Kawato M, Sato T, Nonomura A, Hashimoto T, Matsubara F. Chronic thyroiditis: thyroid function and histologic correlations in 601 cases. Hum Pathol. 1992;23(9):980–8. https://doi.org/10.1016/0046-8177(92)90258-5.
Bastman JJ, Serracino HS, Zhu Y, Koenig MR, Mateescu V, Sams SB, et al. Tumor-infiltrating T cells and the PD-1 checkpoint pathway in advanced differentiated and anaplastic thyroid cancer. J Clin Endocrinol Metab. 2016;101(7):2863–73. https://doi.org/10.1210/jc.2015-4227.
Katoh R, Sasaki J, Kurihara H, Suzuki K, Iida Y, Kawaoi A. Multiple thyroid involvement (intraglandular metastasis) in papillary thyroid carcinoma. A clinicopathologic study of 105 consecutive patients. Cancer. 1992;70(6):1585–90.
Russl WO, Ibanez ML, Clark RL, White EC. Thyroid carcinoma. Classification, intraglandular dissemination, and clinicopathological study based upon whole organ sections of 80 glands. Cancer. 1963;16:1425–60.
Koperek O, Asari R, Niederle B, Kaserer K. Desmoplastic stromal reaction in papillary thyroid microcarcinoma. Histopathology. 2011;58(6):919–24. https://doi.org/10.1111/j.1365-2559.2011.03791.x.
Kim BY, Jung CH, Kim JW, Lee SW, Kim CH, Kang SK, et al. Impact of clinicopathologic factors on subclinical central lymph node metastasis in papillary thyroid microcarcinoma. Yonsei Med J. 2012;53(5):924–30. https://doi.org/10.3349/ymj.2012.53.5.924.
Lim YC, Choi EC, Yoon YH, Kim EH, Koo BS. Central lymph node metastases in unilateral papillary thyroid microcarcinoma. Br J Surg. 2009;96(3):253–7. https://doi.org/10.1002/bjs.6484.
Lee E, Jung W, Woo JS, Lee JB, Shin BK, Kim HK, et al. Tumor sprouting in papillary thyroid carcinoma is correlated with lymph node metastasis and recurrence. Korean J Pathol. 2014;48(2):117–25. https://doi.org/10.4132/KoreanJPathol.2014.48.2.117.
Wagner K, Abraham E, Tran B, Roshan D, Wykes J, Campbell P, Ebrahimi A. Lymphovascular invasion and risk of recurrence in papillary thyroid carcinoma. ANZ J Surg. 2020;90(9):1727–32. https://doi.org/10.1111/ans.16202.
Ghossein R, Barletta J, Bullock M, Johnson S, Kakudo K, Lam A, et al. Carcinoma of the thyroid histopathology reporting guide. Sydney, Australia: International Collaboration on Cancer Reporting; 2019.
Tallini G, de Biase D, Durante C, Acquaviva G, Bisceglia M, Bruno R, et al. BRAF V600E and risk stratification of thyroid microcarcinoma: a multicenter pathological and clinical study. Mod Pathol. 2015;28(10):1343–59.
Bai Y, Guo T, Huang X, Wu Q, Niu D, Ji X, Feng Q, Li Z, Kakudo K. In papillary thyroid carcinoma, expression by immunohistochemistry of BRAF V600E, PD-L1, and PD-1 is closely related. Virchows Arch. 2018;472(5):779–87. https://doi.org/10.1007/s00428-018-2357-6.
Fu G, Polyakova O, MacMillan C, Ralhan R, Walfish PG. Programmed death - ligand 1 expression distinguishes invasive encapsulated follicular variant of papillary thyroid carcinoma from noninvasive follicular thyroid neoplasm with papillary-like nuclear features. EBioMedicine. 2017;18:50–5. https://doi.org/10.1016/j.ebiom.2017.03.031.
Shi RL, Qu N, Luo TX, Xiang J, Liao T, Sun GH, et al. Programmed death-ligand 1 expression in papillary thyroid cancer and its correlation with clinicopathologic factors and recurrence. Thyroid. 2017;27(4):537–45. https://doi.org/10.1089/thy.2016.0228.
Angell TE, Lechner MG, Jang JK, Correa AJ, LoPresti JS, Epstein AL. BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid. 2014;24(9):1385–93. https://doi.org/10.1089/thy.2014.0134.
Ulisse S, Tuccilli C, Sorrenti S, Antonelli A, Fallahi P, D’Armiento E, et al. PD-1 ligand expression in epithelial thyroid cancers: potential clinical implications. Int J Mol Sci. 2019;20(6):1405. https://doi.org/10.3390/ijms20061405.
Gibbons Johnson RM, Dong H. Functional expression of programmed death-ligand 1 (B7–H1) by immune cells and tumor cells. Front Immunol. 2017;8:961. https://doi.org/10.3389/fimmu.2017.00961.
Gulubova MV, Ivanova KV. The expression of tumor-associated macrophages and multinucleated giant cells in papillary thyroid carcinoma. Open Access Maced J Med Sci. 2019;7(23):3944–9. https://doi.org/10.3889/oamjms.
Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20(3):174–86. https://doi.org/10.1038/s41568-019-0238-1.
Liu X, Zhang S, Gang Q, Shen S, Zhang J, Lun Y, et al. Interstitial fibrosis in papillary thyroid microcarcinoma and its association with biological behavior. Oncol Lett. 2018;15(4):4937–43. https://doi.org/10.3892/ol.2018.7928.
Inoue C, Miki Y, Saito R, Hata S, Abe J, Sato I, et al. PD-L1 induction by cancer-associated fibroblast-derived factors in lung adenocarcinoma cells. Cancers (Basel). 2019;11(9):1257. https://doi.org/10.3390/cancers11091257.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
BK, DV, and SC contributed to the study conception and design. Material preparation, data collection and analysis were performed by BK, and SC. The first draft of the manuscript was written by BK and all authors commented on previous versions of the manuscript. The scientific editing and final revision of the manuscript were performed by CE. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest with respect to research, authorship, and/or publication of this article.
Ethics Approval
This work was performed in archived paraffin tissue that remained from the diagnosis of the thyroid samples of patients that were anonymized. The materials used for the work are not needed for diagnosis in the present nor in the future. The Ethics Committee of the Military Medical Academy and University of Defence, Serbia, approved this study (Number: 16–71; 5/29/2019).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kovacevic, B., Vucevic, D., Cerovic, S. et al. Peripheral Versus Intraparenchymal Papillary Thyroid Microcarcinoma: Different Morphologies and PD-L1 Expression. Head and Neck Pathol 16, 200–212 (2022). https://doi.org/10.1007/s12105-021-01337-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-021-01337-1