Abstract
Immune checkpoint inhibitor therapies targeting PD-L1/PD-1 have been shown to be effective in treating several types of human cancer. In papillary thyroid carcinoma (PTC), little is known about the expression of PD-L1/PD-1 in the tumor microenvironment or its potential correlation with BRAF V600E mutation status. In this study, we examined the expression of PD-L1, PD-1, and BRAF V600E in PTC by immunohistochemistry and investigated the clinical significance of expression status. We studied the expression of PD-L1, PD-1, and BRAF V600E by immunohistochemical staining in 110 cases of PTC with a diameter > 1 cm. Cases with a background of chronic lymphocytic thyroiditis (CLT) were excluded, as differentiating lymphocytes in the context of CLT from tumor-infiltrating lymphocytes (TILs) is difficult. We classified PD-L1+/PD-1+ expression as type 1 (41%), PD-L1−/PD-1− as type 2 (17%), PD-L1+/PD-1− as type 3 (5%), and PD-L1−/PD-1+ as type 4 (37%). Significant correlations were found between expression of BRAF V600E and that of PD-L1 and PD-1. The positive correlation observed between expression of BRAF V600E and PD-L1/PD-1 suggests that immunotherapies targeting PD-L1/PD-1 might be effective for PTC patients with the BRAF V600E mutation, which are refractory to radioiodine therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer is the most common endocrine malignancy, and it accounts for the majority of endocrine cancer deaths. Papillary thyroid carcinoma (PTC) accounts for approximately 90% of thyroid cancers, and more than 10% of patients with PTC develop cancer recurrence or distant metastasis, and cancer-related death may occur [1, 2]. In the past decade, significant progress has been made in identifying the molecular changes responsible for PTC development and invasiveness. The RET-RAS-RAF-MEK-ERK (mitogen-activated protein kinase) pathway is the most widely accepted signaling pathway responsible for thyroid tumorigenesis and invasiveness, in which the BRAF V600E mutation plays a prominent role, resulting in overactivity of downstream signaling [3,4,5].
Human cancers harbor numerous genetic and epigenetic alterations, generating neoantigens that are potentially recognizable by the immune system [6]. Programmed death 1 (PD-1) is a key immune checkpoint receptor, mainly expressed by activated T cells, and it mediates immunosuppression [7]. PD-L1 (B7-H1) is the ligand of PD-1, of which the expression is greatly increased on some tumor cells. Inhibition of the interaction between PD-1 and PD-L1 can enhance T cell responses in vitro and mediate preclinical antitumor activity [7,8,9,10]. Clinical trials using anti-PD-1 or anti-PD-L1 antibodies to treat patients with cancers, such as melanoma, nonsmall cell lung carcinoma, renal cell carcinoma, prostatic carcinoma, and colorectal carcinoma, have provided encouraging results [10,11,12,13,14,15]. Furthermore, patients with PD-L1-positive tumors had an objectively better response to anti-PD-1 treatment than patients with PD-L1-negative tumors [14]. A positive response to immunotherapy usually relies on dynamic interactions between tumor cells and immunomodulators inside the tumor microenvironment, which plays an important role in dampening or enhancing immune responses [16, 17]. Teng et al. classified tumors into four groups on the basis of their PD-L1 status and presence or absence of the tumor-infiltrating lymphocytes. Different types of immunotherapeutic approaches might have to be applied to different groups of tumors [16].
Surgery and postoperative radioiodine therapy are the basic treatments for most patients with differentiated thyroid carcinoma. However, a subset of PTC patients does not respond to radioiodine therapy. The incidence of radioiodine-refractory PTCs is higher in tumors with the BRAF V600E mutation [18, 19]. For PTC patients who failed to respond to radioiodine therapy, it is important to develop novel treatment modalities. Little is known regarding PD-L1/PD-1 expression status in PTC cases, especially taking into account the BRAF mutation status [20,21,22,23,24]. Therefore, examining the relationship between BRAF V600E status and PD-L1/PD-1 expression is of great significance, as this might be used to select candidates for immunotherapy. Furthermore, it has been reported that combining BRAF inhibitors and anti-PD-L1 antibody therapies improves tumor regression and antitumor immunity in melanoma cell lines as well as in an immunocompetent murine model of anaplastic thyroid carcinoma [25, 26]. This indicates that combination approaches may be potential avenues to increase antitumor efficacy. Some PTC cases harbor a background of chronic lymphocytic thyroiditis (CLT). In these cases, differentiating CLT from tumor-infiltrating lymphocytes (TILs) is difficult, as conceivably changes in immunomodulatory receptor expression may overlap and thus complicate the results. The purpose of the present study is to clarify in PTC lacking a CLT background the expression status of BRAF V600E, PD-L1, and PD-1 and the clinical significance of BRAF V600E, PD-L1, and PD-1 expression and classify microenvironment in PTC and its correlation with BRAF V600E status.
Materials and methods
Patients and specimens
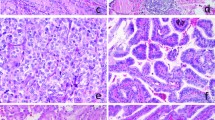
We reviewed the records of patients with a diagnosis of PTC who underwent surgical treatment at Peking University Cancer Hospital, Beijing, China. Patients with a background of CLT or Hashimoto’s disease were excluded from this study. Tumor-bearing thyroid tissues with only a small or focal lymphocytic reaction were not regarded as CLT specimens in the present study (Fig. 1a, b), since they represented types of nonspecific thyroiditis but not autoimmune thyroiditis. Patients with microcarcinoma were excluded from this study because they have a much better clinical outcome than patients with other types of PTC, as noted in our earlier study [27], and may not need additional treatment. In total, 110 patients were enrolled, consisting of 79 females and 31 males. All patients had no evidence of distant metastasis at surgery.
a Papillary thyroid carcinoma (PTC) with papillary structures and typical nuclear features of PTC (×100). b Focal lymphocytic infiltration was noted in the background of PTC, which should not be regarded as chronic lymphocytic thyroiditis (×100). c BRAF V600E was strongly and diffusely expressed on the tumor cell cytoplasm (×100). d Mild but diffuse expression of BRAF V600E was observed on the PTC (×100). e PD-L1 was unequivocally expressed on the tumor cell membrane and in part of the cytoplasm (×100). f PD-1 expression on tumor-infiltrating lymphocytes was identified in the tumor stroma (×100)
This study was approved by the ethics committee of Peking University Cancer Hospital & Institute.
Histological examination
Ninety cases of conventional-type PTC, 18 cases of follicular variant of PTC, one case of a solid variant, and one case of a tall cell variant were selected (n = 110). All sections were stained with hematoxylin and eosin and independently reviewed by two pathologists, YB and DN, to examine the presence of psammoma bodies, stromal calcification, bone formation within the tumor mass, tumor extrathyroidal invasion, and tumor multifocality. The definition of psammoma bodies, stromal calcification, and bone formation has been described in detail in our earlier study [28]. Psammoma bodies, stromal calcification, and bone formation were encountered in 49, 52, and 3% of the samples, respectively.
The pT, pN, and stage grouping were classified according to the cancer staging guidelines in the seventh edition of the American Joint Committee on Cancer.
Immunohistochemical analysis
We stained formalin-fixed, paraffin-embedded (FFPE) whole-tissue 4-μm sections from 110 cases of PTC, including tumor center and tumor invasion front, for BRAF V600E, PD-L1, and PD-1. Two pathologists (YB and DN) independently evaluated all stained sections.
Immunohistochemical staining using a BRAF V600E mutation-specific antibody (VE1) identifies the BRAF V600E gene mutation specifically and sensitively according to previous studies [29,30,31]. Mouse monoclonal anti-VE1 primary antibody (Ventana Medical Systems, Oro Valley, AZ, USA) was used in a working dilution on the Benchmark XT platform (Ventana Medical Systems). Staining was visualized using the ultraView Universal DAB Detection Kit (Ventana Medical Systems). Prevalidated VE1-positive and VE1-negative PTC specimens were used as positive and negative controls, respectively. The immunoreactivity of VE1 was evaluated as positive if unequivocal diffuse cytoplasmic staining of tumor cells was identified [29,30,31,32]. Focal staining, any type of isolated nuclear staining, and weak staining of single interspersed cells were evaluated as negative.
PD-L1 staining was performed with an anti-human PD-L1 rabbit monoclonal antibody (SP142; Zhongshan Golden Bridge Biotechnology, Beijing, China) in a working dilution on an automated staining platform (BOND-III, Leica Biosystems Ltd., Newcastle, UK) using a Bond Polymer Refine Detection kit (Leica Biosystems Ltd., Newcastle, UK) according to the manufacturer’s instructions. Primary antibody was replaced with phosphate-buffered saline in the control sections to verify immunostaining specificity. Staining of 5% or more tumor cells was considered as positive and of less than 5% cells as negative [33].
For PD-1, antigen retrieval was carried out in a PTLink machine (Dako) after deparaffinization and rehydration of the FFPE sections. Subsequently, the sections were incubated in 3% H2O2 solution at room temperature for 10 min to block endogenous peroxidase activity. Sections were then incubated with mouse anti-PD-1 monoclonal antibody in a working dilution (UMAB199; Zhongshan Golden Bridge Biotechnology, Beijing, China) at 37 °C for 15 min, followed by incubation with anti-mouse IgG-HRP conjugate (EnVision FLEX/HRP; Dako) at room temperature for 25 min. Antibody binding was visualized using an EnVision FLEX DAB CHROMOGEN kit (Dako) according to the manufacturer’s instructions. As negative control, primary antibodies were replaced with phosphate-buffered saline. Immunostaining of PD-1 was scored as positive when in a hot spot inside the tumor, or in the tumor invasive front, 3 or more TIL/high power field (HPF) were identified, using an Olympus BX43 microscope.
Statistical analysis
Data analysis was performed using SPSS 17.0 statistical software. Time-independent and categorical data were evaluated using the chi-squared test, continuity correction, or Fisher’s exact probability test as appropriate. Statistical significance was defined as P < 0.05.
Results
Immunohistochemical expression of BRAF V600E, PD-L1, and PD-1 in papillary thyroid carcinoma without a background of CLT
VE1 staining was considered positive and was diffuse with either strong, moderate, or weak intensity (Fig. 1c, d) in 76% of PTC cases. Even tumors with weak diffuse staining have been shown to harbor the BRAF V600E gene mutation [29].
PD-L1 expression was located on the plasma membrane of tumor cells, with or without cytoplasmic staining. The staining pattern was either diffuse or focal (Fig. 1e). Strong staining on histiocytes inside the glandular lumina and in the fibrous stroma of PTC was not considered as positive. In six out of 18 follicular variant cases, the staining was focal. In the solid-type variant, the staining was diffuse, and the tall cell variant was negative. PD-L1 expression was considered positive in 46% of all PTC cases.
Expression of PD-1 on TILs was found both in the tumor center and in the invasive front of the tumor in 78% of PTC cases (Fig. 1f).
Clinicopathological correlation of BRAF V600E, PD-L1, and PD-1 expression
BRAF V600E expression was not significantly correlated with the clinicopathological parameters patient age, gender, tumor size, extrathyroidal invasion, pT, pN, stage grouping, the presence of psammoma bodies, stromal calcification, bone formation, and tumor multifocality (data not shown).
PD-L1 expression significantly correlated with the absence of psammoma bodies (P = 0.007), but not with the other clinicopathological parameters (Table 1). Expression of PD-1 significantly correlated with small tumor size and absence of stromal calcification (P = 0.022 and 0.010, respectively), but not with the other clinicopathological parameters (Table 2).
The correlation between BRAF V600E, PD-L1, and PD-1 expression
BRAF V600E expression was correlated with both PD-L1 and PD-1 expression (P = 0.004 and 0.001, respectively) (Tables 1 and 2, Fig. 2a, b). PD-L1 expression correlated significantly with that of PD-1 (P = 0.005) (Fig. 2c).
Classification of tumor microenvironments based on PD-L1 andPD-1 expression
PD-L1 and PD-1 expression in the tumor microenvironment was classified as type 1 (PD-L1+/PD-1+, 41%), type 2 (PD-L1−/PD-1−, 17%), type 3 (PD-L1+/PD-1−, 5%), and type 4 (PD-L1−/PD-1+, 37%). BRAF V600E expression, the presence of psammoma bodies, and stromal calcification were significantly different between the four types. The BRAF V600E was positive in 89% of type 1, 37% of type 2, 80% of type 3, and 78% of type 4, respectively (P = 0.001). According to type, psammoma bodies were found in 33, 47, 40, and 68% of types 1, 2, 3, and 4, respectively (P = 0.013), while for stromal calcification, this was 47, 79, 60, and 44%, respectively (P = 0.065). In 8% of the 83 BRAF V600E-positive cases, PD-L1−/PD-1− (type 2) expression was found.
Discussion
We studied immunohistochemical expression status of both PD-L1 and PD-1 in a large number of human PTC cases. Expression of PD-L1 and PD-1 and BRAF status have been studied in follicular cell-derived thyroid carcinomas, as listed in Table 3. Bastman et al. found in 92 cases of differentiated thyroid carcinoma expression of PD-L1/PD-1 mRNA; PD-L1 expression was higher in patients with nodal metastases [24]. This is different from our findings, most likely due to methodological differences. They also found no association of the BRAF V600E mutation with immunohistochemical expression of PD-L1, but on a small cohort of 22 advanced pT4 cases. Ahn et al. found PD-L1 expression in approximately 6% of PTCs, without correlation with PD-L1 status or oncogen mutation profile [21]. We found much more frequent expression of PD-L1 (46%) in PTCs, positively correlated with the expression of BRAF V600E and PD-1. The data of Ahn et al. should be interpreted with caution, since they used tissue microarrays which may not be representative of the whole tumor.
In recent years, a novel antibody specifically recognizing the BRAF V600E protein (clone VE1) has become available. Its specificity and sensitivity toward the BRAF V600E protein has been verified in many series of tumors, including in melanoma and PTC [29,30,31], and immunohistochemical staining with this antibody can be used as a surrogate for detection of the BRAF V600E mutation. We used this antibody to detect the BRAF V600E protein in PTC by immunohistochemistry, and our results were in accordance with those of Angell et al. [36] in that expression of BRAF V600E was associated with increased PD-L1 expression. We also found expression of BRAF V600E and PD-1 to be positively correlated. This provides a molecular basis for immunotherapy, targeting PD-L1/PD-1, for the subset of PTC patients who are refractory to radioiodine therapy. It should be noted that our cases were mostly conventional PTCs, and the correlation between BRAF V600E and PD-L1/PD-1 in specific PTC variants should be investigated in larger case series. We found expression of PD-L1 to be correlated with the absence of psammoma bodies, and of PD-1 with small tumor size and absence of stromal calcification. Stromal calcification arises by deposition of calcium phosphate in the fibrous stroma. The formation of both psammoma bodies and stromal calcification requires a certain amount of time. That PD-L1 is more often expressed in the absence of psammoma bodies, and PD-1 more often in the absence of stromal calcification, suggests that expression of PD-L1 and PD-1 is associated with a short clinical course and both may be involved in tumor growth, as has been shown in ovarian cancer [37].
A previous study has classified the tumor microenvironment into four groups based on PD-L1 expression and the presence of TILs [16]. Type 1 is PD-L1-positive in the presence of TILs which drives adaptive immune resistance, type 2 is PD-L1-negative in the absence of TIL which indicates immune ignorance, type 3 is PD-L1-positive in the absence of TILs indicating intrinsic induction, and type 4 is PD-L1-negative in the presence of TILs indicating the role of other suppressors in promoting immune tolerance. Patients with a type 1 tumor are most likely to benefit from single agent anti-PD-1/L1 blockage. In patients with a type 2 tumor, single agent checkpoint blockage would most likely not be successful, given the absence of pre-existing T cells, and combination therapies for this group should therefore be designed to bring T cells into tumors, such as a combination of anti-CTLA4 and anti-PD-1. For type 3 patients, a similar approach to that used for type 2 patients might be employed to recruit lymphocytes into tumors. For patients with a type 4 tumor, other immunosuppressor pathways such as metabolites and non-T cell effector strategies might be considered. The expression of PD-1 in TILs provides a more direct indicator of the tumor immunosuppression status. Therefore, we classified the tumor microenvironment into four groups, according to the expression of PD-L1 in tumor cells and the expression of PD-1 in the TILs, which might direct immunotherapy approaches more effectively. We found low incidence of BRAF V600E expression in PD-L1−/PD-1− presumably immune ignorant tumors, and lack of psammoma bodies in PD-L1+/PD-1+ presumably immune-resistant tumors, which may help to improve the prediction of the efficacy of anti PD-L1/PD-1 immunotherapy.
In conclusion, we found that immunohistochemical expression of BRAF V600E was correlated with that of both PD-L1 and PD-1 in this series of PTC (of > 1 cm and without a background of lymphocytic thyroiditis). Some distinctive pathological features in PTC, including psammoma bodies and stromal calcification, were predictive of PD-L1 or PD-1 expression. Furthermore, patterns of PD-L1 and PD-1 expression might be helpful to predict antitumor efficacy of immunotherapy targeting PD-L1/PD-1 in a subset of PTC patients.
References
Bai Y, Kakudo K, Li Y, Liu Z, Ozaki T, Ito Y, Kihara M, Miyauchi A (2008) Subclassification of non-solid-type papillary thyroid carcinoma identification of high-risk group in common type. Cancer Sci 99:1908–1915
Carcangiu ML, Zampi G, Pupi A, Castagnoli A, Rosai J (1985) Papillary carcinoma of the thyroid. A clinicopathologic study of 241 cases treated at the University of Florence, Italy. Cancer 55:805–828
Xing M (2005) BRAF mutation in thyroid cancer. Endocr Relat Cancer 12:245–262
Baquero P, Sanchez-Hernandez I, Jimenez-Mora E, Orgaz JL, Jimenez B, Chiloeches A (2013) (V600E) BRAF promotes invasiveness of thyroid cancer cells by decreasing E-cadherin expression through a Snail-dependent mechanism. Cancer Lett 335:232–241
Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA (2003) High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 63:1454–1457
Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JK, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE (2006) The consensus coding sequences of human breast and colorectal cancers. Science 314:268–274
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 99:12293–12297
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L (2002) Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800
Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L (2012) Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4:127ra37
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366:2455–2465
Ilie M, Hofman V, Dietel M, Soria JC, Hofman P (2016) Assessment of the PD-L1 status by immunohistochemistry: challenges and perspectives for therapeutic strategies in lung cancer patients. Virchows Arch 468:511–525
Massari F, Santoni M, Ciccarese C, Santini D, Alfieri S, Martignoni G, Brunelli M, Piva F, Berardi R, Montironi R, Porta C, Cascinu S, Tortora G (2015) PD-1 blockade therapy in renal cell carcinoma: current studies and future promises. Cancer Treat Rev 41:114–121
Sznol M, Chen L (2013) Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res 19:1021–1034
Zhang T, Xie J, Arai S, Wang L, Shi X, Shi N, Ma F, Chen S, Huang L, Yang L, Ma W, Zhang B, Han W, Xia J, Chen H, Zhang Y (2016) The efficacy and safety of anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory cancers: a meta-analysis. Oncotarget 7:73068–73079
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454
Teng MW, Ngiow SF, Ribas A, Smyth MJ (2015) Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res 75:2139–2145
Tang H, Qiao J, Fu YX (2016) Immunotherapy and tumor microenvironment. Cancer Lett 370:85–90
Ricarte-Filho JC, Ryder M, Chitale DA, Rivera M, Heguy A, Ladanyi M, Janakiraman M, Solit D, Knauf JA, Tuttle RM, Ghossein RA, Fagin JA (2009) Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res 69:4885–4893
Xing M (2013) Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 13:184–199
Chowdhury S, Veyhl J, Jessa F, Polyakova O, Alenzi A, MacMillan C, Ralhan R, Walfish PG (2016) Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget 31:32318–32328
Ahn S, Kim TH, Kim SW, Ki CS, Jang HW, Kim JS, Kim JH, Choe JH, Shin JH, Hahn SY, Oh YL, Chung JH (2017) Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr Relat Cancer 24:97–106
Cunha LL, Marcello MA, Morari EC, Nonogaki S, Conte FF, Gerhard R, Soares FA, Vassallo J, Ward LS (2013) Differentiated thyroid carcinomas may elude the immune system by B7H1 upregulation. Endocr Relat Cancer 20:103–110
Wu H, Sun Y, Ye H, Yang S, Lee SL, de las Morenas A (2015) Anaplastic thyroid cancer: outcome and the mutation/expression profiles of potential targets. Pathol Oncol Res 21:695–701
Bastman JJ, Serracino HS, Zhu Y, Koenig MR, Mateescu V, Sams SB, Davies KD, Raeburn CD, McIntyre RC Jr, Haugen BR, French JD (2016) Tumor-infiltrating T cells and the PD-1 checkpoint pathway in advanced differentiated and anaplastic thyroid cancer. J Clin Endocrinol Metab 101:2863–2873
Atefi M, Avramis E, Lassen A, Wong DJ, Robert L, Foulad D, Cerniglia M, Titz B, Chodon T, Graeber TG, Comin-Anduix B, Ribas A (2014) Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res 20:3446–3457
Brauner E, Gunda V, Vanden Borre P, Zurakowski D, Kim YS, Dennett KV, Amin S, Freeman GJ, Parangi S (2016) Combining BRAF inhibitor and anti PD-L1 antibody dramatically improves tumor regression and anti tumor immunity in an immunocompetent murine model of anaplastic thyroid cancer. Oncotarget 7:17194–17211
Zuo H, Tang W, Yasuoka H, Nakamura Y, Ito Y, Miyauchi A, Kakudo K (2007) A review of 227 cases of small papillary thyroid carcinoma. Eur J Surg Oncol 33:370–375
Bai Y, Zhou G, Nakamura M, Ozaki T, Mori I, Taniguchi E, Miyauchi A, Ito Y, Kakudo K (2009) Survival impact of psammoma body, stromal calcification, and bone formation in papillary thyroid carcinoma. Mod Pathol 22:887–894
Sun J, Zhang J, Lu J, Gao J, Lu T, Ren X, Duan H, Liang Z (2015) Immunohistochemistry is highly sensitive and specific for detecting the BRAF V600E mutation in papillary thyroid carcinoma. Int J Clin Exp Pathol 8:15072–15078
Pyo JS, Sohn JH, Kang G (2015) BRAF immunohistochemistry using clone VE1 is strongly concordant with BRAF (V600E) mutation test in papillary thyroid carcinoma. Endocr Pathol 26:211–217
Liu H, Li Z, Wang Y, Feng Q, Si L, Cui C, Guo J, Xue W (2014) Immunohistochemical detection of the BRAF V600E mutation in melanoma patients with monoclonal antibody VE1. Pathol Int 64:601–606
Koperek O, Kornauth C, Capper D, Berghoff AS, Asari R, Niederle B, von Deimling A, Birner P, Preusser M (2012) Immunohistochemical detection of the BRAF V600E-mutated protein in papillary thyroid carcinoma. Am J Surg Pathol 36:844–850
Li Z, Lai Y, Sun L, Zhang X, Liu R, Feng G, Zhou L, Jia L, Huang X, Kang Q, Lin D, Gao J, Shen L (2016) PD-L1 expression is associated with massive lymphocyte infiltration and histology in gastric cancer. Hum Pathol 55:182–189
Zwaenepoel K, Jacobs J, De Meulenaere A, Silence K, Smits E, Siozopoulou V, Hauben E, Rolfo C, Rottey S, Pauwels P (2017) CD70 and PD-L1 in anaplastic thyroid cancer-promising targets for immunotherapy. Histopathology 71:357–365
Chintakuntlawar AV, Rumilla KM, Smith CY, Jenkins SM, Foote RL, Kasperbauer JL, Morris JC, Ryder M, Alsidawi S, Hilger C, Bible KC (2017) Expression of PD-1 and PD-L1 in anaplastic thyroid cancer patients treated with multimodal therapy: results from a retrospective study. J Clin Endocrinol Metab 102:1943–1950
Angell TE, Lechner MG, Jang JK, Correa AJ, LoPresti JS, Epstein AL (2014) BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid 24:1385–1393
Clark CA, Gupta HB, Sareddy G, Pandeswara S, Lao S, Yuan B, Drerup JM, Padron A, Conejo-Garcia J, Murthy K, Liu Y, Turk MJ, Thedieck K, Hurez V, Li R, Vadlamudi R, Curiel TJ (2016) Tumor-intrinsic PD-L1 signals regulate cell growth, pathogenesis, and autophagy in ovarian cancer and melanoma. Cancer Res 76:6964–6974
Funding
This work was supported by the National Nature Science Foundation of China (Nos. 81202114, 81301874, and 81301879), the National Science Foundation of Beijing (No. 7132051), and the General Program, Research Fund for the Doctoral Program of Higher Education (No. 2012001120135).
Author information
Authors and Affiliations
Contributions
BY, GT, KK, and LZ conceived and designed the experiments. HX and WQ performed the experiments. BY and ND reviewed the slides; JX and FQ analyzed the data. BY and GT wrote the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bai, Y., Guo, T., Huang, X. et al. In papillary thyroid carcinoma, expression by immunohistochemistry of BRAF V600E, PD-L1, and PD-1 is closely related. Virchows Arch 472, 779–787 (2018). https://doi.org/10.1007/s00428-018-2357-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-018-2357-6