Abstract
Salivary duct carcinoma (SDC) commonly expresses androgen receptor (AR) and HER2, giving rise to treatment implications. SDC may also express programmed-death-ligand-1 (PD-L1), a predictive marker of response to checkpoint inhibitors. PD-L1 can be associated with genomic instability and high density of tumor infiltrating lymphocytes (TILs). Evaluation of HER2 immunohistochemistry (IHC) in SDC is not standardized, and relationships between ERBB2 copy numbers, PD-L1 expression and TILs in SDC are unknown. We evaluated 32 SDCs for HER2, AR and PD-L1 expression (IHC), ERBB2 status (FISH) and TILs (slide review). HER2 was scored with three different systems (breast, gastric, proposed salivary gland). PD-L1 was evaluated with the combined positive score. Most patients were older men, presenting at advanced clinical stage with nodal or distant metastases. During follow-up (mean 5 years, range 6 months to 21 years), 25 of the 32 patients (78%) died of SDC. We propose a HER2 IHC scoring system which accurately predicts underlying ERBB2 amplification or increased copy numbers in SDC. Most tumors had increased ERBB2 copy numbers (19/32 amplification, 6/32 aneusomy), a finding associated with higher TIL densities (p = 0.045) and PD-L1 expression (p = 0.025). Patients with TILs ≥ 40% had better prognoses (Log-Rank p = 0.013), with TILs being favorable prognosticators in univariate analysis (Hazard ratio: 0.18, p = 0.024). A subset of SDCs with increased ERBB2 copy numbers have higher TILs and PD-L1 expression. TILs ≥ 40% are associated with better prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salivary duct carcinoma (SDC) is a rare malignant salivary gland tumor, most commonly arising in the parotid gland of older patients [1]. It was initially described in 1968 by Kleinsasser, who highlighted its morphologic resemblance with mammary ductal carcinoma [2]. SDC behaves aggressively, with more than half of patients presenting with cervical lymph node or distant metastases at the time of diagnosis [3]. Prognosis for these patients is very poor, with an estimated 5-year overall survival of around 40% for those with nodal disease and essentially 0% for those with distant metastases [4]. Histopathologically, the majority of tumors show perineural, vascular and extraparenchymal invasion consistent with their aggressive biological behavior [1].
Interestingly, more than 70% of SDCs express Androgen Receptor (AR) [5, 6] and about 30% express HER2 [1] through underlying ERBB2 gene amplification that can be detected by fluorescent in situ hybridization (FISH) [7].
The hormone receptor expression profile, as well as the mutational landscape of SDCs resemble apocrine breast cancer, which also often expresses AR and HER2 [8] and has similar genetic alterations as defined by gene expression clustering. These similarities may be useful for extending research findings of apocrine breast cancer to SDCs [9].
Androgen deprivation therapy is commonly used in patients with SDC [5, 10, 11] and there is evidence that some of them can benefit from treatment with trastuzumab when their tumors overexpress HER2 [12, 13].
Trastuzumab is used for treating eligible patients with breast or gastric cancer. The immunohistochemical (IHC) evaluation of HER2 expression has been standardized with guidelines that differ for those two malignancies [14,15,16]. There is no scoring system or consensus regarding HER2 IHC evaluation in SDC. Most past studies have used the scoring system for breast [7, 17, 18], arbitrarily designating positivity based on the intensity of stain, or deferring to ERBB2 amplification status by FISH [1].
Many cancer patients have recently benefited from treatment with checkpoint inhibitors [19]. Tumor cells expressing Programmed Death-Ligand 1 (PD-L1) can escape anti-tumor immune response through PD-L1 interaction with Programmed Death-1 (PD-1) molecule found on immune cells [20, 21]. Anti-PD-1 or anti-PD-L1 medications block this interaction, boosting the anti-cancer immune response [21]. Three recent studies reported the expression of PD-L1 in about 25–50% of SDCs, without any clear associations with morphologic or hormone receptor expression [22,23,24]. Recent data showed clinical benefit for patients with PD-L1-expressing, high-grade salivary gland carcinomas treated with pembrolizumab (anti-PD-1) [25].
PD-L1 expression correlates with the density of tumor infiltrating lymphocytes (TILs) in many cancers including laryngeal squamous cell carcinoma [26] and HER2 positive breast cancer [27]. TILs are favorable prognosticators in many cancers, especially those with high mutation burden such as colorectal cancer [28], breast cancer [29] and malignant melanoma [30]. In a series of 30 high-grade salivary gland cancers, which included 8 SDCs, Nakano et al. did not demonstrate any significant relationship between TILs and PD-L1 expression [23]. No studies have confirmed any prognostic significance of TILs and/or PD-L1 in SDC so far.
We investigated the effectivity of the major existing HER2 IHC scoring systems (breast and gastric) in predicting underlying ERBB2 gene amplification and underlying gene copy number alterations and we evaluated the associations and prognostic role of TILs and PD-L1 in patients with SDC.
Materials and Methods
Case Selection
Archival surgical pathology material from patients with a diagnosis of SDC was recovered from institutional Tissue Registry and evaluated for adequacy. 4 μm thick sections were obtained from Formalin Fixed Paraffin Embedded (FFPE) tissues and stained with hematoxylin and eosin (H&E), then reviewed by two surgical pathologists (KC and JJG) for adequacy and representativeness. Clinical data were obtained through chart review.
TIL Evaluation
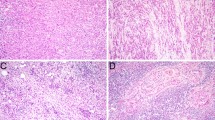
We evaluated TILs as the % ratio of the area occupied by TILs to the area of stromal cells in whole H&E sections (Fig. 1), as it has been recommended in recently published guidelines [31, 32]. We reported the values as continuous variables, which facilitates statistical analysis [33].
Immunohistochemistry (IHC)
Unstained sections were stained for the following antigens in an automated immunostaining processor (Ventana Medical Systems, Tuscon, AZ) with the following antibodies: AR (clone AR27, Novocastra, Newcastle Upon Tyne, United Kingdom); HER2 (clone 4B5, Ventana, Tuscon, AZ, USA); PD-L1 (clone 22C3, Dako North America Inc., Carpinteria, CA, USA); CD4 (clone 4B12, Dako North America Inc., Carpinteria, CA, USA); CD8 (clone 144B, Dako North America Inc., Carpinteria, CA, USA). Normal tonsil tissue was used as positive and negative control.
Evaluation was performed by two surgical pathologists (KC and JJG).
For AR IHC, tumors displaying nuclear reactivity were classified as positive (Fig. 2).
For HER2 IHC we evaluated membranous reactivity with 3 different systems (Breast, Gastric and Proposed Salivary Gland) as follows:
Breast Scoring System [Wolf et al., endorsed by the College of American Pathologists (CAP)] [14]
0: No staining or incomplete weak staining in ≤ 10% of tumor cells; 1+: Incomplete weak staining in > 10% of tumor cells; 2+: Weak to moderate complete staining in > 10% of tumor cells; 3+: Complete strong staining in > 10% of tumor cells.
Gastric Scoring System (Bartley et al. Endorsed by CAP) [16]
0: No staining or staining in < 10% of tumor cells; 1+: Weak, partial staining in ≥ 10% of tumor cells; 2+: Weak to moderate complete, lateral or basolateral staining in > 10% of tumor cells; 3+: Strong, complete, lateral or basolateral staining in ≥ 10% of tumor cells.
Salivary Gland Scoring System (suggested by our group) (Fig. 3b, e, h, k with corresponding H&Es in Fig. 3a, d, f, j)
SDC H&E (left column) with corresponding HER2 IHC (middle column) and FISH (right column). Evaluation according to the proposed salivary gland HER2 IHC scoring system showed values of 0 (b), 1+ (e), 2+ (h) and 3+ (k) with corresponding negative FISH (c, f, i) in 0, 1+ and 2+ and positive FISH (l) in 3+
0: No staining; 1+: Weak staining in < 50% of tumor cells; 2+: Weak staining in ≥ 50% or strong staining in < 50% of tumor cells; 3+: Strong staining in ≥ 50% of tumor cells.
Regarding PD-L1, tumor or immune cells were considered positive when they displayed membranous reactivity (Fig. 4b). We used the combined positive score (CPS) which is calculated by assessing the number of PD-L1 positive tumor cells and immune cells divided by the total number of viable tumor cells [34]. In addition to being more suitable for predicting clinical outcomes, CPS also has excellent inter-observer agreement reproducibility [35].
CD4 and CD8 IHC was evaluated manually (JJG and KC) and the CD4:CD8 ratio was calculated by dividing the average number of CD4 expressing and CD8 expressing cells in “hotspot” areas of expression, as previously described [36].
Fluorescent In Situ Hybridization (FISH)
FISH was performed with the PathVysion HER2 DNA dual probe set (Abbott Molecular Inc. Des Plaines, IL, USA) on 4 μm thick FFPE sections. The probes included CEP17, targeting chromosome 17 centromere (green color) and HER2, targeting chromosomal region 17q11.2-q12 (orange color). Interpretation followed the most recent American Society of Clinical Oncology (ASCO)/CAP recommendations [14] as follows:
First, the cases were classified in five groups according to the HER2 signals per cell and HER2 to CEP17 ratio. Group 1: HER2:CEP17 ≥ 2.0 and HER2 signals/cell ≥ 4.0; Group 2: HER2:CEP17 ≥ 2.0 and HER2 signals/cell < 4.0; Group 3: HER2:CEP17 < 2.0 and HER2 signals/cell ≥ 6.0; Group 4: HER2:CEP17 < 2.0 and HER2 signals/cell ≥ 4.0 and < 6.0; Group 5: HER2:CEP17 < 2.0 and HER2 signals/cell < 4.0. Then, the tumors were classified into positive (Group 1, Group 2 or 4 with concurrent IHC 3+, Group 3 with concurrent IHC 2 + or 3 +) (Fig. 3l) or negative (all other cases) (Fig. 3c, f, i) for ERBB2 amplification.
Chromosome 17 copy numbers that are detected by FISH can vary either artifactually, because of nuclear sectioning during tissue preparation, or truly because of genetic instability or presence of true aneusomy [37]. For these reasons we further sub-classified non-amplified cases, depending on the average number of CEP17 (green) and HER2 (orange) signals as follows: Monosomy (CEP17 and HER2 ≥ 1 and 1.5); Normal (CEP17 and HER2 ≥ 1.5 and ≤ 2.5) and Aneusomy (CEP17 and HER2 ≥ 2.5 and < 4.0).
Statistical Analysis
Assessment of Normality
Kolmogorov–Smirnov or Shapiro–Wilk tests were used as applicable to detect normal distribution of continuous variables and triage selection of the relevant parametric or non-parametric tests.
Frequency Distributions
We used the Chi-square or Fisher’s exact test to detect differences in frequency distributions, as applicable.
Mean Values
We used t-test or the non-parametric Mann–Whitney U-test, as applicable, in order to assess differences in mean values.
Correlations
Pearson’s correlation coefficient or Spearman’s rho was used to detect correlations.
Agreement
Cohen’s kappa coefficient was used to assess the level of agreement between categorical variables.
Cut-Off Values
We used the median to group cases in those with high versus low values. In order to detect additional statistically significant associations we occasionally used the mean value plus or minus one (or more) standard deviations as a cut-off.
Survival Analysis
Log-rank test and Cox proportional hazards model were used to assess the impact of certain variables on survival. The end point was defined as the time of death or the time of patient last follow up and the censored event was “death from SDC” versus “alive or death from other cause”.
Statistical Significance
p-values < 0.05 were considered as statistically significant in all the above mentioned statistical assessments.
Software
IBM SPSS Statistics Version 25 was used for statistical analysis.
Ethical Considerations
We received approval by the Mayo Clinic Institutional Review Board (Application Number 12-001311; last approval date: 2/28/2017).
Results
Patient and Tumor Characteristics
Our cohort included 32 patients diagnosed with SDC between years 1961 and 2007. A synopsis of the main clinicopathologic characteristics can be found in Table 1. The majority of patients were older men with a small or medium-sized tumor of the parotid gland, presenting at an advanced pathologic stage, with the majority of them having T3 or T4a disease with nodal involvement and more than a third with distant metastases. Half of the tumors were pure SDCs while the rest were the malignant component of carcinoma ex pleomorphic adenoma (ex-PA) (Fig. 5). More than half of the tumors showed extraparenchymal extension and many had perineural invasion. The majority of patients were initially treated with surgery and adjuvant radiotherapy and few received chemotherapy. The tumor recurred in 21 of 32 patients (65%) within an average of 17 months (earliest 49 days, latest 79 months) from the initial diagnosis. Most of these patients were treated with a combination of radiotherapy, chemotherapy and surgery for their recurrence. Most patients were diagnosed and treated before the trastuzumab era and only one of them (initially diagnosed in 2007) was treated with trastuzumab and experienced partial response, before eventually developing liver and bone metastases and succumbing to the disease. The majority of chemotherapy regimens contained cisplatin, 5-fluorouracil or a combination of both with other medications. Androgen deprivation was administered in one of the patients, who eventually died of the disease after a course of 7 years. The total follow-up period in our cohort ranged between 6 months and 21 years, averaging 5 years. At the end of the follow-up period only 5 of the patients (16%) were alive, 2 (6%) had passed away of other causes and the majority of them (25 patients or 78%) had died of complications of SDC, most commonly extensive metastatic disease to the lung (6 patients or 24%), bone (5 patients or 20%), liver (4 patients or 16%), brain or skin (2 patients each or 8% each).
Tumor Infiltrating Lymphocytes (TILs)
We assessed TILs in 28 of 32 tumors. The remaining 4 were from intraparotid lymph nodes with metastases or direct extension of the tumor and were not appropriate for evaluation. TILs showed a wide range (1–70%) and a relatively high mean value of 23.1% (standard deviation 16.7%), with a median of 20%. Representative examples of tumors with high (70%) and low (1%) levels of TILs can be seen in Fig. 1.
Immunohistochemistry (IHC)
AR
All 32 cases (100%) displayed nuclear expression of AR (Fig. 2). Staining was almost always strong and diffuse. In a few circumstances there were a few heterogeneous areas with lesser intensity and scattered groups of non-reactive cells. A few tumors showed cytoplasmic reactivity in addition to nuclear reactivity.
HER2
Evaluation was performed with 3 different scoring systems (breast, gastric and salivary gland). The scores of each case are listed in Supplementary Table 1. Most tumors were classified as 3+ with all 3 scoring systems and more specifically 18/32 (56%) with the breast, 22/32 (69%) with the gastric and 19/32 (59%) with the salivary gland scoring system. 7/32 (22%) tumors were classified as 0 with all 3 systems and a minority of tumors were classified as 1+ (3 cases with breast and 4 with salivary gland system) or 2+ (4 cases with breast, 3 with gastric and 2 with salivary gland system). Representative pictures of cases evaluated with the salivary gland system can be seen in Fig. 3b, e, h, k.
We found statistically significant agreement between the 3 scoring systems, more robust between salivary gland with breast (Kappa = 0.896, p < 0.01) than salivary gland with gastric (Kappa = 0.652, p < 0.01) or breast with gastric (Kappa = 0.605, p < 0.01).
PD-L1
The majority of cases (19/32, 59%) were negative (Fig. 4d) for PD-L1 as determined by the combined positive score (CPS). The rest 13/32 (41%) were classified as positive (Fig. 4b) and displayed a wide range of CPS scores ranging from 1.5 to 34 (median 6.00, mean 8.04, standard deviation 8.55). All positive cases showed patchy staining pattern.
CD4 and CD8
Five cases with TILs ≥ 40% were further evaluated by IHC for CD4 and CD8 and showed a consistently elevated CD4:CD8 ratio ranging from 2:1 to 10:1 (Fig. 6) (ratios in the remaining three cases were 4:1; 5:1 and 6:1).
Fluorescent In Situ Hybridization (FISH)
Most tumors had an ERBB2 copy number alteration, either in the form of amplification, which was the most common (19/32, 59%), followed by aneusomy (6/32, 19%). A single tumor (1/32, 3%) had monosomy and the rest (6/32, 19%) did not have any detectable abnormality. A complete list of FISH findings can be found in Supplementary Table 1.
Statistical Analysis
Salivary Gland HER2 IHC Scoring System Accurately Predicts ERBB2 Amplification Status as Determined by FISH
ERBB2 amplification status showed statistically significant positive correlations with HER2 IHC interpretation regardless of the scoring system (Table 2). We obtained a higher Spearman’s Rho correlation coefficient with our Proposed Salivary Gland scoring system than with the other 2 scoring systems. In addition, salivary gland scoring system flagged all 19 cases with ERBB2 amplification as 3+, with the rest of cases scoring 2+ or lower. This was not observed with the gastric scoring system, which evaluated three cases without amplification as 3+, nor for the breast scoring system which had a case with amplification scored as 2+.
Increased ERBB2 Copy Numbers are Associated with Higher TIL Densities and PD-L1 Expression
We grouped the cases according to the underlying ERBB2 status in those harboring aneusomy or amplification (25/32, 78%), therefore having increased ERBB2 gene dosage and those with monosomy or disomy (7/32, 22%), having normal or decreased gene dosage. We then compared for various clinicopathologic factors (Table 3). Cases with monosomy or normal FISH were all negative for HER2 (salivary gland scoring system) and PD-L1 by IHC and had low TIL densities. This was markedly different in the cases with aneusomy or ERBB2 amplification, none of which scored 0 for HER2 by IHC. These cases also had higher TIL densities, and more than half were positive for PD-L1. We did not detect any significant differences regarding the patients’ age, tumor size or histologic subtype (pure SDC versus SDC ex-PA). The frequency of ERBB2 amplification in the SDC ex-PA group was slightly higher than in pure SDCs (62.5% versus 56.25%), but the finding was not statistically significant (Chi-square p = 0.719).
High TIL Densities are Favorable Prognosticators for Patients with SDC
We attempted to identify any impact of the studied variables on patients’ survival. We were limited by the small amount of cases included in the cohort and the resulting small number of censored events. However, we were able to detect a small subset (5/32, 16%) of patients, whose tumors had TILs of 40% or more and had significantly better overall survival than the rest of the cohort (Log-Rank test p = 0.013) (Supplementary Fig. 1), despite the underlying ERBB2 amplification (4/5, 80%) or aneusomy (1/5, 20%) identified by FISH.
In univariate analysis, higher TILs were predictors of better overall survival in those patients (Hazard Ratio: 0.18, p = 0.024. We also examined various other factors (PD-L1, HER2 expression, ERBB2 status, tumor size, patient age, tumor histology, pathologic stage, extraparenchymal extension and perineural invasion) and were unable to obtain any statistically significant results. Finally, we did not find any statistically significant correlation between TILs and PD-L1 or any other of the above mentioned factors.
Discussion
Clinicopathologic Characteristics
Our cohort included 32 patients with an average age of 61.78 years, a male propensity (72%) and localization in the parotid gland. These findings are in accordance with those described in larger cohorts (Table 4) [1, 3, 4, 38, 39]. Half of the tumors showed histology of SDC ex-PA. In various case series, the percentage of SDCs ex-PA ranges from 20% [40] to 47.5% [38]. In the latter study, Stodulski et al. reported a statistically significant association between SDC ex-PA histology and shorter disease-free survival, but not overall survival [38]. We did not find any such association in our cohort. Dalin et al. identified two cases of SDC ex-PA with PLAG1 fusions (CTNNB1-PLAG1 and LIFR-PLAG1) [9]. PLAG1 rearrangements are extremely common in pleomorphic adenomas and carcinomas ex-PA [41]. Bahrami et al. suggest that a combination of FISH and IHC can be helpful in distinguishing carcinomas ex-PA from de novo malignancies [42]. Although we did not find any outcome differences between pure SDCs and SDCs ex-PA, PLAG1 studies may help distinguish between the two groups and potentially identify differences in larger cohorts.
Our study confirms that despite the small size of SDCs (mean 3.03 cm in our cohort and 2.8 cm in the series of 228 cases by Jayaprakash et al. [3]), they have aggressive histology, such as perineural invasion and extraparenchymal extension and are diagnosed in a clinically advanced stage with nodal or distant metastases. In our cohort, 65% of patients had nodal involvement and 37% had distant metastases at the time of diagnosis. In a total of 723 patients from the two large series by Osborn [4] and Jayaparakash [3], the aggregate frequency of nodal and distant metastatic involvement was 47% and 12% respectively and treatment with a combination of surgery with radiotherapy and chemotherapy was the rule.
SDC prognosis is poor on the long term. In our cohort, 25 of 32 patients (78%) died at the end of the follow-up period and a mortality rate of 50% or more seems to be common [3, 38, 40]. Interestingly, in the cohort by Gilbert et al. no patients had recurrence or distant metastasis after being free of disease for 5 years or more [1].
Tumor Infiltrating Lymphocytes (TILs)
High densities of TILs have been associated with better prognosis in many cancers including breast [29], lung [43, 44], colon [45], ovarian [46], endometrial [47], gastric [48] and malignant melanoma [30]. Similarly, TILs are important in patients with head and neck cancer, with most published studies concentrating on laryngeal [26, 49], pharyngeal [50], or oral cavity squamous cell carcinomas [51]. Comprehensive studies of TILs in salivary gland malignancies are currently lacking. Karja et al. reported no prognostic significance of lymphoplasmacytic infiltrates in their case series of 216 patients with benign and malignant salivary gland tumors [52]. Nakano et al., in their cohort of 30 patients with salivary gland cancer, 8 of whom with SDC, did not find any significant survival differences in patients with low versus high TILs [23]. Chang et al., in a cohort of 70 patients including 11 with SDC, reported a possible prognostic impact of CD8 + TILs in relapse-free survival, but not in overall survival. This effect was not retained in multivariate analysis which also included PD-1 and PD-L2 status [53]. Our study is the first demonstrating a clear patient survival benefit for SDCs with TILs ≥ 40%, as morphologically assessed in whole H&E sections according to the established guidelines [31, 32].
Further subtyping of TILs by IHC in cases with TILs ≥ 40% showed a consistently increased CD4:CD8 ratio. Previous studies have shown that under normal conditions, the CD4:CD8 ratio is 2:1 or above and decreases with immunodeficiency of variable etiologies, most notably aging [54]. An increased CD4:CD8 ratio is indicative of a competent immune reaction [55] and is in keeping with the brisk TILs observed in a subset of SDCs. Studies in other tumors have shown that the anti-tumor immune effect is mostly due to the presence of CD8 + T-cells, with CD4 + T-cells having a more regulatory role [56]. On the other hand, morphologically assessed TILs are more representative of the overall anti-tumor immune reaction and have been proven to be a superior prognostic tool to IHC for specific T-cell subsets [57].
Androgen Receptor (AR) Status
All of our 32 cases showed nuclear expression of AR. The underlying molecular mechanism of AR expression is not entirely clear. Mitani et al. identified the presence of an additional copy of chromosome X, where the AR gene is located, in around 40% of SDCs. The resulting increased gene copy dosage may be a good explanation, but is not supported by the accompanying IHC findings, which showed tumors with extra copies of the gene staining negative for AR [58]. Alternative mechanisms of AR expression in SDC may be forkhead box protein A1 (FOXA1) or fatty acid synthase (FASN) gene mutations or amplification, observed in AR-expressing SDCs [9]. Both FOXA1 and FASN have been described as critical mediators in steroid receptor signaling of human cancers, most notably prostate [59, 60]. A few of our cases showed cytoplasmic staining of AR in addition to nuclear, a finding also mentioned by Mitani et al. [58]. Some cases showed a heterogeneous AR staining pattern with scattered non-reactive neoplastic nuclei. We were not able to identify an association of these findings with any other clinicopathologic characteristics and the explanation for these staining patterns remains unclear at this moment.
ERBB2 Copy Numbers and HER2 Immunohistochemistry
About 30% of SDCs express HER2 with underlying gene amplification [1]. ERBB2 gene amplification is also observed in 30% of patients with breast cancer and is associated with a worse overall prognosis [61], but also responsiveness to treatment with trastuzumab, a humanized monoclonal antibody against HER2 protein [62]. In contrast, ERBB2 amplification is not clearly associated with worse outcomes in patients with SDC [1, 7], although there have been some small case series showing adverse prognosis for SDC patients with ERBB2 amplification [63].
Many of the past HER2 IHC studies in SDC have used the breast scoring system for evaluating staining patterns [7, 64], which can be misleading as HER2 is expressed in a variety of heterogeneous carcinomas with markedly different clinical course and treatment [65].
A constant challenge of IHC interpretation is reaching consensus on what constitutes a positive result. Clarity of definition of a positive immunostain is important in reaching interobserver agreement. HER2 scoring has traditionally followed a semi-quantitative system, which takes into consideration both the number of positive cells and the pattern of staining observed [66, 67]. Meyerholz and Beck discuss in detail the several advantages and limitations of developing semi-quantitative scoring systems for IHC in research. Ease of use without special equipment, cost-effectiveness and the ability to identify group related differences are cited as the most important characteristics. The biggest limitation mentioned is observer bias and lack of interobserver agreement that can be reduced with implementing clear definitions and criteria [68].
We proposed a scoring system which can accurately predict the underlying ERBB2 status, as all cases that scored 3+ had underlying amplification, cases that scored 0 had either diploidy or monosomy and those which scored 1+ or 2+ had aneusomy. This can be a useful tool for inferring the underlying ERBB2 status from IHC results and also identifying tumors which are more likely to express PD-L1, as discussed below. Also, our proposed system uses definitions that are conceptually easy to implement in every day practice, as the quantity of immunoreactive cells is stratified into “none” and “less or more than half” and the quality of staining into “none”, “weak” and “strong”. Although this suggested system has not been validated in larger case series, or between different observers we expect interobserver variability to be comparable, if not better than that of the existing scoring systems.
Dogan et al. recently reported significantly increased frequency of ERBB2 amplification in SDCs ex-PA in comparison to pure SDCs [69]. In our case series we also noticed slightly increased prevalence of ERBB2 amplification in the SDC ex-PA group (62.5% versus 56.25%), which was not statistically significant (Chi-square p = 0.719).
Although amplification is the most common genetic aberration of ERBB2 in SDCs, point mutations have also been described and are important because they may provide treatment implications. The mutation, ERBB2 p.S310F reported by Dogan et al. [69] has a known association with responsiveness to trastuzumab in breast cancer patients, even without concurrent ERBB2 amplification [70]. Breast tumors with ERBB2 p.V842I, identified in SDC by Khoo et al. [71], respond to lapatinib [72].
Aneuploidy in SDC is not a new finding. In 1994 Barnes et al. described aneuploidies in 9 of 13 (69%) and in 1995 Grenko et al. in 10 of 12 (83%) patients, but in neither of those two studies did the authors find statistically significant associations with outcomes or other clinicopathologic characteristics [73, 74]. In 2010 Williams et al. detected chromosome 17 polysomy in 15.7% of SDC cases (8 of 51), one of which had concurrent ERBB2 amplification and two of which had HER2 IHC expression. Chromosome 17 polyploidies were not associated with any other clinicopathologic factors or outcomes, in contrast to chromosome 7 polysomy which was associated with EGFR expression and more aggressive clinical course [64]. Genomic instability in cancer can often arise in a background of aneuploidy [75], although a direct causal relationship between the two has been notoriously challenging to establish because aneuploidy is frequently observed in karyotypically stable tumors and also in healthy tissues [76]. Tumors with genomic instability tend to accumulate a higher mutational burden and elicit a stronger anti-tumor inflammatory response, making patients more likely to benefit from treatment with checkpoint inhibitors [77]. PD-L1 IHC is an established tool to screen eligible patients in a variety of tumors [78], with a number of recent publications exploring the role of PD-L1 expression in SDCs.
PD-L1 Status
Sato et al. described expression of PD-L1 in the tumor cells of 50% (9 of 18) of SDCs and also identified unfavorable prognosis [79]. Mikaigawa et al. also mention worse prognosis in 22.8% (50 of 219) of patients with PD-L1 expressing SDCs [22]. These prognostic findings were not validated in our study, nor by the cohorts of Hamza et al. (frequency of PD-L1 reactivity 26% of 113 cases) [24] or by the most recent study by Xu et al. In fact, Xu et al. reported conflicting results depending on the scoring system, with adverse outcomes for patients with higher than 25% of PD-L1 staining tumor cells, but better outcomes for those with a CPS ≥ 1 [80]. This finding highlights the need for standardization in PD-L1 interpretation in SDC, as IHC evaluation may be affected by pre-analytical factors (fixation, tissue handling) and variations in expression due to treatment effect or tumor heterogeneity [81].
Limitations
Our study included a small number of patients (N = 32) and uncensored events (N = 25), which makes it unsuitable for unbiased multivariate analysis, as the most accepted recommendation for a multivariate model is to include one variable per ten uncensored events [82]. SDC is a rare tumor and availability of surgical pathology material and comprehensive clinical information can be challenging. Most of the published case series included a comparable number of cases with ours, with the exceptions of the big epidemiological studies by Osborn et al. (National Cancer Database) [4] and Jayaprakash et al. (Surveillance, Epidemiology and End Results database) [3] as well as the clinicopathologic studies by Boon et al. from the Netherlands [39] and Hamza from MD Anderson Cancer Center [24]. Validation and further analysis of our findings in a larger series, comparable to the latter two is necessary.
PD-L1 IHC can be performed with many different commercially available antibodies. Stains can be hard to interpret because of the variable staining patterns seen with different clones and the multiple cell types present [83]. We used clone 22C3 (Dako North America Inc., Carpinteria, CA, USA) which is most suitable for predicting response to pembrolizumab [84]. A definitive study assessing the interchangeable use of all available antibodies is currently missing, probably with the exception of Hamza et al. who used both clones 22C3 and 28–8 (Dako North America Inc. Carpinteria, CA, USA) and identified higher frequency of positivity with clone 28–8 in SDC [24]. The experience from treating patients with non-small cell lung carcinoma has shown that different clones are suitable for predicting response to different checkpoint inhibitors [83, 84]. It would be an interesting future study to compare staining patterns of different PD-L1 clones in SDCs.
Apart from PD-L1 IHC, other tests may be more suitable for detection of clinically significant associations and determination of eligibility for receiving checkpoint inhibitors among patients with SDC. Multiple recent studies on salivary gland malignancies have been focusing on PD-L2, also a ligand of PD-1. Chang et al. found PD-L2 expression to be associated with poor prognosis in a series of 70 salivary gland malignancies, 15.7% of which were SDCs [53]. These findings were validated by Nakano et al. who reported poor prognosis in patients with malignant salivary gland tumors co-expressing PD-L1 and PD-L2, with all SDCs, mucoepidermoid carcinomas or carcinomas ex-PA with PD-L2 expression developing distant metastases [23].
Conclusions
In summary, the major findings and conclusions from our study of 32 patients with SDCs are the following:
-
TILs ≥ 40% are associated with better overall prognosis.
-
A high CD4:CD8 T-cell ratio is observed in SDCs with TILs ≥ 40%.
-
We proposed a HER2 IHC Salivary Gland scoring system accurately predicts ERBB2 aneusomy or amplification.
-
Increased ERBB2 gene copy numbers, as detected by FISH and inferred from HER2 IHC, are associated with higher TIL densities and PD-L1 expression.
-
HER2 IHC alone can triage further testing for PD-L1, as tumors with HER2 IHC of 1+, 2+ or 3+ are more likely to co-express PD-L1.
Abbreviations
- SDC:
-
Salivary duct carcinoma
- AR:
-
Androgen receptor
- FISH:
-
Fluorescent in situ hybridization
- IHC:
-
Immunohistochemistry
- PD-L1:
-
Programmed death-ligand 1
- PD-1:
-
Programmed death 1
- TILs:
-
Tumor infiltrating lymphocytes
- FFPE:
-
Formalin fixed paraffin embedded
- H&E:
-
Hematoxylin and eosin
- CAP:
-
College of American Pathologists
- CPS:
-
Combined positive score
- ASCO:
-
American Society of Clinical Oncology
- PA:
-
Pleomorphic adenoma
References
Gilbert MR, Sharma A, Schmitt NC, et al. A 20-year review of 75 cases of salivary duct carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142:489–95.
Kleinsasser O, Klein HJ. Hubner G [Salivary duct carcinoma. A group of salivary gland tumors analogous to mammary duct carcinoma]. Arch Klin Exp Ohren Nasen Kehlkopfheilkd. 1968;192:100–5.
Jayaprakash V, Merzianu M, Warren GW, et al. Survival rates and prognostic factors for infiltrating salivary duct carcinoma: analysis of 228 cases from the surveillance, epidemiology, and end results database. Head Neck. 2014;36:694–701.
Osborn V, Givi B, Lee A, et al. Characterization, treatment and outcomes of salivary ductal carcinoma using the National Cancer Database. Oral Oncol. 2017;71:41–6.
Dalin MG, Watson PA, Ho AL, et al. androgen receptor signaling in salivary gland cancer. Cancers (Basel). 2017;9:17.
Yeoh CC, Dabab N, Rigby E, et al. Androgen receptor in salivary gland carcinoma: A review of an old marker as a possible new target. J Oral Pathol Med. 2018;47:691–5.
Skalova A, Starek I, Vanecek T, et al. Expression of HER-2/neu gene and protein in salivary duct carcinomas of parotid gland as revealed by fluorescence in-situ hybridization and immunohistochemistry. Histopathology. 2003;42:348–56.
Guo W, Wang W, Zhu Y, et al. HER2 status in molecular apocrine breast cancer: associations with clinical, pathological, and molecular features. Int J Clin Exp Pathol. 2015;8:8008–177.
Dalin MG, Desrichard A, Katabi N, et al. Comprehensive molecular characterization of salivary duct carcinoma reveals actionable targets and similarity to apocrine breast cancer. Clin Cancer Res. 2016;22:4623–33.
Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29:e473–e476476.
Boon E, van Boxtel W, Buter J, et al. Androgen deprivation therapy for androgen receptor-positive advanced salivary duct carcinoma: a nationwide case series of 35 patients in The Netherlands. Head Neck. 2018;40:605–13.
Takahashi H, Tada Y, Saotome T, et al. Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2-positive salivary duct carcinoma. J Clin Oncol. 2019;37:125–34.
Li BT, Shen R, Offin M, et al. Ado-trastuzumab emtansine in patients with HER2 amplified salivary gland cancers (SGCs): results from a phase II basket trial. J Clin Oncol. 2019;37:6001.
Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline focused update. Arch Pathol Lab Med. 2018;142:1364–82.
Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805.
Bartley AN, Washington MK, Ventura CB, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. Arch Pathol Lab Med. 2016;140:1345–63.
Glisson B, Colevas AD, Haddad R, et al. HER2 expression in salivary gland carcinomas: dependence on histological subtype. Clin Cancer Res. 2004;10:944–6.
Cornolti G, Ungari M, Morassi ML, et al. Amplification and overexpression of her2/neu gene and HER2/neu protein in salivary duct carcinoma of the parotid gland. Arch Otolaryngol Head Neck Surg. 2007;133:1031–6.
Webb ES, Liu P, Baleeiro R, et al. Immune checkpoint inhibitors in cancer therapy. J Biomed Res. 2018;32:317–26.
Dong H, Zhu G, Tamada K, et al. B7–H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9.
Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375:1767–78.
Mukaigawa T, Hayashi R, Hashimoto K, et al. Programmed death ligand-1 expression is associated with poor disease free survival in salivary gland carcinomas. J Surg Oncol. 2016;114:36–433.
Nakano T, Takizawa K, Uezato A, et al. Prognostic value of programed death ligand-1 and ligand-2 co-expression in salivary gland carcinomas. Oral Oncol. 2019;90:30–7.
Hamza A, Roberts D, Su S, et al. PD-L1 expression by immunohistochemistry in salivary duct carcinoma. Ann Diagn Pathol. 2019;40:49–52.
Cohen RB, Delord JP, Doi T, et al. Pembrolizumab for the treatment of advanced salivary gland carcinoma: findings of THE Phase 1b KEYNOTE-028 study. Am J Clin Oncol. 2018. https://doi.org/10.1097/COC.0000000000000429.
Vassilakopoulou M, Avgeris M, Velcheti V, et al. Evaluation of PD-L1 expression and associated tumor-infiltrating lymphocytes in laryngeal squamous cell carcinoma. Clin Cancer Res. 2016;22:704–13.
Kim A, Lee SJ, Kim YK, et al. Programmed death-ligand 1 (PD-L1) expression in tumour cell and tumour infiltrating lymphocytes of HER2-positive breast cancer and its prognostic value. Sci Rep. 2017;7:11671.
Naito Y, Saito K, Shiiba K, et al. CD8+ T Cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–4.
Kotoula V, Chatzopoulos K, Lakis S, et al. Tumors with high-density tumor infiltrating lymphocytes constitute a favorable entity in breast cancer: a pooled analysis of four prospective adjuvant trials. Oncotarget. 2016;7:5074–87.
Fu Q, Chen N, Ge C, et al. Prognostic value of tumor-infiltrating lymphocytes in melanoma: a systematic review and meta-analysis. Oncoimmunology. 2019;8:1593806.
Hendry S, Salgado R, Gevaert T, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for a standardized method from the international immunooncology biomarkers working group: Part 1: Assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv Anat Pathol. 2017;24:235–51.
Hendry S, Salgado R, Gevaert T, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immunooncology biomarkers working group: Part 2: TILs in melanoma, gastrointestinal tract carcinomas, non–small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24:311–35.
Dieci MV, Radosevic-Robin N, Fineberg S, et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol. 2018;52:16–25.
Kulangara K, Zhang N, Corigliano E, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143:330–7.
Kulangara K, Hanks DA, Waldroup S, et al. Development of the combined positive score (CPS) for the evaluation of PD-L1 in solid tumors with the immunohistochemistry assay PD-L1 IHC 22C3 pharmDx. J Clin Oncol. 2017;35:e14589–e1458914589.
Huang Y, Ma C, Zhang Q, et al. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget. 2015;6:17462–78.
Wang S, Hossein Saboorian M, Frenkel EP, et al. Aneusomy 17 in breast cancer: its role in HER-2/neu protein expression and implication for clinical assessment of HER-2/neu status. Mod Pathol. 2002;15:137–45.
Stodulski D, Mikaszewski B, Majewska H, et al. Parotid salivary duct carcinoma: a single institution's 20-year experience. Eur Arch Otorhinolaryngol. 2019;276:2031–8.
Boon E, Bel M, van Boxtel W, et al. A clinicopathological study and prognostic factor analysis of 177 salivary duct carcinoma patients from The Netherlands. Int J Cancer. 2018;143:758–66.
Johnston ML, Huang SH, Waldron JN, et al. Salivary duct carcinoma: Treatment, outcomes, and patterns of failure. Head Neck. 2016;38(Suppl 1):E820–E826826.
Martins C, Fonseca I, Roque L, et al. PLAG1 gene alterations in salivary gland pleomorphic adenoma and carcinoma ex-pleomorphic adenoma: a combined study using chromosome banding, in situ hybridization and immunocytochemistry. Mod Pathol. 2005;18:1048–55.
Bahrami A, Dalton JD, Shivakumar B, et al. PLAG1 alteration in carcinoma ex pleomorphic adenoma: immunohistochemical and fluorescence in situ hybridization studies of 22 cases. Head Neck Pathol. 2012;6:328–35.
Hiraoka K, Miyamoto M, Cho Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–80.
Ye S-L, Li X-Y, Zhao K, et al. High expression of CD8 predicts favorable prognosis in patients with lung adenocarcinoma: A cohort study. Medicine (Baltimore). 2017;96:e6472.
Kwak Y, Koh J, Kim D-W, et al. Immunoscore encompassing CD3+ and CD8+ T cell densities in distant metastasis is a robust prognostic marker for advanced colorectal cancer. Oncotarget. 2016;7:81778–90.
Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–43.
Kondratiev S, Sabo E, Yakirevich E, et al. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10:4450–6.
Lee HE, Chae SW, Lee YJ, et al. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99:1704–11.
Starska K, Glowacka E, Kulig A, et al. Prognostic value of the immunological phenomena and relationship with clinicopathological characteristics of the tumor–the expression of the early CD69+, CD71+ and the late CD25+, CD26+, HLA/DR+ activation markers on T CD4+ and CD8+ lymphocytes in squamous cell laryngeal carcinoma. Part II. Folia Histochem Cytobiol. 2011;49:593–603.
Karpathiou G, Casteillo F, Giroult JB, et al. Prognostic impact of immune microenvironment in laryngeal and pharyngeal squamous cell carcinoma: Immune cell subtypes, immuno-suppressive pathways and clinicopathologic characteristics. Oncotarget. 2017;8:19310–22.
Chen W-Y, Wu C-T, Wang C-W, et al. Prognostic significance of tumor-infiltrating lymphocytes in patients with operable tongue cancer. Radiat Oncol (London, England). 2018;13:157.
Karja VJ, Syrjanen KJ, Syrjanen SM. Immunocompetent cells in benign and malignant salivary gland tumors. Gen Diagn Pathol. 1996;142:75–81.
Chang H, Kim JS, Choi YJ, et al. Overexpression of PD-L2 is associated with shorter relapse-free survival in patients with malignant salivary gland tumors. Onco Targets Ther. 2017;10:2983–92.
Wikby A, Månsson IA, Johansson B, et al. The immune risk profile is associated with age and gender: findings from three Swedish population studies of individuals 20–100 years of age. Biogerontology. 2008;9:299–308.
Amadori A, Zamarchi R, De Silvestro G, et al. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med. 1995;1:1279–83.
Van der Leun AM, Thommen DS, Schumacher TN. CD8+ T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer. 2020;20:218–32.
Chatzopoulos K, Kotoula V, Manoussou K, et al. Tumor infiltrating lymphocytes and CD8+ T cell subsets as prognostic markers in patients with surgically treated laryngeal squamous cell carcinoma. Head Neck Pathol. 2019. https://doi.org/10.1007/s12105-019--01101-6.
Mitani Y, Rao PH, Maity SN, et al. Alterations associated with androgen receptor gene activation in salivary duct carcinoma of both sexes: potential therapeutic ramifications. Clin Cancer Res. 2014;20:6570–81.
Augello MA, Hickey TE, Knudsen KE. FOXA1: master of steroid receptor function in cancer. EMBO J. 2011;30:3885–944.
Wen S, Niu Y, Lee SO, et al. Targeting fatty acid synthase with ASC-J9 suppresses proliferation and invasion of prostate cancer cells. Mol Carcinog. 2016;55:2278–90.
Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82.
Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83.
Schmitt NC, Kang H, Sharma A. Salivary duct carcinoma: An aggressive salivary gland malignancy with opportunities for targeted therapy. Oral Oncol. 2017;74:40–8.
Williams MD, Roberts DB, Kies MS, et al. Genetic and expression analysis of HER-2 and EGFR genes in salivary duct carcinoma: empirical and therapeutic significance. Clin Cancer Res. 2010;16:2266–74.
Yan M, Schwaederle M, Arguello D, et al. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev. 2015;34:157–64.
Leong AS. Quantitation in immunohistology: fact or fiction? A discussion of variables that influence results. Appl Immunohistochem Mol Morphol. 2004;12:1–7.
Seidal T, Balaton AJ, Battifora H. Interpretation and quantification of immunostains. Am J Surg Pathol. 2001;25:1204–7.
Meyerholz DK, Beck AP. Principles and approaches for reproducible scoring of tissue stains in research. Lab Invest. 2018;98:844–55.
Dogan S, Ng CKY, Xu B, et al. The repertoire of genetic alterations in salivary duct carcinoma including a novel HNRNPH3-ALK rearrangement. Hum Pathol. 2019;88:66–77.
Chumsri S, Weidler J, Ali S, et al. Prolonged response to trastuzumab in a patient with HER2-nonamplified breast cancer with elevated HER2 dimerization harboring an ERBB2 S310F mutation. J Natl Compr Canc Netw. 2015;13:1066–70.
Khoo TK, Yu B, Smith JA, et al. Somatic mutations in salivary duct carcinoma and potential therapeutic targets. Oncotarget. 2017;8:75893–903.
Bose R, Kavuri SM, Searleman AC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3:224–37.
Barnes L, Rao U, Krause J, et al. Salivary duct carcinoma. Part I. A clinicopathologic evaluation and DNA image analysis of 13 cases with review of the literature. Oral Surg Oral Med Oral Pathol. 1994;78:64–73.
Grenko RT, Gemryd P, Tytor M, et al. Salivary duct carcinoma. Histopathology. 1995;26:261–6.
Duesberg P, Rausch C, Rasnick D, et al. Genetic instability of cancer cells is proportional to their degree of aneuploidy. Proc Natl Acad Sci USA. 1998;95:13692–7.
Zasadil LM, Britigan EMC, Weaver BA. 2n or not 2n: aneuploidy, polyploidy and chromosomal instability in primary and tumor cells. Semin Cell Dev Biol. 2013;24:370–9.
Yaghmour G, Pandey M, Ireland C, et al. Role of genomic instability in immunotherapy with checkpoint inhibitors. Anticancer Res. 2016;36:4033–8.
Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–e551551.
Sato F, Akiba J, Kawahara A, et al. The expression of programed death ligand-1 could be related with unfavorable prognosis in salivary duct carcinoma. J Oral Pathol Med. 2018;47:683–90.
Xu B, Jungbluth AA, Frosina D, et al. Immune microenvironment and expression of PD-L1, PD-1, cancer testis antigen PRAME and MHC I in salivary duct carcinoma. Histopathology. 2019;75:672–82.
Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87.
Bradburn MJ, Clark TG, Love SB, et al. Survival analysis Part III: multivariate data analysis—choosing a model and assessing its adequacy and fit. Br J Cancer. 2003;89:605–11.
Troncone G, Gridelli C. The reproducibility of PD-L1 scoring in lung cancer: can the pathologists do better? Transl Lung Cancer Res. 2017;6:S74–S7777.
Scheel AH, Schäfer SC. Current PD-L1 immunohistochemistry for non-small cell lung cancer. J Thorac Dis. 2018;10:1217–9.
Acknowledgements
The authors thank Mohamed A. Ibrahim with the Mayo Clinic DLMP Research and Innovation Office for the administrative support.
Funding
Institutional Research Funds from the Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN. The funders had no role in designing the study, collecting and analyzing the data, drafting the manuscript or making the decision to publish.
Author information
Authors and Affiliations
Contributions
KC reviewed the histologic material, interpreted immunohistochemical stains, performed the statistical analysis and wrote the manuscript. AC participated in data interpretation and drafted portions of the manuscript. SS participated in data interpretation and critically edited the manuscript. MK collected the histologic material, reviewed immunohistochemical stains and interpreted the data. DV participated in data interpretation and critically edited the manuscript. MR interpreted the data, critically revised and edited the manuscript. DS-W assisted in data interpretation, critically revised and edited the manuscript. JL assisted in data interpretation, critically revised and edited the manuscript. PG interpreted cytogenetics data. WS interpreted cytogenetics data and guided their incorporation into the study. AC collected clinical data, critically revised and edited the manuscript. KP collected clinical data. JG designed the study, collected histologic material, interpreted immunohistochemical stains and cytogenetics data, critically reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest relevant to the present study to disclose.
Ethical Approval
The study was approved by the Mayo Clinic Institutional Review Board (Application Number 12-001311; Last approval date: 2/28/2017).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chatzopoulos, K., Collins, A.R., Sotiriou, S. et al. Increased ERBB2 Gene Copy Numbers Reveal a Subset of Salivary Duct Carcinomas with High Densities of Tumor Infiltrating Lymphocytes and PD-L1 Expression. Head and Neck Pathol 14, 951–965 (2020). https://doi.org/10.1007/s12105-020-01163-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-020-01163-x