Abstract
Salivary duct carcinoma (SDC) is an aggressive neoplasm that resembles high-grade invasive ductal carcinoma of the breast. It can develop de novo or from the malignant transformation of pleomorphic adenoma (PA). We performed immunohistochemical stains for phosphatase and tensin homologue [PTEN androgen receptor (AR)], HER2/neu, cytokeratin 5/6, estrogen receptor-beta, high-mobility group AT-hook 2 (HMGA2), and pleomorphic adenoma gene 1 (PLAG1) on tissue microarray samples of 75 SDCs and 31 adenocarcinomas, not otherwise specified (NOS). Our data showed the following in SDC samples: loss of PTEN was found in 17 of 60 (28.3%); AR was expressed in 43 of 62 (69.4%); HER2/neu was overexpressed in 25 of 58 (43.1%); cytokeratin 5/6 was expressed in 14 of 54 (25.9%); estrogen receptor-beta was expressed in 37 of 56 (66.1%); HMGA2 was expressed in 29 of 63 (46.0%); and PLAG1 was expressed in 0 of 62 (0%). In addition, there was no statistically significant difference in the age at onset between patients with HMGA2-positive SDCs (range 32–85 years; mean: 64.3 years; median: 64.5 years) and those with HMGA2-negative SDCs (range 41–79 years; mean: 62.5 years; median: 64.5 years). There was also no statistically significant difference in overall survival between patients with HMGA2-positive and HMGA2-negative SDCs (follow-up period range 3–201 months; mean: 49.8 months; median: 30 months). Among 10 patients with a definite PA component (SDC ex-PA), 6 were positive and 4 were negative for HMGA2. Our data were consistent with previous findings that AR and estrogen receptor-beta are expressed in most SDCs, whereas HER2/neu overexpression and loss of PTEN are expressed in a subset of SDCs. In our cohort of patients, HMGA2 was expressed in approximately half of SDCs. HMGA2 and PTEN are promising therapeutic targets for salivary gland tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salivary duct carcinoma (SDC) is an aggressive salivary gland neoplasm that resembles high-grade invasive ductal carcinoma of the breast. SDCs often occur in elderly patients and are frequently associated with soft tissue extension, perineural and lymphovascular invasions, a high rate of local recurrence, and distant metastasis. SDCs show a variety of growth patterns, including cribriform (Roman-bridge architecture), papillary, solid, tubular, trabecular, and single cells, typically with central necrosis. Apocrine morphology with eosinophilic cytoplasm and apical snouts is often seen in SDCs. Sarcomatoid, mucin-rich, micropapillary, and oncocytic variants have also been described [1,2,3], but are rare and do not seem to be found in “pure” form, intimately admixed with conventional areas. The diagnosis of the basal-like variant of SDC remains challenging, and the difficulties of identifying a basal-like phenotype in SDC have been previously recognized [3, 4]. SDCs often express breast markers, such as gross cystic disease fluid protein-15 (GCDFP-15), mammaglobin, and GATA3; however, SDCs show a hormone receptor expression pattern different from typical invasive duct carcinoma of the breast and are usually negative for estrogen receptor α and progesterone receptor [2].
Making an accurate diagnosis of SDC remains a challenge. We have retrospectively reviewed outside referral/consultation cases that were diagnosed as SDCs in our institution (these data have not been published yet) and found that many SDCs were diagnosed descriptively as poorly differentiated carcinoma or carcinoma with oncocytic features, whereas some cases were misdiagnosed as mucoepidermoid carcinoma, acinic cell carcinoma, or non-keratinizing squamous cell carcinoma. The presence of intercellular bridge and/or focal keratinizing should lead to the correct diagnosis of metastatic squamous cell carcinoma. Moreover, metastatic melanoma, breast, or prostate cancers are also in the differential diagnosis of SDCs.
SDCs can develop de novo or from malignant transformation of pleomorphic adenoma (PA). Recently, high-mobility group AT-hook 2 (HMGA2) and pleomorphic adenoma gene 1 (PLAG1) abnormalities have been identified in PA and carcinoma-ex-PA [5]. HMGA2 is a DNA binding protein that binds with the minor groove of AT-rich DNA sequences. PLAG1 is a zinc finger protein. HMGA2 is an upstream regulator of PLAG1. However, few large-scale studies have examined the expression of PLAG1 and HMGA2 in salivary duct carcinomas [6, 7].
Current therapy for SDCs includes surgery with or without adjuvant radiation. Systemic chemotherapy is mainly for palliative care. Most patients die of the disease within 3 years despite conventional therapy [7, 8]. Therefore, SDCs warrant new therapeutic strategies.
The aim of this study was to examine the expression of phosphatase and tensin homologue (PTEN), androgen receptor (AR), epidermal growth factor receptor 2 (ERBB2, also known as HER2/neu), cytokeratin 5/6 (CK5/6), estrogen receptor-beta (ER-beta), HMGA2, and PLAG1 on tissue microarray samples of SDCs and adenocarcinomas, not otherwise specified (NOS). Our study included a panel of both diagnostic markers and biologic markers that may guide future targeted therapies.
Materials and Methods
Tissue Microarray and Sample Selection
Patients’ tissue blocks were obtained from archived material at The University of Texas MD Anderson Cancer Center. Each tissue microarray (TMA) was created by using two 1.0-mm diameter cores taken from each case and was used for immunohistochemical analyses. TMAs included samples of 75 SDCs and 31 adenocarcinomas NOS, collected during 1998–2014; these were major salivary gland malignancies and treatment naive.
In brief, SDC showed variable growth patterns with comedonecrosis present; along with prominent nucleoli and prominent apocrine features. For basaloid SDC (basal-like phenotype) comedonecrosis was present, while other mimickers were excluded (high-grade adenoid cystic carcinoma, solid or with high-grade transformation). The adenocarcinoma NOS constituted a mix of dominant intermediate- and a few high-grade tumors, which lacked comedonecrosis, had none/ focal apocrine features, and at that time did not fit any subtype (“conventional” secretory carcinoma with ETV6/NTRK3, cystadenocarcinoma).
Immunohistochemical Analyses
Immunohistochemistry was performed on 4-µm-thick unstained slides, using the avidin-biotin-peroxidase complex technique, and commercially available antibodies against PTEN (Vector; Burlingame, CA, USA; 1:50), AR (Dako, Carpinteria, CA,USA; 1:100), Her2/neu and CK5/6 (Ventana System, Tucson, AZ, USA; prediluted), ER-beta (Cell Signaling, MA, USA; 1:100), HMGA2 (Novus Bio, USA; 1:50), PLAG1 (NovusBio, USA; 1:200), on a Ventana XT instrument (Ventana System). Semiquantitative analysis was performed for CK5/6, HMGA2, PLAG1 using scores based on the number of cells that stained positively and the staining intensity: 0, negative staining (up to 10% of cells stained and visible at 40×); 1, positive staining (more than 10% of cells stained and easily detectable t 10×). PTEN was accounted as complete loss of expression (lack of staining) and retained expression (homogeneous or heterogeneous staining pattern). Her/2neu scoring followed the ASCO/CAP guidelines: negative (score 0, 1 +, with < 10%, incomplete/fainth membrane staining), weakly positive/ equivocal (score 2 +, with circumferential membrane staining that is incomplete or weak in > 10% of cells, or complete strong membranous staining in < 10% of cells), positive (score 3 +, with homogeneous dark “chicken-wire” staining in10% of carcinoma cells); only cases with score 3 + were considered. ER-beta was done in a similar fashion to the ER—alpha (negative < 1%; positive > 1%). Quantification of AR staining was performed using the Allred system (Allred total score, ranging from 0 to 8: determined by adding an intensity score of 0, 1, 2, 3 corresponding to negative, weak, moderate, strong staining respectively to a proportion score of 0, 1, 2, 3, 4, 5 corresponding to negative, < 1%, > 1–10%, > 10–33%, > 33–66%, > 66% of tumor cells with positive staining); 6 was the cut-off for total score.
Statistical Analysis
A Fisher exact test was used to compare the two groups of patients (SDC vs. adenocarcinoma, NOS). A P value of < 0.05 was considered statistically significant.
Results
Expression of PTEN, AR, HER2/neu, CK5/6, ER-beta, HMGA2, and PLAG1 in SDCs and Adenocarcinoma, NOS
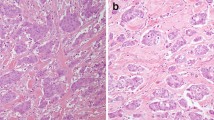
Our data showed that AR was expressed in 43 of 62 of SDCs (69.4%) and in 8 of 25 (32.0%) adenocarcinomas, NOS (Table 1). The difference was statistically significant (P = 0.0018). ER-beta was expressed in 37 of 56 SDCs (66.1%) and in 7 of 25 adenocarcinomas, NOS (28.0%). The difference was statistically significant (P = 0.0018). Therefore, both AR and ER-beta are useful diagnostic markers for SDCs (Fig. 1).
Loss of PTEN was found in 17 of 60 SDCs (28.3%) and in 14 of 27 adenocarcinomas, NOS (51.9%) (P = 0.0521). HER2/neu was overexpressed in 25 of 58 SDCs (43.1%) and in 6 of 28 adenocarcinomas, NOS (21.4%) (P = 0.0584). Therefore, both PTEN and HER2/neu are biologic markers that can guide targeted therapy.
CK5/6 was expressed in 14 of 54 SDCs (25.9%) and in 5 of 21 adenocarcinomas, NOS (23.8%) (P = 1.0000). HMGA2 was expressed in 29 of 63 SDCs (46.0%) and in 11 of 23 adenocarcinomas, NOS (47.8%) (P = 1.0000). PLAG1 was expressed in 0 of 62 SDCs (0%) and in 1 of 24 adenocarcinomas, NOS (4.2%) (P = 0.2791).
HMGA2 Expression in SDCs Not Associated with Patients’ Age at Onset or Overall Survival
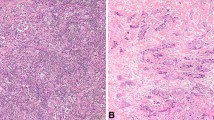
We further studied the expression of HMGA2 in patients with SDCs. There was no statistically significant difference in the age at onset between patients with HMGA2-positive SDCs (range 32–85 years; mean: 64.3 years; median: 64.5 years) and those with HMGA2-negative SDCs (range 41–79 years; mean: 62.5 years; median: 64.5 years). Furthermore, there was no statistically significant difference in overall survival between patients with HMGA2-positive and HMGA2-negative SDCs (follow-up period range 3–201 months; mean: 49.8 months; median: 30 months). Among 10 patients with a definite PA component (SDC ex-PA), 6 were positive and 4 were negative for HMGA2 (Fig. 2).
Discussion
Consistent with results from previous studies [4, 8,9,10,11,12,13,14,15,16], our results showed that AR was expressed in 69.4% of SDCs, ER-beta was expressed in 66.1% of SDCs, whereas overexpression of HER2/neu was found in 43.1% of SDCs. AR and ER-beta are useful diagnostic markers for SDCs. ER-beta is the predominantly ER expressed form in salivary gland tumors [17] and we previously shown that lack of ER-beta expression correlated with increased local and regional recurrence, indicating that ER-beta down-regulation is associated with adverse clinical features in SDCs [16]. Androgen deprivation therapy is a potential therapy modality for AR-positive SDCs [18, 19]. HER2/neu is a useful biologic marker for guiding targeted therapy against HER2 (i.e., trastuzumab and lapatinib) [20,21,22].
PTEN is a tumor suppressor gene located on chromosome 10q23 [23]. PTEN suppresses the phosphoinositide 3-kinase (PI3K) pathway, which is often activated in SDCs. We found loss of PTEN expression in a subset of both SDCs (28.3%) and adenocarcinoma, NOS (51.9%). Previously, Ettl et al. [24] found homozygous deletion of PTEN in 29% of SDCs (7/24) by fluorescent in situ hybridization (FISH), hemizygous deletion in 38% of SDCs (9/24) by FISH, and loss of PTEN expression in 42% of SDCs (10/24) by IHC. Griffith et al. found loss of PTEN in 50% of SDCs (8/16) by FISH [25]. These findings suggest that the PTEN inhibitor is a promising therapy modality in SDCs.
PLAG1 gene rearrangement has been found in more than half of PAs, and the fusion partners include CTNNB1, FGFR1, LIFR, CHCHD7, and TCEA1 [26]. SDC is the most commonly identified malignant component in carcinoma ex-PAs. However, only one case of adenocarcinoma, NOS in our series was positive for PLAG1. There are several possibilities for this finding: (1) the antibody may not have worked as expected; (2) PLAG1 immunohistochemistry may not have correlated with PLAG1 rearrangement; or (3) PLAG1 expression may have been lost after malignant transformation of PA. Developing an anti-PLAG1 antibody with better performance or using alternative methods, such as FISH or molecular testing, is necessary to further study the expression of PLAG1 in SDCs.
The second most common gene rearrangement in PAs is HMGA2, and the partner genes include NFIB, FHIT, and WIF1 [26]. Previously, Mito et al. showed that HMGA2 is a specific but not sensitive marker for PA and carcinoma ex-PA [5]. Our data showed that HMGA2 was expressed in approximately half of SDCs and adenocarcinoma, NOS. However, only 6 of 10 SDCs with definite a PA component (SDC ex-PA) expressed HMGA2. Therefore, HMGA2 negativity cannot exclude the possibility of malignant transformation of PA. In contrast, HMGA2 expression was seen in SDCs with no obvious PA component. This may be due to either a sampling issue or because SDCs completely replaced the benign component. We speculate that many previously considered de novo SDCs may actually be SDCs ex-PA. Similar concept was postulated by Chiosea et al. [7]. The overall prevalence of salivary duct carcinoma ex-pleomorphic adenoma in this series was 79% (52/66) and was is higher than previously reported by Williams et al. and Bahrani et al. [3, 6]. Moreover, our data showed that expression of HMGA2 was not associated with patients’ age at onset or overall survival. Our findings did not support HMGA2 immunohistochemistry alone as a useful diagnostic marker for differentiating SDCs ex-PA from SDC de novo and adenocarcinoma NOS. However, HMGA2 may be a potential novel therapeutic target in SDCs. For example, microRNA let-7 has been shown to suppress HMGA2 in cell culture [27].
Our findings show that immunohistochemical evaluations of SDC and salivary adenocarcinomas, NOS have overlapping characteristics with no single marker being entirely specific. It is debatable whether some of the adenocarcinomas, NOS that expressed androgen receptor should be reclassified as salivary duct carcinoma, since morphologic features remain the key to diagnosis of salivary gland tumor.
In conclusion, our data support previous findings that AR and ER-beta are expressed in most SDCs, whereas HER2/neu and PTEN are actionable therapeutic targets in SDCs. HMGA2 is expressed in approximately half of SDCs, and HMGA2 inhibitor is a potential novel therapeutic modality in salivary gland tumors. In addition, future studies may be performed to stratify SDCs based on biomarkers, such as hormone receptor and HER2 status.
References
Schmitt NC, Kang H, Sharma A. Salivary duct carcinoma: an aggressive salivary gland malignancy with opportunities for targeted therapy. Oral Oncol. 2017;74:40–8.
Simpson RH. Salivary duct carcinoma: new developments—morphological variants including pure in situ high grade lesions; proposed molecular classification. Head Neck Pathol. 2013;7(Suppl 1):48–58.
Williams L, Thompson LD, Seethala RR, et al. Salivary duct carcinoma: the predominance of apocrine morphology, prevalence of histologic variants, and androgen receptor expression. Am J Surg Pathol. 2015;39(5):705–13.
Di Palma S, Simpson RH, Marchio C, et al. Salivary duct carcinomas can be classified into luminal androgen receptor-positive, HER2 and basal-like phenotypes. Histopathology 2012;61(4):629–43.
Mito JK, Jo VY, Chiosea SI, Dal Cin P, Krane JF. HMGA2 is a specific immunohistochemical marker for pleomorphic adenoma and carcinoma ex-pleomorphic adenoma. Histopathology 2017;71(4):511–21.
Bahrami A, Perez-Ordonez B, Dalton JD, Weinreb I. An analysis of PLAG1 and HMGA2 rearrangements in salivary duct carcinoma and examination of the role of precursor lesions. Histopathology 2013;63(2):250–62.
Chiosea SI, Thompson LD, Weinreb I, et al. Subsets of salivary duct carcinoma defined by morphologic evidence of pleomorphic adenoma, PLAG1 or HMGA2 rearrangements, and common genetic alterations. Cancer 2016;122(20):3136–44.
Williams MD, Roberts DB, Kies MS, Mao L, Weber RS, El-Naggar AK. Genetic and expression analysis of HER-2 and EGFR genes in salivary duct carcinoma: empirical and therapeutic significance. Clin Cancer Res 2010;16(8):2266–74.
Felix A, El-Naggar AK, Press MF, et al. Prognostic significance of biomarkers (c-erbB-2, p53, proliferating cell nuclear antigen, and DNA content) in salivary duct carcinoma. Hum Pathol. 1996;27(6):561–6.
Glisson B, Colevas AD, Haddad R, et al. HER2 expression in salivary gland carcinomas: dependence on histological subtype. Clin Cancer Res. 2004;10(3):944–6.
Masubuchi T, Tada Y, Maruya S, et al. Clinicopathological significance of androgen receptor, HER2, Ki-67 and EGFR expressions in salivary duct carcinoma. Int J Clin Oncol 2015;20(1):35–44.
Mitani Y, Rao PH, Maity SN, et al. Alterations associated with androgen receptor gene activation in salivary duct carcinoma of both sexes: potential therapeutic ramifications. Clin Cancer Res. 2014;20(24):6570–81.
Nardi V, Sadow PM, Juric D, et al. Detection of novel actionable genetic changes in salivary duct carcinoma helps direct patient treatment. Clin Cancer Res. 2013;19(2):480–90.
Skalova A, Starek I, Vanecek T, et al. Expression of HER-2/neu gene and protein in salivary duct carcinomas of parotid gland as revealed by fluorescence in-situ hybridization and immunohistochemistry. Histopathology 2003;42(4):348–56.
Vadlamudi RK, Balasenthil S, Sahin AA, et al. Novel estrogen receptor coactivator PELP1/MNAR gene and ERbeta expression in salivary duct adenocarcinoma: potential therapeutic targets. Hum Pathol. 2005;36(6):670–5.
Williams MD, Roberts D, Blumenschein GR Jr, et al. Differential expression of hormonal and growth factor receptors in salivary duct carcinomas: biologic significance and potential role in therapeutic stratification of patients. Am J Surg Pathol. 2007;31(11):1645–52.
Ohshiro K, Rayala SK, Williams MD, Kumar R, El-Naggar AK. Biological role of estrogen receptor beta in salivary gland adenocarcinoma cells. Clin Cancer Res. 2006;12(20 Pt 1):5994–9.
Boon E, van Boxtel W, Buter J, et al. Androgen deprivation therapy for androgen receptor-positive advanced salivary duct carcinoma: a nationwide case series of 35 patients in The Netherlands. Head Neck. 2018;40(3):605–13.
Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol. 2011;29(16):e473–6.
Falchook GS, Lippman SM, Bastida CC, Kurzrock R. Human epidermal receptor 2-amplified salivary duct carcinoma: regression with dual human epidermal receptor 2 inhibition and anti-vascular endothelial growth factor combination treatment. Head Neck. 2014;36(3):E25–7.
Haddad R, Colevas AD, Krane JF, et al. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncology. 2003;39(7):724–7.
Limaye SA, Posner MR, Krane JF, et al. Trastuzumab for the treatment of salivary duct carcinoma. Oncologist 2013;18(3):294–300.
Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997;275(5308):1943–7.
Ettl T, Baader K, Stiegler C, et al. Loss of PTEN is associated with elevated EGFR and HER2 expression and worse prognosis in salivary gland cancer. Br J Cancer. 2012;106(4):719–26.
Griffith CC, Seethala RR, Luvison A, Miller M, Chiosea SI. PIK3CA mutations and PTEN loss in salivary duct carcinomas. Am J Surg Pathol. 2013;37(8):1201–7.
Stenman G. Fusion oncogenes in salivary gland tumors: molecular and clinical consequences. Head Neck Pathol. 2013;7(Suppl 1):12–9.
Lee YS, Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007;21(9):1025–30.
Acknowledgements
This work was supported by MD Anderson start-up funds (DB). Presented at Annual Meeting USCAP 2018, Vancouver.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Liang, L., Williams, M.D. & Bell, D. Expression of PTEN, Androgen Receptor, HER2/neu, Cytokeratin 5/6, Estrogen Receptor-Beta, HMGA2, and PLAG1 in Salivary Duct Carcinoma. Head and Neck Pathol 13, 529–534 (2019). https://doi.org/10.1007/s12105-018-0984-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-018-0984-5