Abstract
Positron emission tomography/computed tomography (PET/CT) has shown prognostic significance in head and neck cancer patients. The underlying pathologic features that could explain the mechanisms associated with this observation are not clear. To analyze the correlation between 18-F-fluoro-2-deoxy-d-glucose (18F-FDG) uptake assessed by PET/CT in head and neck cancer and histopathologic prognostic factors. Ninety-nine patients with laryngeal and pharyngeal squamous cell carcinoma were retrospectively reviewed for pretreatment PET/CT measurements, namely standardized uptake value (SUV), metabolic tumor volume (MTV), and total lesion glycolysis (TLG). The corresponding histologic material was evaluated for tumor stroma-related prognostic factors such as the amount and type of stroma, lymphocytic response, tumor budding activity, and size of tumor cell nests in the tumor core area and tumor front. TLG and MTV were associated with tumor localization, as they were higher in oropharyngeal tumors. These values were also associated with tumor cell nest size in the tumor core with higher values corresponding to tumors with smaller nests. MTV40% was marginally associated with fibroblastic stroma type and higher budding activity. SUVmax was not associated with the histological factors in the whole sample, but higher values trended with higher tumor budding activity and stroma-rich tumors of the oropharynx. 18F-FDG PET measurements in head and neck squamous cell carcinomas are associated with prognostic histopathologic factors and suggest a possible correlation of glucose metabolism to epithelial-to-mesenchymal transition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Analysis of microenvironments in head and neck cancer has revealed various histologic factors of prognostic significance. For example, a favorable prognostic factor is the histologic finding of an intense lymphocytic reaction at the tumor front with high numbers of T lymphocytes, especially cytotoxic T lymphocytes [1,2,3]. Aggressive behavior is seen in tumors rich in fibroblastic stroma with smaller tumor cell nests and budding at the tumor front [4]. These important prognostic factors also have predictive value on the response to induction chemotherapy [1,2,3,4].
Similarly, PET evaluation performed upon diagnosis of head and neck cancer has prognostic significance. A recent meta-analysis studying the association between semiquantitative metabolic parameters and outcomes of patients with advanced head and neck squamous cell carcinoma (HNSCC) showed that high pretreatment metabolic tumor volume (MTV) was significantly associated with worse overall survival, progression-free survival, and locoregional control [5]. The standardized uptake value (SUV) did not show any association with outcome measures [5]. To the best of our knowledge, the relationship between PET values and histologic characteristics with prognostic significance has not been previously investigated in head and neck cancer. In other forms of cancer, a few studies do correlate histologic findings with PET values and suggest that high baseline 18Ffluorodeoxyglucose (18FDG) uptake, as assessed by high SUV, may be associated with poor prognostic factors such as high histological grade in breast cancer [6].
Accordingly, PET may be used for diagnostic staging, evaluation or prediction of treatment response, and also as a prognostic method that, based on tumor glucose metabolism, could predict tumor aggressiveness [7]. In the present study, we investigate the association between PET image parameters and histopathologic prognostic factors in head and neck cancer.
Material and Methods
Patients
A retrospective, single-center study of patients with primary HNSCC treated at the University Hospital of Saint-Etienne, France was conducted. The study was approved by the institutional review board with waivers of informed written consent given the retrospective nature of the study and the anonymization of all data (IRBN452018/CHUSTE).
We reviewed the pretreatment FDG PET/CT scans of HNSCC patients and the corresponding histology. Excision and biopsy specimens with adequate material were used. Pretreatment biopsies were used for patients treated with neoadjuvant chemoradiotherapy to avoid any treatment-induced changes. All patients with a diagnosis of laryngeal or pharyngeal squamous cell carcinoma with sufficient histologic material for review and an available PET/CT scan were included (n = 120). Cases where a biopsy was performed at least 6 days before the PET/CT were excluded in order to avoid biopsy-induced changes on the PET values. Cases where the delay between the PET/CT and the histologic evaluation was more than 45 days were also excluded to ascertain correspondence between histology and PET values. Finally, 99 patients were further analyzed.
PET/CT
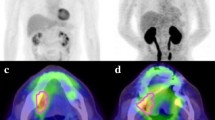
FDG PET/CT was performed from 2010 to 2017 using the Biograph6® LSO PICO 3D PET/CT HI-REZ system (Siemens Medical, Knoxville, TN, USA) as previously described [7]. All patients underwent 18F-FDG PET/CT for initial staging. PET/CT images were assessed visually and quantitatively by a consensus of two experienced nuclear medicine physicians who were informed of all standard imaging as well as clinical and laboratory findings. All patients fasted for at least 6 h before FDG PET/CT, and their serum glucose levels at the time of FDG injection were < 150 mg/dL. CT was performed 60 min after an injection of 4 MBq/kg FDG: high-resolution head and neck acquisition comprised CT without contrast enhancement (85 mAs; 130 kV; slice thickness, 3 mm; pitch, 0.85; care dose) followed by two 16.2-cm PET steps of 10 min each, with arms along the body; thoracic-abdominal-pelvic acquisition comprised CT without contrast enhancement (52 mAs; 130 kV; slice thickness, 5 mm; pitch, 1.5; care dose) followed by 7 or 8 16.2-cm PET steps of 6 min each with arms behind the head. The post-treatment console Leonardo; Siemens was used for image reading and processing (Figs 1, 2).
Standardized uptake values (SUV), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) were defined according to guidelines as follows [8]: SUV: the concentration of FDG in a given region of interest (ROI) or volume of interest (VOI; expressed in kilobecquerels per millilitre) divided by the ratio between administered activity (corrected for radioactive decay at the time of scanning) and the body weight of the patient; SUVmax: the highest SUV of pixels (or voxels) in a given ROI (or VOI); TLG: the product of the SUV and the corresponding MTV. MTV was defined as the total tumor volume segmented via threshold. For determining the VOI boundary, fixed SUV values of 2.5 and a fixed threshold (40% of SUVmax) were adopted.
Histopathologic Parameters
Histologic evaluation was conducted as previously described [4]. Briefly, hematoxylin, eosin, saffron (HES) slides were evaluated, by the consensus of two pathologists (GK, JMD), for the following parameters: (a) keratinizing versus nonkeratinizing, (b) tumor budding activity (TBA), (c) tumor to stroma ratio (TSR), (d) cell nest size (CNS) at both the core and tumor front areas, (e) fibroblastic or fibrotic stroma type, and (f) the lymphocytic host response. Specifically, keratinizing tumors showed keratin pearl formation, ‘mature’ eosinophilic cytoplasm, and polygonal-shaped cells with distinct borders and intercellular bridges. Non-keratinizing carcinomas comprised hyperchromatic cells with indistinct cell borders that formed nests with pushing borders and showed absent or limited squamous maturation [3, 9]. Oropharyngeal tumors and a subset of the hypopharyngeal tumors (n = 31) were tested for HPV infection at the time of diagnosis and were all negative.
Tumor budding was defined as small tumor nests of less than 5 tumor cells invading surrounding tissue at the tumor front. The TSR classifies tumors as stroma-poor, when the stroma consists of < 50% of the examined tumor field, or stroma-rich (≥ 50%). A cut-off value of 30% can be also used and both cut off values were used for statistical analysis [4]. Stroma is classified as fibroblastic when comprised of loose connective tissue or as fibrotic when a dense, homogeneous, collagenous stroma is present. Cell nests are defined as clusters of tumor cells surrounded by stroma, and they are classified as large cell nests when they contain > 15 tumor cells, intermediate when they include 5–15 tumor cells, and small cell nests when they contain 2–4 tumor cells. CNS was assessed at the invasive margin and separately in the tumor core area. Lymphocytic host response was evaluated at the tumor front as mild or absent when few or no lymphocytes were seen, moderate when some lymphoid nodules were seen at the advancing edge but not in all fields, and severe when a dense complete reaction with lymphoid nodules was seen.
Statistical Analysis
Data were analyzed using the StatView® software (Abacus Concepts, Berkley, California). We used the factorial analysis of variances to consider the effect of at least one factor on a continuous parameter studied and simple regression analysis to explore a possible relationship between two continuous parameters. A p-value < 0.05 was considered statistically significant.
Results
The cohort included 99 patients with male predominance (84.8%) and a mean age of 61.7 years (range 42–84, median 62). Most tumors (68.6%) were laryngeal with 16.2% localized to the oropharynx and 19.2% to the hypopharynx. Specimens included biopsies (n = 22) and resections (n = 77). No difference in PET values was seen between these two groups. Patients characteristics, PET values, and histologic features are shown in Table 1.
SUVmax was not associated with age (p = 0.7) while TLG40% was inversely associated with age (p = 0.01, r2 = 0.05). Similarly, TLG2.5 also trended to an inverse association with age (p = 0.07, r2 = 0.27), but this was not statistically significant. TLG40%, TLG2.5, MTV40%, and MTV2.5 were all associated with tumor localization (p = 0.01, p = 0.02, p = 0.001 and p = 0.01, respectively); these values were higher in oropharyngeal tumors followed by laryngeal and hypopharyngeal ones. TLG40%, TLG2.5, MTV40% and MTV2.5 were associated with tumor CNS in tumor cores, with higher values identified in tumors with smaller tumor cell nests (Table 2). MTV40% showed a strong trend (p = 0.05) to the fibroblastic stroma type. It also trended with higher TBA (p = 0.06) and lesser lymphocytic host response (p = 0.09). The same parameters were separately analyzed by tumor localization and showed further correlation in oropharyngeal tumors. Higher SUV max trended with non-keratinizing squamous cell type (p = 0.09), higher TBA (p = 0.07), and stroma-rich tumors (p = 0.07).
Discussion
PET is an imaging technique based on the pharmacodynamics of the 18F-FDG radiotracer metabolized by neoplastic cells [10]. SUV is the ratio between the amount of radiotracer taken up by a lesion and the amount of radiotracer that would hypothetically be present in a region of equal volume if the tracer was uniformly distributed throughout the body [10]. High SUV values are predictive of malignancy. TLG is theoretically a superior measurement as it considers the level of glucose accumulation within the total volume of all the regions of interest [11]. PET is widely used for the evaluation of cancer patients, and interest for its use in the management of HNSCC patients was recently renewed following results from the PET-NECK trial [12]. This trial showed that post-treatment FDG PET scans have a high negative predictive value after primary chemoradiation and can be used to stratify patients for neck dissection [12].
The prognostic role of PET has been the subject of many studies for various forms of cancer over the last decade. Regarding HNSCC, SUV, MTV, and TLG are all shown to have prognostic significance [13,14,15]. Most of the studies examining the prognostic role of PET in head and neck cancer patients are retrospective with heterogeneous technical issues and values thresholds. This has prohibited set cut-off points, although it is suggested that volumetric parameters, as opposed to SUV which is realized into only one pixel and thus non-representative of the whole lesion, have a more important prognostic role in this patient group and are more reproducible [5, 15].
In the current study, we directly compare PET values with well-established histopathologic prognostic factors in order to find a pathophysiological correlation between cancer cell metabolism, as assessed by PET imaging, and morphologic features of aggressiveness as assessed by microscopy. We show that TLG and MTV are higher in tumors with aggressive histological characteristics, namely tumors with smaller CNS, stroma-rich tumors, and TBA. SUVmax also showed a marginal correlation with higher TBA and stroma-rich tumors but only in oropharyngeal carcinomas. These histopathologic factors have prognostic significance in HNSCC. We have recently shown that stroma-rich tumors, smaller CNS, and TBA are associated with advanced T stage in HNSCC and a worse overall and progression-free survival [4]. These factors can be considered the morphologic expression of epithelial-to-mesenchymal transition (EMT) and thus reveal a more aggressive and invasive tumor [4]. This may be explained by the upregulation of corresponding EMT genes [16]. The results of the current study show that higher glucose metabolism is associated with HNSCCs with a more aggressive, EMT-favoring phenotype.
The possible association of PET values with EMT has been recently suggested in other forms of cancer. In a study of 18 esophageal adenocarcinomas, tumors with high FDG-uptake, as measured by SUVmax, showed significant upregulation of genes associated with extracellular matrix organization [17]. Similarly, esophageal squamous cell carcinomas with higher SUVmax were also associated with upregulation of EMT genes [18]. A similar association between FGD uptake and EMT proteins has been found in hepatocellular carcinomas and non-small cell lung carcinomas [19, 20]. Thus, our results suggest an association of FDG uptake with morphologic features of EMT in HNSCC and reinforces these observations in other forms of cancer.
The possible association of PET parameters with histopathologic prognostic factors in head and neck cancer has been never studied to the best of our knowledge. In a study of 44 patients suffering from HNSCC, FDG PET and MRI were performed to measure textural-based imaging parameters, like coarseness in PET and apparent diffusion coefficient (ADC) in MRI. The results showed that these measurements are associated with stroma proportion [21]. In a study of 15 patients with HNSCC, higher SUV was associated with a lower rate of apoptosis and higher p53 and VEGF expression, resulting in a more aggressive phenotype [22]. The relationship between PET/CT values and histopathological prognostic factors has been mainly studied in breast cancer, where it is suggested that the accumulation of FDG depends on histologic type (ductal vs. lobular), microscopic tumor growth pattern (nodular vs. diffuse), and Ki-67 index of tumor cell proliferation. Accumulation of FDG does not correlate with axillary lymph node status, tumor size, percentage of tumor cells, presence of inflammatory cells, histopathologic grading, or steroid receptor status [23]. PET/MRI values in breast cancer seem to discriminate between luminal A and luminal B subtypes, with the lowest SUV values associated with the luminal A subtype, a subtype with better prognosis [24]. Increased SUV values are also associated with higher histologic grade in pulmonary tumors [10]. Our results in HNSCC are consistent with findings of other forms of cancer and show that higher PET measurements are indeed associated with aggressive histologic features.
This study has certain limitations. First, it is a retrospective study in a single institution and thus subjected to biases, but the same PET protocol has been used over the study period. Second, it enrolls a relatively low number of patients. Nevertheless, it the first study examining the association of PET with histopathologic factors.
In conclusion, we show that FDG PET measurements in HNSCCs are associated with prognostic histopathologic factors and suggest a correlation between glucose metabolism and epithelial-to-mesenchymal transition.
References
Karpathiou G, Casteillo F, Giroult J-B, Forest F, Fournel P, Monaya A, et al. Prognostic impact of immune microenvironment in laryngeal and pharyngeal squamous cell carcinoma: immune cell subtypes, immuno-suppressive pathways and clinicopathologic characteristics. Oncotarget. 2017;8:19310–22.
Karpathiou G, Giroult J-B, Forest F, Fournel P, Monaya A, Froudarakis M, et al. Clinical and histologic predictive factors of response to induction chemotherapy in head and neck squamous cell carcinoma. Am J Clin Pathol. 2016;146:546–53.
Karpathiou G, Monaya A, Forest F, Froudarakis M, Casteillo F, Marc Dumollard J, et al. p16 and p53 expression status in head and neck squamous cell carcinoma: a correlation with histological, histoprognostic and clinical parameters. Pathology. 2016;48:341–8.
Karpathiou G, Vieville M, Gavid M, Camy F, Dumollard JM, Magné N, et al. Prognostic significance of tumor budding, tumor-stroma ratio, cell nests size, and stroma type in laryngeal and pharyngeal squamous cell carcinomas. Head Neck. 2019;41:1918–27.
Bonomo P, Merlotti A, Olmetto E, Bianchi A, Desideri I, Bacigalupo A, et al. What is the prognostic impact of FDG PET in locally advanced head and neck squamous cell carcinoma treated with concomitant chemo-radiotherapy? A systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2018;45:2122–38.
Groheux D, Giacchetti S, Moretti J-L, Porcher R, Espié M, Lehmann-Che J, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–35.
Gavid M, Prevot-Bitot N, Timoschenko A, Gallet P, Martin C, Prades J-M. [18F]-FDG PET-CT prediction of response to induction chemotherapy in head and neck squamous cell carcinoma: preliminary findings. Eur Ann Otorhinolaryngol Head Neck Dis. 2015;132:3–7.
Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Chernock RD, El-Mofty SK, Thorstad WL, Parvin CA, Lewis JS. HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: Utility of microscopic features in predicting patient outcome. Head Neck Pathol. 2009;3:186–94.
Cappabianca S, Porto A, Petrillo M, Greco B, Reginelli A, Ronza F, et al. Preliminary study on the correlation between grading and histology of solitary pulmonary nodules and contrast enhancement and [18F]fluorodeoxyglucose standardised uptake value after evaluation by dynamic multiphase CT and PET/CT. J Clin Pathol. 2011;64:114–9.
McDonald JE, Kessler MM, Gardner MW, Buros AF, Ntambi JA, Waheed S, et al. Assessment of total lesion glycolysis by 18 F FDG PET/CT significantly improves prognostic value of GEP and ISS in myeloma. Clin Cancer Res. 2017;23:1981–7.
Mehanna H, Wong W-L, McConkey CC, Rahman JK, Robinson M, Hartley AGJ, et al. PET-CT Surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med. 2016;374:1444–54.
Xie P, Li M, Zhao H, Sun X, Fu Z, Yu J. 18F-FDG PET or PET-CT to evaluate prognosis for head and neck cancer: a meta-analysis. J Cancer Res Clin Oncol. 2011;137:1085–93.
Pak K, Cheon GJ, Nam H-Y, Kim S-J, Kang KW, Chung J-K, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med. 2014;55:884–90.
Abgral R, Malard O, Salaün PY. Carcinomes des VADS—Adénopathies cervicales métastatiques sans primitif connu—Cancers des glandes salivaires. Médecine Nucléaire. 2019;43:8–29.
Jensen D, Dabelsteen E, Specht L, Fiehn A, Therkildsen M, Jønson L, et al. Molecular profiling of tumour budding implicates TGFβ-mediated epithelial-mesenchymal transition as a therapeutic target in oral squamous cell carcinoma. J Pathol. 2015;236:505–16. https://doi.org/10.1002/path.4550.
Heiden BT, Patel N, Nancarrow DJ, Hermann M, Brown RKJ, Orringer MB, et al. Positron emission tomography 18F-fluorodeoxyglucose uptake correlates with KRAS and EMT gene signatures in operable esophageal adenocarcinoma. J Surg Res. 2018;232:621–8.
Kajiwara T, Hiasa Y, Nishina T, Matsumoto T, Hori S, Nadano S, et al. Maximum standardized uptake value in 18F-fluoro-2-deoxyglucose positron emission tomography is associated with advanced tumor factors in esophageal cancer. Mol Clin Oncol. 2014;2:313–21.
Lee M, Jeon JY, Neugent ML, Kim J-W, Yun M. 18F-Fluorodeoxyglucose uptake on positron emission tomography/computed tomography is associated with metastasis and epithelial-mesenchymal transition in hepatocellular carcinoma. Clin Exp Metastasis. 2017;34:251–60.
Yamamoto S, Huang D, Du L, Korn RL, Jamshidi N, Burnette BL, et al. Radiogenomic analysis demonstrates associations between 18 F-fluoro-2-deoxyglucose PET, prognosis, and epithelial-mesenchymal transition in non-small cell lung cancer. Radiology. 2016;280:261–70.
Choi JW, Lee D, Hyun S, Han M, Kim J-H, Lee SJ. Intratumoural heterogeneity measured using FDG PET and MRI is associated with tumour–stroma ratio and clinical outcome in head and neck squamous cell carcinoma. Clin Radiol. 2017;72:482–9.
Grönroos TJ, Lehtiö K, Söderström K-O, Kronqvist P, Laine J, Eskola O, et al. Hypoxia, blood flow and metabolism in squamous-cell carcinoma of the head and neck: correlations between multiple immunohistochemical parameters and PET. BMC Cancer. 2014;14:876.
García Vicente AM, Castrejón ÁS, Relea Calatayud F, Muñoz AP, León Martín AA, López-Muñiz IC, et al. 18F-FDG retention index and biologic prognostic parameters in breast cancer. Clin Nucl Med. 2012;37:460–6.
Incoronato M, Grimaldi AM, Cavaliere C, Inglese M, Mirabelli P, Monti S, et al. Relationship between functional imaging and immunohistochemical markers and prediction of breast cancer subtype: a PET/MRI study. Eur J Nucl Med Mol Imaging. 2018;45:1680–93.
Acknowledgements
The authors would like to thank Mr Philippe Cosmo from the Tumorothèque/Centre de Ressources Biologiques de CHU Saint-Etienne (BRIF No. BB-0033-00041) for his assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karpathiou, G., Gavid, M., Prevot-Bitot, N. et al. Correlation Between Semiquantitative Metabolic Parameters After PET/CT and Histologic Prognostic Factors in Laryngeal and Pharyngeal Carcinoma. Head and Neck Pathol 14, 724–732 (2020). https://doi.org/10.1007/s12105-019-01116-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-019-01116-z