Abstract
Sclerosing microcystic adenocarcinoma is an exceedingly rare entity occurring in the mucosal surfaces of the head and neck that closely resembles cutaneous microcystic adnexal carcinoma. Here, we report a case of sclerosing microcystic adenocarcinoma that presented as a vague mass at the floor of the mouth in a 55-year-old woman. The pathology features and the diagnostic challenges, especially in the biopsy and margin evaluation are discussed here. Similar cases published in the English literatures are reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microcystic adnexal carcinoma (MAC) is an uncommon, cutaneous-based malignant neoplasm that tends to occur at the head and neck region [1]. It is typically regarded as originating from eccrine sweat glands or a multipotent stem cell. Histologically, it features keratin-filled cysts, nests and cords of basaloid cells, and the formation of ductal structures within a desmoplastic stroma [2]. Recently, Mills et al. described 5 cases of head and neck tumors that closely resembled MAC morphologically, but occurred in the mucosal surface and named the tumor “sclerosing microcystic adenocarcinoma” [3]. Here, we report an additional case to raise the awareness of this rare entity.
Report of a Case
The patient, a 55-year-old woman, presented with left floor of mouth (FOM) fullness initially noted by her dentist. Prior to this presentation, she had numbness and weakness of the tongue for 4–5 months. She recalled no prior injury to the area. She acknowledged fatigue, weakness, frequent sore throat and some soreness of the tongue. Her medical history was significant for multiple sclerosis treated with Glatiramer acetate. A family history of BRCA gene mutation was noted.
Intraoral physical exam revealed healthy-appearing mucosa throughout and firmness upon palpation in the left FOM. Computed tomography of the head and neck with contrast demonstrated an amorphous soft tissue mass in the FOM along the lingual cortex of the left mandible. The mass obscured fat planes with the adjacent genioglossus and mylohyoid muscles and the fat plane around the lingual neurovascular bundle, and measured approximately 2.8 cm in the greatest dimension (Fig. 1a). There was no osseous erosion of the adjacent mandible. Tongue muscle bulk was otherwise symmetric, with no evidence of atrophy. In addition, an enlarged left level 2 lymph node was appreciated (Fig. 1b). The left submandibular gland was severely atrophic.
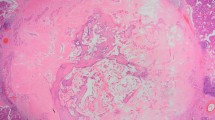
Incisional biopsies of the FOM mass confirmed the malignant nature of the lesion by identifying perineural invasion; however, the classification of the tumor could not be made. A resection of the tumor with selective neck dissection was performed. Gross examination of the surgical resection specimen revealed an ill-defined white-tan submucosal mass measuring 3.0 × 1.0 × 1.0 cm. Microscopically the tumor was paucicellular with a predominant stroma component intermixed with infiltrating cell nests and ducts (Fig. 2a, b). The stroma ranged from densely collagenized stroma to basophilic-desmoplasia which frequently surrounded the infiltrating ducts (Fig. 2b–d). The infiltrating cell nests and ducts were cytologically bland and mitotically inactive (Fig. 2c, f). The ducts were lined by luminal epithelium with abundant eosinophilic cytoplasm and abluminal epithelium and were frequently filled with eosinophilic secretion in the lumen (Fig. 2c). No basophilic or mucinous material was seen. The tumor infiltrated into skeletal muscles, and multiple foci of perineural invasion were identified (Fig. 2e, f). The tumor cells were immunoreactive to cytokeratins (AE1/AE3, CK5/6, CK7), and p63 with a pattern similar to epithelium-myoepithelium pattern in many salivary gland tumors and MAC as well. Immunostain of CD117 (C-kit) is negative. The mass was in close association with minor salivary gland (Fig. 2b). Margin was focally involved by the carcinoma. Limited neck dissection did not reveal nodal involvement. The location and morphology of the tumor are most consistent with sclerosing microcystic adenocarcinoma of the head and neck mucosa. The patient received adjuvant radiation therapy postsurgically due to margin positivity. Ten month post-surgery follow-up showed no evidence of residual/recurrent disease.
Histologic features of the tumor. The overlying squamous epithelium is not dysplastic; the tumor is paucicellular with a predominant stroma component intermixed with infiltrating cell nests or glands (a original magnification ×40). Basophilic desmoplasia stroma (b original magnification ×100) or eosinophilic collagenized stroma (c original magnification ×400). The tumor cells are cytologically bland and mitotically inactive, eosinophilic secretions are present in the lumen of some ducts (c). Stroma around tumor nests mimics atrophic and sclerosing minor salivary gland tissue focally (d original magnification ×200). The tumor widely infiltrates into adjacent skeletal muscle (e original magnification ×200); perineural invasion is present (f original magnification ×400)
Discussion
MAC is an uncommon, cutaneous-based malignant neoplasm that is usually regarded as originating from the eccrine sweat glands. Rare cases of tumors that share striking similarity with MAC but originate from the mucosal surface have been reported in the head and neck region under different names [4, 5]. Mills et al. recently reported five such cases and named them “sclerosing microcystic adenocarcinoma” as a distinct entity [3]. Since then, two additional cases of sclerosing microcystic adenocarcinoma of head neck mucosa have been reported [6].
Table 1 summarizes the clinical and histologic features of the previously published cases in English literatures and this case. Similar lesions occurring in the lips were left out due to somewhat uncertainty in the cutaneous versus mucosal origin [1, 7]. The majority patients are females (8 of 10 cases) with a wide range of age (41–73 years). From the limited case reports and series of this rare entity, patients appear to have relatively indolent clinical courses without lymph node or distant metastasis (Table 1) [3,4,5,6]. Our patient underwent radiotherapy due to a focally positive margin without recurrence for 10 months. There was a speculation about its relationship with some forms of immunosuppression [3]. Our patient was not immunosuppressed by strict definition, but being treated with Glatiramer acetate for multiple sclerosis which is considered an immunomodulator. It is not clear whether this correlation is coincidental.
Microscopically sclerosing microcystic adenocarcinoma is characterized by prominent stroma component intermixed with infiltrating cytologically bland cell nests and ducts. The stroma ranged from densely collagenized stroma to basophilic-desmoplasia, frequently surrounding the ducts. Currently, there is no known molecular alteration of sclerosing microcystic adenocarcinoma, and the immunohistochemistry profile is nonspecific. Therefore, the diagnosis relies heavily on careful morphological evaluation.
Histologic diagnosis of this entity is difficult, especially in small incisional biopsies when the constellation of diagnostic features is not evident. For example, the basophilic desmoplasia surrounding ducts can be mistaken for sclerotic and atrophic lobules of salivary gland tissue when the infiltrating pattern is not present in biopsies (Fig. 2d), therefore interpreted as benign. The paucicellular stroma of the tumor can be mistaken for reactive change especially when the tumor was biopsied previously. In our case, the patient underwent three incisional biopsies without definitive diagnosis despite extensive internal and external pathology consultation. The malignant nature of the tumor was evident in the resection specimen in our case given the widely infiltrative pattern infiltrating into skeletal muscle and unequivocal perineural invasions. Assessment of margins especially during intraoperative evaluation is extremely challenging if not impossible, due to the paucicellular nature of the tumor. In our case, the intraoperative margin assessment was inconclusive; the margin was eventually deemed positive by comparing it to the tumor in the main specimen. It is important to be aware of this pitfall during intraoperative margin assessment if this entity potentially could be the diagnosis.
The differential diagnoses of sclerosing microcystic adenocarcinoma include neoplasms like squamous cell carcinoma, adenoid cystic carcinoma, low grade mucoepidermoid carcinoma, tubular pattern of polymorphous low-grade adenocarcinoma (PLGA) [8], and secretory carcinoma and reactive lesion like chronic sclerosing sialadenitis. Distinguishing this tumor from squamous cell carcinoma is very important since it will impact the management significantly. Sclerosing microcystic adenocarcinoma lacks prominent cytologic atypia and surface epithelial dysplasia, features usually present in squamous cell carcinoma. Although the infiltrating tumor nests have squamoid appearance (Fig. 2f), they do not have keratin, and the presence of ducts lined by two layers of cytologically bland cells argues against squamous cell carcinoma. Although tubular pattern of adenoid cystic carcinoma can overlap with sclerosing microcystic adenocarcinoma, the latter lacks angulated hyperchromatic nuclei and basophilic secretion seen in adenoid cystic carcinoma. In addition, infiltrating small nests or cords of tumor cells are not seen in adenoid cystic carcinoma. CD117 (c-Kit) immunostain, usually positive in adenoid cystic carcinoma but negative in sclerosing microcystic adenocarcinoma, could be helpful [9]. PLGA is usually well circumscribed under low power view and more cellular. On the contrary, sclerosing microcystic adenocarcinoma is widely infiltrative and paucicellular. Lack of architectural variability and the abundant sclerosing stroma also assist in differentiating sclerosing microcystic adenocarcinoma from PLGA. Secretory carcinoma often contains eosinophilic secretion in the lumen, however abundant sclerotic stroma is not typical for secretory carcinoma, which is usually more cellular. Low grade mucoepidermoid carcinoma is often cystic, and can be excluded from the differential diagnosis by the lack of mucinous cells. The constellation of morphology of the tumor in the resection specimen reported here does not fit adenoid cystic carcinoma, low grade mucoepidermoid carcinoma, PLGA or secretory carcinoma, therefore tests for molecular changes commonly seen in these entities were not performed in this case. The widely infiltrative growth pattern beyond the extent of minor salivary gland or presence of unequivocal perineural invasion would help differentiate sclerosing microcystic adenocarcinoma from sclerosing sialometaplasia, which maintains the lobular configuration without perineural invasion. The immunostain of IgG and IgG4 helps to differentiate this entity from IgG4-related sclerosing disease in the small biopsies.
References
Basile JR, Lin YL. A salivary gland adenocarcinoma mimicking a microcystic adnexal carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(4):e28–33.
Hoang MP, Dresser KA, Kapur P, High WA, Mahalingam M. Microcystic adnexal carcinoma: an immunohistochemical reappraisal. Mod Pathol. 2008;21(2):178–85.
Mills AM, Policarpio-Nicholas ML, Agaimy A, Wick MR, Mills SE. Sclerosing microcystic adenocarcinoma of the head and neck mucosa: a neoplasm closely resembling microcystic adnexal carcinoma. Head Neck Pathol. 2016;10(4):501–8.
Schipper JH, Holecek BU, Sievers KW. A tumour derived from Ebner’s glands: microcystic adnexal carcinoma of the tongue. J Laryngol Otol. 1995;109(12):1211–4.
Petersson F, Skogvall I, Elmberger G. Sclerosing sweat duct-like carcinoma of the tongue-a case report and a review of the literature. Am J Dermatopathol. 2009;31(7):691–4.
Wood A, Conn BI. Sclerosing microcystic adenocarcinoma of the tongue: a report of 2 further cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(4):e94–102.
Johnston CA, Toker C. Syringomatous tumors of minor salivary gland origin. Hum Pathol. 1982;13(2):182–4.
Ide F, Kikuchi K, Kusama K. Microcystic adnexal (sclerosing sweat duct) carcinoma of intraoral minor salivary gland origin: an extracutaneous adnexal neoplasm? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(3):284–6.
Mino M, Pilch BZ, Faquin WC. Expression of KIT (CD117) in neoplasms of the head and neck: an ancillary marker for adenoid cystic carcinoma. Mod Pathol. 2003;16(12):1224–31.
Acknowledgements
We would like to thank Dr. Ricardo Lloyd and Dr. Aaron Wieland for their input in this case.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, R., Cagaanan, A., Hafez, GR. et al. Sclerosing Microcystic Adenocarcinoma: Report of a Rare Case and Review of Literature. Head and Neck Pathol 13, 215–219 (2019). https://doi.org/10.1007/s12105-018-0945-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-018-0945-z