Abstract
Cribriform morular variant of PTC (CMV-PTC) frequently shows activation of the CTNNB1/Wnt pathway with nuclear accumulation of beta catenin. The utility of LEF-1, also in the CTNNB1/WNT pathway, in the diagnosis of CMV-PTC has not been previously studied. LEF-1 immunohistochemistry was performed on seven CMV-PTC, 52 benign cases and 101 malignant thyroid neoplasms. LEF-1 was scored by stain intensity (0 = no nuclear stain, 1 = weak nuclear stain, less than lymphocyte and 2 = strong nuclear stain, intense as lymphocyte) and percentage of positive cells at each intensity, for a maximum total score of 200. Sensitivity and specificity of LEF-1 stain for all cases and to differentiate between regular PTC and CMV-PTC was also calculated. Six of the seven CMV-PTCs showed ≥ 30% strong (2+) nuclear LEF-1 staining and a total score over 100. Beta catenin also showed strong and diffuse nuclear staining in these cases. One CMV-PTC was negative for both LEF-1 and beta catenin and did not have a history of FAP. All control PTC cases uniformly lacked LEF-1 staining at 2+ intensity. LEF-1 had a sensitivity of 86% and specificity of 98% for the diagnosis of CMV-PTC. LEF-1 is highly sensitive and specific marker for CMV-PTC, especially when used in the setting of a PTC neoplasm. The pattern of staining is important with ≥ 30% of cells showing strong 2+ nuclear staining having the highest combined sensitivity and specificity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Papillary thyroid cancer (PTC) is the most common malignancy of the thyroid gland and accounts for 80% of all thyroid cancers [1]. Cribriform morular variant of PTC (CMV-PTC) is a rare subtype that is seen in association with familial adenomatosis polyposis (FAP) although sporadic cases are also reported. In 1994, Harach and colleagues first described this distinct tumor in FAP patients, and later in 1999 Cameselle-Teijeino and Chan proposed the term CMV-PTC for this entity [2, 3]. Approximately 1–2% of patients with FAP are affected by thyroid cancer, and in approximately 30% the thyroid cancer precedes the development of adenomatosis polyposis coli by 4–12 years [1]. The familial form of CMV-PTC is often multifocal and indolent while the sporadic form is solitary and occasionally aggressive. Therefore, recognition of CMV-PTC is clinically relevant as it has management implications and the potential to be used as a screening tool for undiagnosed FAP [1, 2, 4, 5].
FAP is caused by autosomal dominant inheritance of a germline mutation in the APC gene located in 5q21 region, which is identified in 60–80% of families with FAP [6]. The APC gene is a component of the Wnt signaling pathway which plays a role in tumorigenesis when aberrantly activated. LEF-1 (lymphoid enhancing factor 1) and beta catenin when coupled together serve as key nuclear mediators of this pathway. Normally the APC gene causes degradation of beta catenin and inhibits the Wnt/CTNNB1 signaling pathway [6, 7].
Mutations in the beta catenin gene (CTNNB1) or APC gene leads to permanent activation of the Wnt/CTNNB1 pathway with nuclear accumulation of beta catenin and activation of TCF4 (T cell factor) and LEF-1 which promotes tumor development [1, 8]. LEF-1 belongs to the LEF/TCF family, is widely expressed in developing tissue and in adulthood it is seen in T and pre-B cells [8, 9]. Its immunohistochemical expression has been studied extensively in lymphoma and leukemia and more recent studies have examined its role in gastrointestinal, pancreatic, breast, prostate and salivary gland tumors [9,10,11]. Beta catenin immunohistochemistry has been previously identified as a reliable marker of dysregulation of the CTNNB1/Wnt pathway and nuclear staining is present in 100% of CMV-PTC [12,13,14]. However the utility of LEF-1 immunohistochemistry in the diagnosis of thyroid tumors has not been previously reported. In the current study we evaluate the utility of LEF-1 as an immunohistochemical marker in thyroid neoplasms, with particular attention to the differentiation of CMV-PTC from other types of PTC.
Materials and Methods
This study was approved by the Cleveland Clinic Institutional Review Board. A retrospective search for all cases diagnosed as CMV-PTC were retrieved from the surgical pathology archives in the department of pathology at the Cleveland Clinic from January 1980 to January 2016. Only cases with surgical pathology slides and blocks available for review were included. Seven cases meeting these criteria were identified and included in the study. For comparison, a selection of 52 benign and 101 malignant thyroid cases was included. This included cases from a previously constructed tissue microarray (TMA) of benign and malignant thyroid tumors, supplemented by a selection of cases of benign and malignant thyroid tumors examined by whole section immunohistochemistry (see Table 1). Of the 30 PTCs included for comparison, the break down by subtype is as follows: 5 classical PTCs with whole slide staining and 30 PTCs from the TMA (12 classical type, 9 follicular variant, 2 tall cell variant, 2 oncocytic variant).
The TMA was constructed from original formalin-fixed, paraffin-embedded (FFPE) tissue blocks as previously described [15]. Briefly, regular 5-µm sections were made from the original tissue blocks and stained with Hematoxylin and Eosin (H&E) to confirm previously rendered histologic diagnoses. Selected areas were marked on the H&E stained slides as a guide for TMA construction by one pathologist (CS). Two tissue cylinders with a diameter of 1.2 mm were punched from each donor tissue block and brought into the recipient paraffin block using “Pathology Devices TMArrayer” (Pathology Devices Inc., Westminster, MD). The cores from each case i.e. NH or thyroid follicular cell-derived carcinomas were arrayed with 1.8 mm spacing. A total of three TMAs were created; TMA1 included nodular hyperplasia (NH) and PTC cases, TMA2 housed follicular carcinoma (FC) and Hürthle cell carcinoma (HCC) cases, and TMA3 had undifferentiated carcinoma (UDC) cases. The first core of each TMA was made from normal liver tissue to allow proper orientation by the scoring pathologists. TMA slides were cut and stained with H&E to ensure that the all of the desired tissue cores were present.
Immunohistochemical Analysis
LEF-1 immunohistochemistry was performed on all cases (whole slide and TMA) with appropriate positive and negative controls. LEF-1 (rabbit monoclonal EPR2029Y, Abcam cat# ab137872) immunohistochemical stains were performed on FFPE tissues cut in 4 micron sections on the Leica Bond III automated immunostainer (Leica Biosystems, Buffalo Grove, IL). Online deparaffinization was followed by online epitope retrieval using high pH buffer for 20 min. The slides were incubated with the primary antibody at 1:25 dilution for 15 min with no heat. Localization of the antigen–antibody complex was achieved using Leica Bond Polymer Refine DAB Detection kit.
Beta catenin immunohistochemistry was performed on all study cases (whole slide and TMA), with appropriate positive and negative controls. The immunohistochemical stains for beta catenin were performed on formalin- fixed paraffin-embedded tissues cut in 4 µm sections on the Ventana Benchmark Ultra automated immunostainer [Ventana Medical Systems (VMS), Tucson, AZ]. Online deparaffinization was followed by online epitope retrieval using a high pH tris based solution (VMS Ultra CC1) for 32 min. The slides were incubated with the anti-beta catenin primary antibody (mouse monoclonal 14 from BD Biosciences, cat# 610153) at 1:250 dilution for 20 min at 37 °C. Localization of the antigen–antibody complex was achieved using the VMS OptiView DAB detection kit.
Scoring Parameters
LEF-1 was scored based on the overall intensity of the nuclear stain compared to the intensity of a lymphocyte nuclear stain as follows: 0 = no nuclear stain, 1 = nuclear staining present, but intensity less than that of a lymphocyte, 2 = nuclear stain as strong as a lymphocyte. The percentage of positive cells for each staining intensity was also tabulated and a total maximum combined score of 200 was calculated for each case by multiplying the percent of cells by the staining intensity score [for example 20% of cells staining at 1+ and 10% of cells staining at 2+ equals a total score of 40 (20 × 1 + 10 × 2 = 40)]. Only nuclear staining within the pathologic area of interest (within PTC nuclei, within NH nuclei, etc.) was included in the score.
For beta catenin, only nuclear expression was considered positive staining. Diffuse (> 75%) nuclear staining within the neoplasm or area of interest was considered a positive result.
Results
Morphology
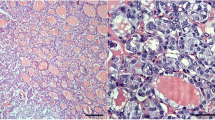
Microscopic examination of all seven whole slide sections of CMV-PTC showed features similar to that previously reported [1–3]: an infiltrative cellular epithelial neoplasm with branching/cribriform growth lacking colloid production and showing focal squamoid morules. The nuclei were enlarged with pale chromatin, nuclear grooves, occasional pseudoinclusions and peculiar nuclear clearing (Fig. 1b).
Immunohistochemistry
Results of LEF-1 immunohistochemistry by total score and mean percentage of cells staining according to intensity is summarized in Table 2.
Six of the 7 (86%) CMV-PTC neoplasms showed patchy to diffuse strong nuclear LEF-1 at 2+ staining intensity (average 75% cells with 2+ staining, range 30–100%) and a total score over 100 (average total score of 172, range 110–200, Fig. 1d). In these six cases, all of the tumor nuclei stained with at least 1+ staining, and all six showed strong and diffuse beta catenin nuclear staining. Interestingly, one CMV-PTC was negative for LEF-1 (2% of cells with 2+ staining, 15% of cells with 1+ staining, total score 19); this case was also negative for beta catenin, and this patient did not have a history of FAP (Fig. 2).
All control PTC cases (100%) lacked LEF-1 staining at 2+ intensity with an average total score of 19 (range 0–95, Fig. 1a, c). The majority had < 25% staining at even 1+ (24 cases) but six cases showed patchy to diffuse 1+ staining (average 77%, range 50–95% 1+ staining, total score average 77, range 50–95). These six cases on morphologic review were from the TMA and were a mix of classical and follicular variants. The five cases of classical PTC with whole section immunohistochemistry performed showed similar findings to that seen in the TMA cohort; none had 2+ staining, and focal patchy 1+ staining was seen in four cases with an average of 18% of cells staining (range 0–60% of cells 1+ staining) and an average total score 18 (range 0–60). Beta catenin was negative in all control cases of PTC.
Patchy focal LEF-1 staining was seen in the benign thyroid follicles in all cases (100%) of chronic lymphocytic thyroiditis (n = 6), (see Fig. 3a, b), with an average total score of 33 (range 10–52). Focal 2+ LEF-1 staining was seen in four cases, ranging from 1 to 10% of cells (average 4%), and focal 1+ staining was seen in all cases, ranging from 15 to 50% of cells (average 27%). Increased LEF-1 staining was seen most prominently in areas adjacent to a prominent lymphoid aggregate. Beta catenin was negative in all cases of chronic lymphocytic thyroiditis.
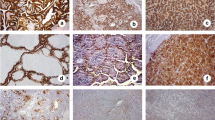
LEF-1 in other benign and malignant cases. Chronic lymphocytic thyroiditis (a H&E, × 200) showing focal strong nuclear LEF-1 staining in follicles adjacent to a lymphoid aggregate (b × 200). Nodular hyperplasia (c H&E, × 200) lacking LEF-1 staining (d × 200). Follicular carcinoma (e H&E, × 200) showing negative LEF-1 staining (f × 200). Undifferentiated thyroid carcinoma (g H&E, × 200) with patchy strong LEF-1 positive nuclear staining (h × 200)
All cases (100%) of NH (n = 25) uniformly lacked LEF-1 staining at 2+ intensity with an average total score of 12 (range 0–100, Fig. 3c, d). The majority had < 25% staining at even 1+ (22 cases) but three cases showed patchy to diffuse 1+ staining (average 80%, range 50–100% 1+ staining, total score average 80, range 50–100). These three cases were from the TMA and on morphologic review showed focal lymphocytic infiltration in the cores adjacent to the hyperplasia. Beta catenin was negative in all cases of NH.
All cases (100%) of normal thyroid (n = 15) had 1% or less LEF-1 staining at 2+ intensity with an average total score of 19 (range 0–42). Nine cases (60%) had patchy 1+ staining ranging from 25 to 40% of normal thyroid follicles. This staining was noted in areas of lymphocytic infiltration, degenerative change and in areas adjacent to other neoplasms. Of note, LEF-1 staining was occasionally seen in endothelial cells and stromal cells, usually focal and at 1+ staining only. Beta catenin was negative in all cases of normal thyroid.
Patchy focal LEF-1 staining was seen in all cases (100%) of Graves’ disease (GD) (n = 6), with an average total score of 33 (range 15–50). Focal 2+ LEF-1 staining was seen in four cases, ranging from 1 to 5% of cells, and focal 1+ staining was seen in all cases, ranging from 15 to 55% of cells (average 30%). Increased LEF-1 staining was seen most prominently in areas adjacent to a prominent lymphoid aggregate. Beta catenin was negative in all cases of GD.
Twenty-four of 25 cases (96%) of FC lacked LEF-1 staining at 2+ intensity with an average total score of 20 (range 0–120, Fig. 3e, f). The majority of FCs had < 25% staining even at 1+ (19 cases). But 6 cases had patchy to diffuse 1+ staining (range 30–90% 1+ staining, total score 30–120). One case of FC had 2+ LEF-1 staining in 20% of tumor cells and 80% of cells with 1+ LEF-1 staining, for a total score of 120, similar to that seen in the cases of CMV-PTC. This case on morphologic review consisted of small closely packed follicles with no unusual histologic features. Beta catenin was negative in all cases of FC, including the case with a LEF-1 total score of 120.
All cases (100%) of HCC (n = 25) lacked LEF-1 staining at 2+ intensity, with an average total score of 17 (range 0–95). The majority had < 25% of cells staining even at 1+ (18 cases), but 7 cases had patchy to diffuse 1+ staining (range 30–95% 1+ staining, total score 30–95). Beta catenin was negative in all cases of HCC.
Overall, the undifferentiated thyroid carcinomas (n = 21) had an average total score of 51 (range 0–120). Twelve undifferentiated thyroid carcinomas (54%) showed at least focal (≥ 5% of cells) 2+ staining with LEF1 (average 20%, range 5–40%), and the majority of the tumors showed patchy to diffuse 1+ staining with LEF1 (average 1+ staining 27%, range 0–90). Two undifferentiated thyroid carcinomas had a total score over 100; one case had 90% of cells with 1+ staining and 10% of cells with 2+ staining for a total score of 110, and the other had 60% of cells with 1+ staining and 30% of cells with 2+ staining for a total score of 120, similar to that seen in the cases of CMV-PTC (Fig. 3g, h). Beta catenin was negative (cytoplasmic or membranous staining only) in all cases of undifferentiated thyroid carcinomas.
Sensitivity and Specificity
The sensitivity and specificity of LEF-1 stain for CMV-PTC was calculated at various thresholds (see Table 3) compared to all the tissue tested (benign and malignant). Calculating a total score is useful for research purposes, but impractical for daily use. The most practical clinical threshold of LEF-1 was ≥ 30% of tumor cells showing nuclear staining with 2+ intensity (similar to a lymphocyte). This has a sensitivity of 85.7% and specificity of 98% for CMV-PTC. In the specific setting of PTC, the sensitivity and specificity of LEF-1 at various thresholds was calculated to differentiate between classical PTC and CMV-PTC (Table 3). Using the same threshold of ≥ 30% of tumor cells with 2+ staining intensity, the sensitivity and specificity of LEF-1 to identify CMV-PTC was 85.7 and 100% respectively. If the LEF-1 and beta catenin negative CMV-PTC case was excluded, then the sensitivity and specificity both increased to 100%.
Discussion
Cribriform morular variant is a rare subtype of papillary thyroid carcinoma usually associated with FAP. The APC gene, a component of the CTNNB1/Wnt pathway, normally inhibits the Wnt pathway [6]. Mutations in the APC or beta-catenin gene leads to activation of Wnt/CTNNB1 pathway with nuclear accumulation of beta-catenin and activation of TCF4 and LEF-1. In this study we document the utility of LEF-1 as an immunohistochemical marker for thyroid neoplasms and especially with respect to CMV-PTC.
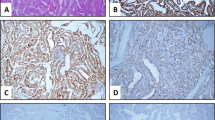
Although CMV-PTC has a distinct morphology, there are overlapping features between conventional PTC and CMV-PTC. Therefore, ancillary studies can be very helpful in establishing the diagnosis. Beta catenin immunohistochemistry has been well established in CMV-PTC [12] with uniform nuclear staining in FAP associated CMV-PTC and only membranous staining in normal thyroid tissue, PTC and other neoplasms. More recent studies [5, 13, 14] have supported the diagnostic role of nuclear beta-catenin in the cytologic diagnosis of CMV-PTC. Ancillary studies also have an important role to play in cases with limited tissue. One case of CMV-PTC in our study (Fig. 4) was composed of a small cyst, which morphologically looked like a classical PTC with squamous metaplasia. However, based on knowledge that the patient had FAP, immunohistochemistry was performed and was strongly positive for beta catenin and LEF-1 supporting a diagnosis of CMV-PTC.
CMV-PTC PTC mimicking a cystic classical PTC. The tumor was mostly cystic (a, b H&E, × 40) with focal projections that appear to have squamous metaplasia (b H&E, × 400). However, strong nuclear beta catenin stain staining (c × 400) and strong nuclear LEF-1 staining (d × 400) identify it as a CMV-PTC
Our results support LEF-1 as an adjuvant marker of CMV-PTC. LEF-1 may be easier to interpret compared to beta catenin as it is a nuclear stain, which lacks background cytoplasmic or membranous staining. Furthermore, the presence of an internal control (strength of lymphocyte staining) makes standardization possible regardless of institutional variation. The best threshold for clinical practice identified in this study was ≥ 30% of tumor cells with 2+ staining intensity, which had a sensitivity of 85.7% and specificity of 98% for CMV-PTC.
Interestingly one case of CMV-PTC lacked both beta catenin and LEF-1 nuclear staining. On morphologic review, this case showed usual features of CMV-PTC (Fig. 2): an unusual morphology with cribriform, papillary architecture and solid growth, and minimal to absent colloid within follicular structures. However, the peculiar nuclear clearing typical of CMV-PTC was not appreciated. This patient did not have a diagnosis of FAP; all other patients with CMV-PTC included in this study did have a clinical diagnosis of FAP. It is difficult to determine if this case should continue to be classified as CMV-PTC based on the H&E morphology or reclassified based on the immunohistochemical findings. For the purposes of this study, this case was kept in the CMV-PTC cohort to prevent bias.
Most other thyroid neoplasms including conventional PTC, FCs and HCCs showed no significant LEF-1 staining at 2+ intensity. One case of FC had strong LEF-1 staining but negative beta-catenin staining which suggests upregulation of Wnt pathway in this tumor, but possibly through a different mechanism. A significant number (54%) of undifferentiated thyroid carcinomas showed at least focal (≥ 5% of cells) 2+ staining with LEF1 and many of the tumors showed diffuse 1+ staining with LEF1. Beta catenin was negative in all cases of undifferentiated thyroid carcinomas. Upregulation of beta catenin has been previously reported in undifferentiated thyroid carcinomas. Garcia-Rostan et al. [12, 16] demonstrated nuclear beta catenin immunofluorescence in 15 of 36 anaplastic carcinoma specimens with focal nuclear expression. Beta catenin mutations were detected in 19 of 31 patients analyzed with many cases showing multiple activating point mutations which might reflect the increased genetic instability in this tumor type. The abnormal expression of beta catenin in undifferentiated thyroid carcinomas appears to be different from FAP associated cases as it was only focally observed in all the carcinomas. However, given the dismal prognosis of undifferentiated thyroid carcinoma, the presence of LEF-1 upregulation, even in a small subset of tumors, raises the possibility of targeted therapy against the Wnt pathway for these patients.
Interpretation of LEF-1 staining in the thyroid must be performed carefully, as we noted focal patchy staining for LEF-1 in both benign processes and in the background stroma/endothelial cells. Increased staining for LEF-1 was seen most commonly in areas with other reactive changes present, including lymphocyte aggregates, tissue injury (ex. aspiration biopsy site) and degeneration. Thus, interpretation of LEF-1 in small biopsies may be less useful, particularly if there is a prominent background thyroiditis.
This study has some limitations. First, performing immunohistochemistry to determine protein expression on a TMA can result in sampling errors, and the majority of tumors examined in this study were included in the TMA. Even though TMA is a very practical and effective tool for research, the small cores of tissue may not be representative of the entire tumor and heterogeneity of protein expression within tumors could lead to sampling errors. It is possible the TMA cases may have falsely high or low scores due to sampling a “patch” of high or low staining. We attempted to control for this by performing immunohistochemistry on whole-tissue section on 39 cases, which showed similar overall results to the TMA. While the whole-tissue section staining did show some protein expression heterogeneity, it was typically on the order of 0–1+ staining, and not 2+ staining (as is typically seen in CMV-PTC) except in areas of marked chronic inflammation, an issue discussed above. Thus, the potential to over diagnose CMV-PTC on the basis of LEF-1 staining is felt to be unlikely, particularly in the appropriate morphologic and clinical setting. A second limitation is that given the previous construction of the TMAs, some tumor subtypes (follicular adenoma, poorly differentiated thyroid carcinoma) were not included in the study. Finally, CMV-PTC cases were diagnosed morphologically but no mutational analysis was performed.
In summary, CMV-PTC is a rare variant of PTC which is associated with familial adenomatosis polyposis and should be recognized to ensure patients are appropriately managed. In this study we have shown LEF-1 immunostain is a highly sensitive and specific marker of CMV-PTC, especially when used in the setting of a PTC neoplasm, and may be easier to interpret than beta catenin. Furthermore, we have demonstrated that the pattern of staining is important and we recommend using a threshold of ≥ 30% of cells showing strong nuclear staining for a positive result to have the highest combined sensitivity and specificity. An unexpected number of anaplastic carcinomas showed positive staining with LEF-1, which may have implications for future therapy.
References
Pradhan D, Sharma A, Mohanty SK. Cribriform-morular variant of papillary thyroid carcinoma. Pathol Res Pract. 2015;211(10):712–6.
Harach HR, Williams GT, Williams ED. Familial adenomatous polyposis associated thyroid carcinoma: a distinct type of follicular cell neoplasm. Histopathology. 1994;25(6):549–61.
Cameselle-Teijeiro J, Chan JK. Cribriform-morular variant of papillary carcinoma: a distinctive variant representing the sporadic counterpart of familial adenomatous polyposis-associated thyroid carcinoma? Mod Pathol. 1999;12(4):400–11.
Boonyaarunnate T, Olson MT, Bishop JA, Yang GC, Ali SZ. Cribriform morular variant of papillary thyroid carcinoma: clinical and cytomorphological features on fine-needle aspiration. Acta Cytol. 2013;57(2):127–33.
Levy RA, Hui VW, Sood R, Fish S, Markowitz AJ, Wong RJ, et al. Cribriform-morular variant of papillary thyroid carcinoma: an indication to screen for occult FAP. Fam Cancer. 2014;13(4):547–51.
Jung CK, Choi YJ, Lee KY, Bae JS, Kim HJ, Yoon SK, et al. The cytological, clinical, and pathological features of the cribriform-morular variant of papillary thyroid carcinoma and mutation analysis of CTNNB1 and BRAF genes. Thyroid. 2009;19(8):905–13.
Kaya S, Gumus M, Gurbuz Y, Cabuk D, Acikgoz O, Temiz S, et al. The prognostic value of beta-catenin and LEF-1 expression in patients with operable gastric carcinoma. Am J Transl Res. 2016;8(2):1228–36.
Tandon B, Peterson L, Gao J, Nelson B, Ma S, Rosen S, et al. Nuclear overexpression of lymphoid-enhancer-binding factor 1 identifies chronic lymphocytic leukemia/small lymphocytic lymphoma in small B-cell lymphomas. Mod Pathol. 2011;24(11):1433–43.
Singhi AD, Lilo M, Hruban RH, Cressman KL, Fuhrer K, Seethala RR. Overexpression of lymphoid enhancer-binding factor 1 (LEF1) in solid-pseudopapillary neoplasms of the pancreas. Mod Pathol. 2014;27(10):1355–63.
Bilodeau EA, Acquafondata M, Barnes EL, Seethala RR. A comparative analysis of LEF-1 in odontogenic and salivary tumors. Hum Pathol. 2015;46(2):255–9.
Kermanshahi TR, Jayachandran P, Chang DT, Pai R. LEF-1 is frequently expressed in colorectal carcinoma and not in other gastrointestinal tract adenocarcinomas: an immunohistochemical survey of 602 gastrointestinal tract neoplasms. Appl Immunohistochem Mol Morphol. 2014;22(10):728–34.
Kurihara K, Shimizu S, Chong J, Hishima T, Funata N, Kashiwagi H, et al. Nuclear localization of immunoreactive beta-catenin is specific to familial adenomatous polyposis in papillary thyroid carcinoma. Jpn J Cancer Res. 2000;91(11):1100–2.
Hirokawa M, Maekawa M, Kuma S, Miyauchi A. Cribriform-morular variant of papillary thyroid carcinoma–cytological and immunocytochemical findings of 18 cases. Diagn Cytopathol. 2010;38(12):890–6.
Koo JS, Jung W, Hong SW. Cytologic characteristics and beta-catenin immunocytochemistry on smear slide of cribriform-morular variant of papillary thyroid carcinoma. Acta Cytol. 2011;55(1):13 – 8.
Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4(7):844–7.
Garcia-Rostan G, Tallini G, Herrero A, D’Aquila TG, Carcangiu ML, Rimm DL. Frequent mutation and nuclear localization of beta-catenin in anaplastic thyroid carcinoma. Cancer Res. 1999;59(8):1811–5.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohindra, S., Sakr, H., Sturgis, C. et al. LEF-1 is a Sensitive Marker of Cribriform Morular Variant of Papillary Thyroid Carcinoma. Head and Neck Pathol 12, 455–462 (2018). https://doi.org/10.1007/s12105-017-0873-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-017-0873-3