Abstract
Objectives
To evaluate the sensitivity and specificity of inferior vena cava (IVC) distensibility index (∆IVC) and respiratory variation in peak aortic blood flow velocity (∆Vpeak) to predict fluid responsiveness in ventilated children with shock and to find out the best cut-off values for predicting fluid responsiveness.
Methods
In this prospective observational study, conducted in a pediatric ICU from January 2019 through May 2020, consecutive children aged 2 mo to 17 y with shock requiring fluid bolus were included. ∆IVC and ∆Vpeak were measured before and immediately after 10 ml/kg fluid bolus administration. ∆IVC and ∆Vpeak were compared between responders and non-responders, defined by a change in stroke volume index (SVI) of ≥10%.
Results
Thirty-seven ventilated children [26 (70.4%) boys] with median age of 60 (36, 108) mo were included. The median (IQR) ∆IVC was 21.7% (14.3, 30.9) and the median (IQR) ΔVpeak was 11.3% (7.2, 15.2). Twenty-three (62%) children were fluid responsive. The median (IQR) ∆IVC was higher in responders compared to non-responders [26% (16.9, 36.5) vs. 17.2% (8.4, 21.9); p = 0.018] and mean (SD) ΔVpeak was higher in responders [13.9% (6.1) vs. 8.4% (3.9), p = 0.004]. The prediction of fluid responsiveness with ΔIVC [ROC curve area 0.73 (0.56–0.9), p = 0.01] and ΔVpeak [ROC curve area 0.78 (0.63–0.94), p = 0.002] was similar. The best cut-off of ∆IVC to predict fluid responsiveness was 23% (sensitivity, 60.8%; specificity, 85.7%) and ΔVpeak was 11.3% (sensitivity, 74%; specificity, 86%).
Conclusions
In this study, authors found that ∆IVC and ΔVpeak were good predictors of fluid responsiveness in ventilated children with shock.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shock can be due to multiple etiologies including hypovolemic, cardiogenic, hemorrhagic and septic [1]. Fluid therapy remains the first step in resuscitation of children with shock [2]. However, not all children with shock respond to fluid bolus administration. The adverse consequences of overzealous fluid administration and fluid overload are well studied and lead to multiple complications including prolonged ventilation and increased mortality [3, 4].
Predicting fluid responsiveness is a challenging task in the intensive care unit and currently various parameters are in use for the same. The utility of static parameters including central venous pressures (CVP) and pulmonary capillary wedge pressure (PCWP) is limited. Dynamic parameters are a result of physiological heart lung interactions and are thought to be better predictors of fluid responsiveness [5]. However, there is no single gold standard method for predicting fluid responsiveness.
IVC distensibility index (∆IVC) and respiratory variation in peak aortic blood flow velocity (∆Vpeak) are two common parameters used for predicting fluid responsiveness. These are measured by transthoracic echocardiography and are simple and easily available at the bedside. Gan et al., in a systematic review, found that only ∆Vpeak was able to predict fluid responsiveness whereas other parameters including ∆IVC did not predict fluid responsiveness [6]. However, ∆IVC is one of the commonly assessed parameter at the bedside in an emergency setting requiring lower skill and expertise than that is required in assessing ∆Vpeak. There is conflicting evidence on the utility of ∆IVC to predict fluid responsiveness and data is evolving on the utility of these parameters. Such data will inform the clinicians and researchers on the utility and reliability of these parameters in assessing volume status and fluid responsiveness at the bedside. The objective of the study was to evaluate the sensitivity and specificity of ∆IVC and ∆Vpeak to predict fluid responsiveness in children with shock.
Material and Methods
This prospective observational study was conducted between January 2019 and May 2020, in the Pediatric Intensive Care Unit (PICU), AIIMS, New Delhi. The study protocol was approved by Institute ethics committee of AIIMS, New Delhi (IECPG-211/23.08.2017, RT-40/28.09.2017). Written informed consent was taken from parents/guardian before enrollment.
Children aged 2 mo to 17 y with shock requiring fluid bolus administration were screened for inclusion. Children with rhythm disturbances, congenital heart disease, on high frequency ventilation, and those with increased intrabdominal pressure were excluded. Children with poor image window on echocardiography were also excluded. All eligible children were screened and were included in the study.
The primary outcome was to evaluate the sensitivity and specificity of ∆IVC to predict fluid responsiveness in children with shock. The secondary outcomes were to evaluate the sensitivity and specificity of ∆Vpeak to predict fluid responsiveness, to identify the best cut-off value of ∆IVC and ∆V peak to predict fluid responsiveness and to identify the proportion of children responding to fluid bolus administration.
All consecutive mechanically ventilated children with shock requiring fluid bolus administration were enrolled after screening for exclusion criteria. Tissue hypoperfusion was defined by the presence of two or more of the following – altered mental status, capillary refill time greater than 2 s, cool mottled extremities or flash capillary refill, diminished pulses or bounding peripheral pulses, decreased urine output <1 ml/kg/h (previous 6 h) and/or low blood pressure <5th centile [2, 7]. Patient details including age, demographic details, clinical presentation were recorded. Hemodynamic variables including heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean blood pressure (MBP), capillary refill time, sensorium and urine output were recorded. Intra-abdominal pressure was measured through Foley catheter using two three-way adapters and pressure transducer and children with elevated intra-abdominal pressure (>10 mm Hg) were excluded [8]. All children were ventilated to target a tidal volume of 8–10 ml/kg (as per unit protocol) to maintain adequate oxygenation. As per unit policy, children were sedated with midazolam and fentanyl. Utmost care was taken to identify children with spontaneous respiratory efforts and neuromuscular paralysis was used in these children as required.
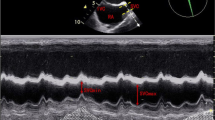
Echocardiographic parameters were recorded using Philips Ultrasound CX-50 machine (Philips Ultrasound, Bothell, Washington), using S5-1 probe. The echocardiographic parameters were assessed by primary investigator (KKB) and are approved in a blinded manner by a co-author (DK). Assessment of IVC parameters was done in the subcoastal view. After attaining a good quality image, IVC diameter (IVCD) was measured at 2 cm from the right atrial margin at end inspiration and end expiration. IVC distensibility index was measured using the formulae: IVCDinspiration – IVCDexpiration / IVCDexpiration × 100. Three readings were taken during three consecutive respiratory cycles and the mean was calculated [9].
Velocity time integral (VTI) and respiratory variation in peak aortic blood flow (∆V peak) velocity were measured in the apical five chamber view. After attaining a good quality image, using pulse-wave doppler, aortic blood flow was recorded at the level of aortic annulus. VTIao was measured as a mean of three consecutive measurements over a single respiratory cycle. Stroke volume (SV) was measured using the formula: VTIao × aortic area. Aortic diameter (D) was measured in parasternal long axis view and aortic area was measured by the formula π × (D2)/4. Stroke volume index (SVI) was measured using the formula: Stroke volume/ Body surface area. ∆Vpeak was calculated as the percentage change in peak aortic blood flow velocity in one respiratory cycle. ∆Vpeak was calculated using the formula: (Vpeakmax –Vpeakmin)/[(Vpeakmax + Vpeakmin)/2] × 100 where Vpeakmax and Vpeakmin are the maximum and minimum aortic flow peak velocities respectively. The mean ∆Vpeak was measured for three consecutive breaths [10].
Enrolled children were given crystalloid bolus at 10 ml/kg over 20 min. Hemodynamic variables and echocardiographic variables were recorded just before the administration of fluid bolus and immediately after the completion of the fluid bolus. Fluid responsiveness was defined by an increase of SVI by ≥10% after fluid bolus administration.
The authors planned to enroll all the children with shock requiring fluid boluses in pediatric ICU with no exclusion criteria during the study period. In authors’ PICU average number of children admitted per year with shock is 80. The authors targeted a sample size of 40 during the study period based on high number of exclusion criteria.
Data were entered into Microsoft Excel and analyzed using Stata 13 (StataCorp, College Station, TX). Categorical variables are presented as numbers (%), while continuous variables are presented as mean (SD), if normally distributed and median (IQR), if non-normally distributed. Tests of statistical significance were applied based on the type of variables. Variables before and after intervention were compared using paired ‘t’ test if normally distributed and by Wilcoxon matched-pairs test, if non-normally distributed. Continuous variables, if normally distributed were analyzed using ‘t’ test and if not normally distributed, were compared using Wilcoxon rank-sum test. ROC curves were plotted for ∆IVC and ∆Vpeak. The best cut-off values with best sensitivity and specificity were calculated using Youden’s index. Linear correlation was tested using spearman rank method. A p value less than 0.05 was taken as significant.
Results
A total of 109 children were screened of which 63 were ventilated. Of these 26 were excluded as per prespecified exclusion criteria (Fig. 1). A total of 37 mechanically ventilated children were enrolled during the study period. The median age was 60 (36, 108) mo and 70% were boys. The most common focus of infection was central nervous system (27%) followed by respiratory infection (22%). Of the enrolled children, 16 (43.2%) had hypotensive shock and 21 (56.8%) had compensated shock. The median (IQR) ∆IVC of the study population was 21.7% (14.3, 30.9) and the median (IQR) ΔVpeak was 11.3% (7.2, 15.2). Other key baseline characteristics are summarized in Table 1.
For the entire study population, there was a significant change in the heart rate, systolic blood pressure, diastolic blood pressure and mean blood pressure after volume expansion (Table 2). There was a significant change in ∆IVC and ΔVpeak after volume expansion (Table 2). Stroke volume increased from 11.6 ml (9.1, 22.7) to 14.1 ml (11.1, 26.9) (p < 0.001) after volume expansion for the whole study population (Table 2).
Twenty-three (62%) children were responsive to fluid bolus as per the study definition and 14 (38%) did not respond. The baseline characteristics including heart rate, blood pressures were similar in responders and non-responders (Supplementary Table S1). The median (IQR) ∆IVC was higher in responders when compared to non-responders [26% (16.9, 36.5) vs. 17.2% (8.4, 21.9); p = 0.01]. Also, the mean (SD) ΔVpeak was significantly higher in responders [13.9% (6.1) vs. 8.4% (3.9), p = 0.004]. In fluid responders, there was a significant change in the heart rate, systolic blood pressure, diastolic blood pressure and mean blood pressure after volume expansion (Table 3). Also, there was a significant change in ∆IVC [from 26% (16.9, 36.5) to 15.8% (10.1, 23.9), p = 0.001] and the ΔV peak [from 15% (10.1, 16.5) to 8% (5.5, 11.1); p <0.001]. In comparison, parameters including heart rate, blood pressure, IVC distensibility index and ΔVpeak were not different after volume expansion in non-responders (Table 3). Of the enrolled children, 28 (75%) children required further fluid boluses and 25 (67%) required vasoactive agents during the hospital stay.
The prediction of fluid responsiveness with ΔVpeak [ROC curve area 0.78 (95% CI: 0.63–0.94), p = 0.002] and IVC distensibility index [ROC curve area 0.73 (95% CI: 0.56–0.9). p = 0.01) was similar (Fig. 2). There was a moderate correlation of pre-bolus ΔVpeak with percentage increase in stroke volume index after volume expansion (Rho = 0.42, p = 0.008). However, there was only weak correlation of pre-bolus ∆IVC with percentage increase in stroke volume index (Rho = 0.26, p = 0.1). The best cut-off of ΔVpeak to predict fluid responsiveness was 11.3% which had a sensitivity of 74% and specificity of 86%. The best cut-off of ∆IVC to predict fluid responsiveness was 23% which had a sensitivity and specificity of 60.8% and 85.7%, respectively. There was no difference in ventilator parameters including tidal volume, peak inspiratory pressure and positive end expiratory pressure between fluid responders and non-responders (Supplementary Table S1).
Discussion
In this prospective observational study assessing the utility of dynamic parameters to assess fluid responsiveness in children with shock, authors found that ΔVpeak and ∆IVC are reliable predictors of fluid responsiveness in mechanically ventilated children.
Fluid therapy remains crucial in the resuscitation of children with shock. Static parameters have long been used to predict fluid responsiveness in adults and children with shock. However, these have limitations and recent evidence showed that they are not reliable indicators of fluid responsiveness [5, 11]. Dynamic parameters are a result of cardiopulmonary interactions and reflect the changes in cardiac output/stroke volume with mechanical ventilation. These are more prominent in hypovolemic state. Various dynamic parameters including IVC distensibility index and respiratory variation in peak aortic blood flow velocity are being used for predicting fluid responsiveness. However, there is no single best parameter available for predicting fluid responsiveness and is an area of ongoing research.
Various studies have found that ΔVpeak is a useful predictor of fluid responsiveness and cut-off values of 12–13% (range: 7–20%) are most commonly used [12]. Wang et al. in a recent systematic review including 302 children found that ΔVpeak has good ability to predict fluid responsiveness with a sensitivity and specificty of 89% and 85% respectively with summary ROC of 0.91 (0.88–0.93) [13]. They also found that in children less than 25 mo, the predictive ability of ΔVpeak was low compared to older children. Gan et al., in a systematic review evaluating the predictors of fluid responsiveness in children, found that ΔVpeak was the best parameter to predict fluid responsiveness [6]. In the present study, authors found that, a ΔVpeak cut-off of 11.3% had a sensitivity and specifity of 74% and 86%, respectively with AUC of 0.78 (0.63–0.94) which is lower compared to previous studies. This could be because of multiple reasons. A greater proportion of children (75.6%) included in present study had septic shock. These children could have had early cardiac dysfunction (systolic or diastolic) which could have affected the results. About 25% of children in present study were <2 y old which could have affected the results as highlighted by Wang et al. Also, a greater proportion of children (67%) had underlying illness which could have affected the response to acute illness. Although a useful parameter, measuring ΔVpeak has some limitations. It requires technical expertise and is operator dependent. It is also affected by various parameters including tidal volume, presence of rhythm abnormality, heart rate/ventilatory rate ratio and the timing of measurement. Also, the question of optimal cut-off for predicting fluid responsiveness still remains unclear.
IVC distensibility index is a commonly used parameter to predict fluid responsiveness in ventilated children. It is easily available at bedside, requires minimal training and is non-invasive. Although initial studies have shown that it is a useful marker for predicting fluid responsiveness, subsequent studies found mixed results [14, 15]. Si et al. in their systematic review, have found that IVC distensibility index has a moderate predictive ability to predict fluid responsiveness with a sensitivity and specificity of 75% and 82% respectively [16]. In another systematic review, Xavier Filho et al. have observed that the cut-offs used varied widely (10–20.5%) with varying sensitivity and specificity [17]. Weber et al., in 31 pediatric patients, found that IVC distensibility index did not predict fluid responsiveness with AUC of 0.50 (0.29–0.71) [15]. Assessment of IVC distensibility index has some limitations. It is not reliable in conditions of increased abdominal pressure. It is difficult to measure in very young children, in patients with spontaneous efforts and in children with poor quality image.
In mechanically ventilated children undergoing surgery, ΔVpeak and ∆IVC were found to be reliable predictors of fluid responsiveness [9]. Choi et al. in post-op cardiac children found that both ΔVpeak and IVC distensibility index are reliable predictors of fluid responsiveness [18]. However, Byon et al. have found that ΔVpeak is a reliable predictor and IVC distensibility index was not a reliable predictor of fluid responsiveness in children [19]. In the present study in critically ill children with shock, authors found that ΔVpeak and IVC distensibility index almost had similar ability to predict fluid responsiveness in critically ill children with AUC of 0.78 (0.63–0.94) and 0.73 (0.56–0.9) respectively. The cut-off with best sensitivity and specificity for ΔVpeak was 11.3% and for IVC distensibility index was 23%. With wide range of cut-offs as observed in various studies, large prospective studies with larger sample size are required to find out the best cut-off values for these parameters.
The present study has a few limitations. First, the sample size is small. Second, all the echocardiographic measurements were done by a single investigator; potential for introducing operator bias. However, the authors used a mean of three measurements which would have decreased the bias. Also, the images were reviewed by a second investigator which minimized the bias. Third, a greater proportion of children had underlying illness which could have affected the response to fluid therapy.
Conclusions
In this prospective study, authors found that ΔIVC and ΔVpeak reliably predicted fluid responsiveness in critically ill mechanically ventilated children with shock.
References
Yager P, Noviski N. Shock. Pediatr Rev. 2010;31:311–9.
Davis AL, Carcillo JA, Aneja RK, et al. American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med. 2017;45:1061–93.
Alobaidi R, Morgan C, Basu RK, et al. Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:257–68.
Raina R, Sethi SK, Wadhwani N, Vemuganti M, Krishnappa V, Bansal SB. Fluid overload in critically ill children. Front Pediatr. 2018;6:306.
Cherpanath TGV, Geerts BF, Lagrand WK, Schultz MJ, Groeneveld ABJ. Basic concepts of fluid responsiveness. Neth Heart J. 2013;21:530–6.
Gan H, Cannesson M, Chandler JR, Ansermino JM. Predicting fluid responsiveness in children: a systematic review. Anesth Analg. 2013;117:1380–92.
Kleinman ME, Chameides L, Schexnayder SM, et al. Part 14: pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S876-908.
Thabet FC, Ejike JC. Intra-abdominal hypertension and abdominal compartment syndrome in pediatrics. a review. J Crit Care. 2017;41:275–82.
Achar SK, Sagar MS, Shetty R, et al. Respiratory variation in aortic flow peak velocity and inferior vena cava distensibility as indices of fluid responsiveness in anaesthetised and mechanically ventilated children. Indian J Anaesth. 2016;60:121–6.
Feissel M, Mangin I, Ruyer O, Faller JP, Michard F, Teboul JL. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest. 2001;119:867–73.
Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? an updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41:1774–81.
Desgranges FP, Desebbe O, Pereira de Souza Neto E, Raphael D, Chassard D. Respiratory variation in aortic blood flow peak velocity to predict fluid responsiveness in mechanically ventilated children: a systematic review and meta-analysis. Pediatr Anesth. 2016;26:37–47.
Wang X, Jiang L, Liu S, Ge Y, Gao J. Value of respiratory variation of aortic peak velocity in predicting children receiving mechanical ventilation: a systematic review and meta-analysis. Crit Care. 2019;23:372.
Kim DW, Chung S, Kang WS, Kim J. Diagnostic accuracy of ultrasonographic respiratory variation in the inferior vena cava, subclavian vein, internal jugular vein, and femoral vein diameter to predict fluid responsiveness: a systematic review and meta-analysis. Diagnostics (Basel). 2021;12:49.
Weber T, Wagner T, Neumann K, Deusch E. Low predictability of three different noninvasive methods to determine fluid responsiveness in critically ill children. Pediatr Crit Care Med. 2015;16:e89.
Si X, Cao D, Xu H, Guan X. Meta-analysis of ventilated versus spontaneously breathing patients in predicting fluid responsiveness by inferior vena cava variation. Int J Clin Med. 2018;9:760–77.
Xavier Filho DG, Coutinho ALN, Barbosa RH de A, Lopes MR, Tenório AP de O. Inferior vena cava ultrasound for assessing volume status and fluid responsiveness in critically ill patients: a systematic review. Arq Bras Cardiol Imagem Cardiovasc. 2021;34:eabc193.
Choi DY, Kwak HJ, Park HY, Kim YB, Choi CH, Lee JY. Respiratory variation in aortic blood flow velocity as a predictor of fluid responsiveness in children after repair of ventricular septal defect. Pediatr Cardiol. 2010;31:1166–70.
Byon HJ, Lim CW, Lee JH, et al. Prediction of fluid responsiveness in mechanically ventilated children undergoing neurosurgery. Br J Anaesth. 2013;110:586–91.
Acknowledgements
The authors are thankful to PICU senior residents and junior residents for their contribution to the conduct of the study.
Author information
Authors and Affiliations
Contributions
KKB, JS conceptualized the study, enrolled patients, analyzed the data and drafted the manuscript; DK conceptualized the study, verified all the echocardiographic data and helped with the manuscript; PG helped with patient enrollment, collected data and provided inputs to the manuscript; MP helped with statistical analysis and provided inputs to the manuscript; SKK and RL provided critical inputs to the manuscript and supervised the study. SKK will act as the guarantor for this manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Banothu, K.K., Sankar, J., Pathak, M. et al. Utility of Inferior Vena Cava Distensibility and Respiratory Variation in Peak Aortic Blood Flow Velocity to Predict Fluid Responsiveness in Children with Shock. Indian J Pediatr 90, 1077–1082 (2023). https://doi.org/10.1007/s12098-023-04585-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-023-04585-x