Abstract
Objectives
To describe the diagnostic test properties of Cardiac Troponin-T (cTnT) in predicting myocardial dysfunction in asphyxiated term neonates by taking echocardiography as the gold standard and to establish the optimum cut-off values of cTnT for myocardial dysfunction, shock, severe hypoxic ischemic encephalopathy (HIE) and mortality by receiver operator characteristic (ROC) curve analysis.
Methods
This was a prospective study based on diagnostic test evaluation. The study included 120 term asphyxiated neonates in a tertiary care neonatal intensive care unit (NICU) in Southern India from June 2011 through June 2015. All the neonates were clinically evaluated. Venous blood was taken at 4 h of life for cTnT estimation. Echocardiography was done within 24 h of birth.
Results
The mean cTnT level of asphyxiated term neonates was 0.207±0.289 ng/ml (mean ± SD). Asphyxiated neonates with myocardial dysfunction had higher cTnT levels (0.277±0.231) as compared to those without myocardial dysfunction (0.061±0.036, p = 0.0001). Using ROC curve, the cut-off cTnT values for myocardial dysfunction was 0.1145 ng/ml with sensitivity 92.4% and specificity 94.1%. Cardiac Troponin-T levels were significantly higher among asphyxiated neonates with shock (0.378±0.348, p = 0.0001) and the levels also correlated positively with increasing grades of HIE. The cut-off cTnT value for mortality was 0.2505 ng/ml with sensitivity 83.9% and specificity 96.6%.
Conclusions
In asphyxiated term neonates, early cTnT elevation is a marker for predicting myocardial dysfunction and elevated cTnT levels had high sensitivity and specificity. There was significant relation with increasing cTnT values and increasing grades of HIE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite advancement in perinatal and neonatal intensive care in the last two decades, perinatal asphyxia still remains a major cause of mortality and morbidity in India, accounting for 23% of neonatal deaths [1]. Asphyxial event can compromise the function of many vital organs like brain, heart and kidneys and progress to hypoxic ischemic encephalopathy (HIE), cardiac dysfunction and multiorgan damage. Cardiac dysfunction results from hypoxic ischemic injury to subendocardial tissue, papillary muscle and myocardium [2]. Neurological dysfunction is usually the most obvious clinical presentation which distracts our attention from diagnosing associated myocardial dysfunction. The incidence of clinical cardiac dysfunction (shock) varies from 25% to 60% [3]. This myocardial dysfunction is a contributing factor to the postnatal neurological damage resulting in adverse neurological outcome and increased mortality. Hence appropriate targeted treatment for myocardial dysfunction is essential to improve the neurological outcome.
Cardiac Troponin-T (cTnT) is a highly sensitive and specific biochemical marker of myocardial injury which helps in early detection of myocardial damage [4]. cTnT is a useful early marker of cardiac injury in newborns and its levels are related to severity of asphyxia [5]. cTnT has a high positive predictive value in postnatal diagnosis of asphyxia [6]. Elevated cTnT levels are found in HIE which can lead to poor outcome due to myocardial damage [7]. But, cTnT is detectable in the blood of many healthy neonates. It could be that the perinatal period causes minimal myocardial compromise and the rise may be a result of perinatal cardiovascular remodeling, in the course of adapting to extra uterine life. Also, neonatal cTnT values are unlikely to be influenced by maternal levels since T subunit has a molecular mass of 37 kDa and is therefore too large to diffuse freely across the placenta [8]. Sick neonates have significantly higher levels than healthy neonates [9]. The Troponins appear in blood 2 to 4 h after myocardial injury and remain detectable for up to 21 d. Previous studies in neonates have used creatine kinase isoforms as biochemical indices of myocardial injury. However these markers have been largely discarded because gestation, mode of delivery, sex and birth weight all can affect creatine kinase levels [10, 11]. But, the cTnT levels are unaffected by gestation, birth weight, sex and mode of delivery [12].
Echocardiographic evaluation substantiates the findings of myocardial dysfunction in birth asphyxia [13]. cTnT also benefit centres without onsite echocardiographic machines, with evidence showing good correlation with echo derived markers of myocardial function [14]. cTnT can be used as a screening test in diagnosing myocardial dysfunction in term asphyxiated neonates especially in resource limited situations where facilities for echocardiography are not available. Early detection of myocardial injury in neonates with HIE will help in early treatment and can also predict morbidity and mortality. Therefore the present study was planned to explore and assess the diagnostic role of cTnT as a biomarker for myocardial dysfunction in term asphyxiated neonates.
Material and Methods
This was a prospective study conducted at a tertiary level neonatal intensive care unit (NICU) of Government Medical College Thiruvananthapuram from June 2011 through June 2015 (4 years). The study was approved by the Institute Ethics Committee and written informed consent was obtained from the parents of each neonate. One hundred twenty term asphyxiated neonates satisfying the inclusion criteria for the study were enrolled. Asphyxia is indicated by any 4 of the following [3];
-
1.
5 min APGAR ≤ 6/ 10
-
2.
Meconium stained amniotic fluid
-
3.
pH within 1 h < 7. 2
-
4.
Changes in fetal heart rate
-
5.
Evidence of neurological or multiorgan dysfunction
Preterm neonates <37 wk, those with major malformations, chromosomal anomalies, intrauterine infections/ sepsis and major structural heart disease were excluded.
The neonates were resuscitated according to National Resuscitation Program (NRP) guidelines and were admitted in the NICU. Myocardial involvement was assessed by monitoring the heart rate, capillary filling time, blood pressure and cardiac murmurs. The severity of HIE was graded based on Sarnat & Sarnat staging. They were managed in NICU as per hospital protocol. None of the neonates underwent therapeutic hypothermia at authors’ institute. Venous blood was taken for cTnT at 4 h of birth and was estimated by electro chemiluminescent sandwich enzyme linked immunosorbent assay (ECLIA) with ECLIA machine manufactured by HITACHI High - Technologies Corporation, Tokyo Japan having product name – Cobras e 411. It has a lower limit of detection of 0.01 ng/ml, with minimal cross reactivity with cTnI (0.002%) and skeletal Troponin T (0.001%). The coefficient of repeatability for paired samples was less than 10% and the coefficient of variation for precision analysis was 6.4%. The cTnT value of more than 0.1 ng/ml was taken as positive. Echocardiography was done within 24 h of birth using Power vision 6000, TOSHIBA with a 3.75 MHz probe incorporating pulsed wave and continuous wave Doppler by a Pediatric cardiologist. Myocardial dysfunction was assessed by American Society of Echocardiography (ASE) standards [15] as given in Table 1.
All neonates were followed up till death or survival in the NICU.
The sample size was calculated using n- Master sample size software developed by CMC Vellore. The sensitivity of the new test is 80%.
Sensitivity of the reference test (Gold standard – echocardiographic diagnosis) is 95% with Alpha error 5%, (1 – β) 80%. With the above values, using two tale test, the sample size came to 75: cases of myocardial dysfunction in birth asphyxia. Considering the 60% incidence of myocardial dysfunction in perinatal asphyxia, the total sample size of birth asphyxia neonates would come to approximately 120. Analysis was done using SPSS version 20 statistical software. Diagnostic test evaluation was done to find out the sensitivity and specificity of cTnT in diagnosing myocardial dysfunction taking echocardiographic evidence of myocardial dysfunction as the gold standard. Independent sample student T test was used to compare the mean cTnT values in myocardial dysfunction, shock, HIE and mortality. P value <0.05 taken as statistically significant. Receiver operator characteristic (ROC) curve was constructed to find out the optimum cut-off value of cTnT in myocardial dysfunction, shock, HIE 3 and death. Analysis of variance (ANOVA) was used to find out the relation of cTnT between the groups of HIE.
Results
A total of 120 term neonates with mean gestation (SD) of 37(0.73) wk and mean (SD) birth weight of 2.816(0.61) kg fulfilled the inclusion criteria and were enrolled in the study. Of the total, 40% were male neonates and 45% were born by cesarean section. All neonates required positive pressure ventilation and 85% of them required endotracheal intubation in the delivery room. The median (IQR) 5 min Apgar score was 5(4, 6) and mean (SD) pH at 1 h was 7.136(0.112). Antenatal complications were seen in 48% mothers. Natal complications were present in 77.5% babies with meconium stained amniotic fluid in 37.5%.
All babies had respiratory distress and 43.3% were ventilated. Clinical shock was present in 55.8% of the babies and all required ionotropic support. No HIE was seen in 26 babies (21.7%), 23 babies had HIE 1 (19.2%), 33 had HIE 2 (27.5%) and 38 had HIE 3 (31.6%). cTnT values were positive (value >0.1 ng/ml) in 64.2% (n = 77) of asphyxiated term neonates and echocardiographic evidence of myocardial dysfunction was noted in 55% (n = 66). The highest value of cTnT was 2.380 ng/ml. Echocardiography could not be done for three babies as they were critically ill and could not be shifted for echocardiography and died within 12 h of birth. Out of the total 120 babies, 31 babies died (25.8%) as against 89 babies (74.2%) who survived.
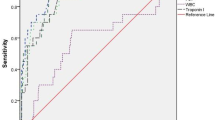
Using diagnostic test evaluation, the sensitivity of cTnT in diagnosing myocardial dysfunction (gold standard as echocardiography and cut-off cTnT as 0.1 ng/ml) was 92.4%; specificity 74.5%; positive predictive value 82.4%; negative predictive value 88.3%; positive LR ratio 3.62 (Table 2). Using ROC curve analysis, cut-off cTnT of 0.1145 ng/ml, increased the specificity to 94.1% with sensitivity remaining at 92.4% (Fig. 1). Using ROC curve analysis, a cut-off cTnT of 0.1345 ng/ml had 83.6% sensitivity and 94.3% specificity for diagnosing clinical shock (Fig. 2). For mortality, a cut-off cTnT of 0.2505 ng/ml had a sensitivity of 83.9% and specificity of 96.6 % (Fig. 3). For severe HIE (HIE 3), a sensitivity of 81.6% and specificity of 91.5% with a cut-off cTnT of 0.188 ng/ml was found (Fig. 4).
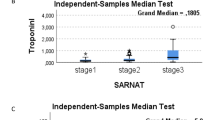
The mean cTnT value in birth asphyxia was 0.207 ± 0.289 (Fig. 5). The mean with standard deviation and p value of cTnT with respect to myocardial dysfunction, shock, HIE and outcome is shown in Table 3. The mean cTnT values increased with increasing grades of HIE; No HIE (0.035±0.013); HIE 1 (0.089±0.042); HIE 2 (0.150±0.0464); HIE 3 (0.447±0.418) [F = 19.879, P = 0.0001] (Table 3).
The box plots shown in Fig. 6 show cTnT in term asphyxiated newborns with and without myocardial dysfunction, with and without clinical shock, mortality and survival and in different grades of HIE.
Discussion
In this study, the mean cTnT value in birth asphyxia was 0.207 ± 0.289 ng/ml. There was statistically significant difference in the mean cTnT values in asphyxiated neonates with myocardial dysfunction (0.277±0.231 ng/ml) compared to asphyxiated neonates without myocardial dysfunction (0.061 ± 0.036 ng/ml) [t = − 7.505; p = 0.0001]. There are several studies published on the behavior of cTnT in newborns, in different clinical situations, and its value in diagnosing cardiovascular dysfunction [3, 5, 9]. According to Rajkumar et al., the mean cTnT in asphyxiated neonates was 0.22 ± 0.28 ng/ml as against controls 0.003 ± 0.018 ng/ml (P < 0.001) [3]. Szymankiewicz et al. tried to relate the cTnT to echocardiographic findings of myocardial damage. Two-dimensional Doppler and color Doppler studies were performed and evaluated fractional shortening (FS), cardiac output (CO), cardiac index (CI), tricuspid (TI) and mitral (MI) insufficiency. The asphyxiated neonates had higher levels of cTnT (0.141 ± 0.226 vs. 0.087±0.111 ng/ml; P < 0.01) and TI (38.5 vs. 11.4% of population; p < 0.05) compared to healthy infants. CO, CI and FS remained in the same range [16]. Costa et al. reported that cTnT levels were found to be elevated in neonates with echocardiographic evidence of myocardial dysfunction. cTnT was higher in asphyxiated vs. controls [0.15(0.10–0.23) and 0.05(0.02–0.13) respectively with P < 0.001]. In asphyxiated neonates with myocardial damage, cTnT was 0.20(0.11–0.28) against 0.11(0.05–0.14)ng/ml in asphyxiated neonates without myocardial damage [17]. In a study by Awada et al., healthy neonates had cTnT 0.044(0.027–0.073 ng/ml) and sick infants 0.121(0.065–0.238) [18]. These studies reinforce the utility of cTnT serving as a marker for myocardial dysfunction in asphyxiated neonates.
In the present study, there was also statistically significant difference in the mean cTnT values in neonates with shock (0.318±0.345 ng/ml) compared to those without shock (0.068±0.046 ng/ml). This shows the increasing cTnT values with clinical shock paralleling with increasing myocardial injury. According to Clark et al., the hypotensive neonates had significantly higher cTnT than normotensive [0.258(0.163–0.514) vs. 0.105(0.069–0.174)ng/ml, P < 0.0001] [12]. Trevisanuto et al. observed a significant relationship of cTnT with the use of vasopressors, mechanical ventilation, and high oxygenation index in patients with respiratory distress syndrome. The mean cTnT was 0.38 ng/ml in patients with respiratory distress vs. 0.13 ng/ml in patients without respiratory distress (P < 0.01) [19].
In the present study, the mean cTnT values for babies who died were significantly higher (0.499±0.447) than those who survived (0.106±0.069) [t = − 4.879; P = 0.0001]. So, cTnT could serve as an early marker for predicting mortality. Boo et al., did find that cTnT levels were significantly higher in term asphyxiated neonates who did not go on to survive (P < 0.0001), thereby suggesting that cardiac troponins can be used to predict short term outcome. According to him, serum cTnT of asphyxiated infants with low ejection fraction <60% was significantly higher at 12 and 24 h than those with normal ejection fraction (P < 0.05) [20]. Kanik et al. reported that survivors of neonatal encephalopathy had significantly lower cardiac Troponin I (cTnI) levels at days 1 and 3. cTnI showed 33% sensitivity and 80% specificity in predicting mortality [21]. Alexandra et al. suggested that cTnI is a more sensitive marker for prediction of death in patients with perinatal asphyxia. Cut-off value for lethal outcome for cTnI blood level was 0.135 μg/l (Sensitivity 0.81; specificity 0.72) [22]. Matter et al. reported that a cTnT cut-off of 0.15 microgm/L gave a specificity of 100% and a sensitivity of 70% in predicting mortality in asphyxiated neonates [23].
In this study, the mean cTnT values increased with increasing grades of HIE. According to Gunes et al., term neonates with severe asphyxia (HIE 3) had significantly higher cTnT than HIE 1 and 2 neonates and healthy neonates within first 2 to 4 h of life; (HIE 3: 0.34 ± 0.21 ng/ml); (HIE 2: 0.12 ± 0.07 ng/ml); (HIE 1: 0.07 ± 0.03 ng/ml); (Healthy: 0.04 ± 0.02 ng/ml) [5]. Other authors have also noted similar relation of increasing cardiac troponin levels with increasing severity of asphyxia [7, 22].
Due to the absolute specificity of cardiac Troponins regarding the myocardial tissue and its high sensitivity, the American College of Cardiology and the European Society of Cardiology recommended cardiac Troponin as the best biological marker for myocardial injury [24]. cTnT and cTnI are used to detect myocardial compromise in the newborn, although the use of cTnI as a marker in newborns is debated [25]. Advantages of cTnT over cTnI are that cTnI is more prone to posttranslational modification and these modifications may prevent some antibodies used in the assay system from binding to the molecules and diminishes the signal. Another possible concern is the ontogeny of troponins. Bodor et al. showed expression of cTnT in skeletal muscles up to 20 wk of gestation. However they could not show cTnT in mature human skeletal muscle by western blot [26]. Sasse et al. showed that, at 38 wk gestation, 75% of TnI in human myocardium may be the slow twitch skeletal type. This falls by 50% by 12 wk postnatal age, and only by 8 mo postnatal age, cTnI is expressed in human myocardium [27]. So cTnT assay was preferred and used in present study. The value of 0.1 ng/ml as a cut-off was chosen according to the studies conducted by Clark et al., Costa et al. and Adamcova et al. in newborns [12, 17, 28].
By diagnostic test evaluation, the sensitivity and specificity of cTnT for predicting myocardial dysfunction were found to be 92.4% and 74.5% respectively. Using ROC curve analysis, cut-off cTnT of 0.1145 ng/ml, increased the specificity to 94.1%, sensitivity remaining at 92.4%. In a study by Clark et al., the median IQR cTnT in healthy infants was 0.025(0.01–0.062)ng/ml, and the 95th centile was 0.153 ng/ml [12]. Adamcova et al. evaluated cord blood cTnT in healthy term newborns and found plasma concentrations of 0.05±0.04 ng/ml in 65% neonates studied. Among these, 50% showed higher concentrations of cTnT (0.19±0.07 ng/ml) [28]. These could explain the higher specificity obtained by raising the cTnT cut-off to 0.1145 ng/ml.
The main limitation in the present study was lack of healthy controls and the mean cTnT of healthy term neonates. The other limitations were:
-
(a)
Serial monitoring of Cardiac Troponin-T values was not done.
-
(b)
The technique of Doppler Tissue Imaging (DTI) and usage of Tei index could not be put to use due to its unavailability in authors’ hospital. The advent of these new methods have drastically improved the interpretation of myocardial performance [22, 23].
-
(c)
Other reasons for myocardial dysfunction in an asphyxiated neonate such as sepsis, Persistent Pulmonary Hypertension of the Newborn (PPHN) and multiorgan dysfunction especially Acute Kidney Injury (AKI) (which could co-exist) were not evaluated [29].
This study is the largest series of term asphyxiated newborns studied, with a third generation cTnT assay. This is the only study in which the sensitivity and specificity of cTnT in diagnosing myocardial dysfunction was found taking echocardiography as the gold standard. Moreover this is the only study in which the ROC curves of cTnT in relation to myocardial dysfunction, clinical shock, grades of HIE and mortality were calculated.
The cut-off values of cTnT for myocardial dysfunction can be used as a predictor of myocardial dysfunction in asphyxiated neonates (sensitivity 92.4%). Also, because of the high specificity (94.1%) for a cut-off cTnT of 0.1145 ng/ml, it can also be used for ruling in myocardial dysfunction. The cTnT levels also correlated with severity of asphyxia and other poor outcomes like shock and mortality. Moreover, the cut-off cTnT values for myocardial dysfunction, HIE and death could be replicated in other neonatal centres.
Conclusions
This study shows a positive correlation between raised cTnT concentration at 4 h of age and myocardial dysfunction in term asphyxiated neonates. Thus early measurement of cTnT may be helpful in predicting myocardial dysfunction in such neonates. The raised cTnT also correlated with poor outcome like shock and mortality. The early detection and prompt treatment of myocardial dysfunction will help in improving the prognosis of these asphyxiated newborns.
References
Lawn JE, Cousins S, Zupan J; Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? where? why? Lancet. 2005;365:891–900.
Tapia-Rombo CA, Carpio-Hernández JC, Salazar-Acuña AH, et al. Detection of transitory myocardial ischemia secondary to perinatal asphyxia. Arch Med Res. 2000;31:377–83.
Rajkumar PS, Bhat BV, Sridhar MG, et al. Cardiac enzyme levels in myocardial dysfunction in newborns with perinatal asphyxia. Indian J Pediatr. 2008;75:1223–5.
Vijlbrief DC, Benders MJ, Kemperman H, van Bel F, de Vries WB. Use of cardiac biomarkers in neonatology. Pediatr Res. 2012;72:337–43.
Güneś T, Oztürk MA, Köklü SM, Narin N, Köklü E. Troponin –T levels in perinatally asphyxiated neonates during the first 15 days of life. Acta Pediatr. 2005;94:1638–43.
Moller JC, Thidson B, Schaible TF. Value of myocardial hypoxic markers and serum creatinine for retrospective diagnosis of perinatal asphyxia. Biol Neonate. 1998;73:367–74.
Agarwal J, Shah GS, Paudel P, Baral N, Agrawal A, Mishra OP. Electrocardiographic and enzymatic correlations with outcome in neonates with hypoxic–ischemic encephalopathy. Ital J Pediatr. 2012;38:33.
EL Khuffash AF, Molloy CT. Serum Troponin in neonatal intensive care. Neonatology. 2008;94:1–7.
Clark SJ, Newland P, Yoxall CW, Subhedar NV. Concentrations of cardiac troponin T in neonates with and without respiratory distress. Arch Dis Child Fetal Neonatal Ed. 2004;89:348–52.
Omokhodion SI, Losekoot TG, Jaiyesimi F. Serum creatine kinase and creatine kinase MB isoenzyme activities in perinatally asphyxiated newborns. Eur Heart J. 1991;12:980–4.
Mandal Ravi RN, Gupta R, Kapoor AK. Evaluation of activity of creatine phosphokinase (CPK) and its isoenzyme CPK-MB in perinatal asphyxia and its implications for myocardial involvement. Bull NNF. 1999;13:2–7.
Clark SJ, Newland P, Yoxall CW, Subhedar NV. Cardiac troponin T in cord blood. Arch Dis Child Fetal Neonatal Ed. 2001;84:F34–7.
Lopes DN, Ramos JMM, Moreira MEL, Cabral JA, de Carvalho M, Lopes JM. Cardiac troponin T and illness severity in very low birth weight infant. Int J Pediatr. 2012;2012:479242.
Goel M, Gohiya P, Yadav BS. Assessment of myocardial function in birth asphyxia. Int J Med Res Rev. 2013;1:228–32.
Mertens L, Seri I, Marek J, et al. Targeted neonatal echocardiography in the neonatal intensive care unit: practice guidelines and recommendations for training. J Am Soc Echocardiogr. 2011;24:1057–78.
Szymankiewicz M, Matuszczak-Wleklak M, Hodgman JE, Gadzinowski J. Usefulness of cardiac troponin- T and echocardiography in the diagnosis of hypoxic myocardial injury of full term neonates. Biol Neonate. 2005;88:19–23.
Costa S, Zecca E, De Rosa G, et al. Is serum troponin-T a useful marker of myocardial damage in newborn infants with perinatal asphyxia? Acta Paediatr. 2007;96:181–4.
Awada H, Ai Tannir M, Ziade, et al. Cardiac troponin T: useful early marker for cardiac and respiratory dysfunction in neonates. Neonatology. 2007;92:105–10.
Trevisanuto D, Zaninott M, Altiniers S, et al. High serum cardiac troponin T concentrations in preterm infants with respiratory distress syndrome. Acta Paediatr. 2000;89:1134–6.
Boo NY, Hafidz H, Nawaui HM, et al. Comparison of serum cardiac troponin T and creatine kinase MB isoenzyme concentration in asphyxiated term infants during the first 48 hrs of life. J Paediatr Child Health. 2005;41:331–7.
Kanik E, Ozer EA, Bakibi AR, et al. Assessment of myocardial dysfunction in neonates with hypoxic ischemic encephalopathy: is it a significant predictor of mortality? J Matern Fetal Neonatal Med. 2009;22:239–42.
Simovic AM, Prijic SM, Knezevic JB, Igrutinovic ZR, Vujic AJ, Kosutic JLJ. Predictive value of biochemical, echocardiographic and electrocardiographic markers in non–surviving and surviving asphyxiated full term neonates. Turk J Pediatr. 2014;56:243–9.
Matter M, Abdel-Hady H, Attia G, Hafez M, Seliem W, Al-Arman M. Myocardial performance in asphyxiated full term infants assessed by Doppler tissue imaging. Pediatr Cardiol. 2010;31:634–42.
Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined --a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–69.
Gaze DC, Collinson PO. Cardiac troponin I should be interpreted with caution in pediatric neonatal patients. Concerning Turker et al: cord blood troponin I as an early predictor of short term outcome in perinatal hypoxia. Biol Neonate. 2005;87:19.
Bodor GS, Survant L, Voss EM, Smith S, Porterfield D, Apple FS. Cardiac troponin T composition in normal and regenerating human skeletal muscle. Clin Chem. 1997;43:476–84.
Sasse S, Brand NJ, Kyprianov P, et al. Troponin I gene expression during human cardiac development and in end – stage heart failure. Circ Res. 1993;72:932–8.
Adamcová M, Kokstein Z, Palicka V, Podholová M, Kostál M. Troponin T levels in the cord blood of healthy term neonates. Physiol Res. 1995;44:99–104.
Martín-Ancel A, García-Alix A, Gayá F, Cabañas F, Burgueros M, Quero J. Multiple organ involvement in perinatal asphyxia. J Pediatr. 1995;127:786–93.
Acknowledgements
Dr Saboora Beegum - Professor & HOD, Department of Biochemistry, Govt. Medical college, Thiruvananthapuram for her role in supervising the biochemical estimation of cTnT.Dr Muralidharan Nair – Asst. Prof. of Medical Statistics (Retd), CERTC, Govt. Medical College, Thiruvananthapuram for his help in the statistical analysis.
Author information
Authors and Affiliations
Contributions
SJ: Designed the study, collected & analysed the data, did the literature work, wrote the paper; SL and ZAM: Conducted the echocardiographic analysis; SK: Permitted to do the study in NICU, critically reviewed the manuscript and approved the final version. SK will act as guarantor for this paper.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
State Board of Medical Research (SBMR), Govt. of Kerala.
Rights and permissions
About this article
Cite this article
Joseph, S., Kumar, S., Ahamed M, Z. et al. Cardiac Troponin-T as a Marker of Myocardial Dysfunction in Term Neonates with Perinatal Asphyxia. Indian J Pediatr 85, 877–884 (2018). https://doi.org/10.1007/s12098-018-2667-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-018-2667-3