Abstract
The Indian Society for Pediatric and Adolescent Endocrinology has formulated locally relevant Clinical Practice Guidelines for newborn screening, diagnosis and management of primary congenital hypothyroidism (CH). Recommendations: Screening should be done for every newborn using cord blood, or postnatal blood, ideally at 48 to 72 h of age. On this screen sample, neonates with TSH > 20 mIU/L serum units (or >34 mIU/L for samples taken between 24 to 48 h of age) should be recalled for confirmation. For screen TSH > 40 mIU/L, immediate confirmatory venous T4/FT4 and TSH, and for milder elevation of screen TSH, a second screening TSH at 7 to 10 d of age, should be taken. Preterm and low birth weight infants should undergo screening at 48–72 h postnatal age. Sick babies should be screened at least by 7 d of age. Venous confirmatory TSH >20 mIU/L before age 2 wk and >10 mIU/L after age 2 wk, with low T4 (<10 μg/dL) or FT4 (<1.17 ng/dL) indicate primary CH and treatment initiation. Imaging is recommended by radionuclide scintigraphy and ultrasonography after CH is biochemically confirmed but treatment should not be delayed till scans are performed. Levothyroxine is commenced at 10 to 15 μg/kg in the neonatal period. Serum T4/FT4 is measured at 2 wk and TSH and T4/FT4 at 1 mo, then 2 monthly till 6 mo, 3 monthly from 6 mo-3 y and every 3–6 mo thereafter. Babies with the possibility of transient congenital hypothyroidism should be re-evaluated at age 3 y, to assess the need for lifelong therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congenital hypothyroidism (CH) is the commonest cause of preventable mental retardation, and satisfies all the criteria for being included in a newborn screening (NBS) program (see Supplementary Table 1) [1, 2]. At present, there is no national NBS program in India. All physicians who want to provide NBS in their own environment should have access to locally relevant guidelines regarding the appropriate sample for screening, the diagnostic cut-offs for best sensitivity and specificity, and optimum therapy of affected infants.
Objectives
To formulate Clinical Practice Guidelines for NBS for primary CH for India.
Methods
The Indian Society for Pediatric and Adolescent Endocrinology (ISPAE) appointed writing and editorial committees in August 2016. A literature search included more than 80 original articles from PubMed and existing guidelines from professional societies. The GRADE method of expressing quality of evidence and strength of recommendation was adopted [3]. A strong recommendation is denoted by 1 and a weak one by 2, while ⊕ to ⊕ ⊕ ⊕ denote weak to strong level of evidence. This part of the Guidelines describes screening and diagnosis of congenital hypothyroidism, and part II, which covers imaging, treatment and follow up, is elsewhere in this issue.
History of Newborn Screening for Congenital Hypothyroidism
The concept of NBS with dried blood spot (DBS) was first conceived by Prof. Guthrie in 1960 for phenylketonuria [4]. To this, screening for CH was added in 1965. Demonstration of improved intellectual outcome in CH with institution of early treatment by investigators in the early 1970’s generated interest worldwide. Pilot screening programs for CH were developed in Quebec [5] and Pittsburgh [6], and subsequently in all developed countries. Today many developing countries undertake NBS for endocrine and metabolic disorders.
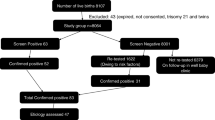
In India, the first NBS programme for CH was at BJ Wadia Hospital, Mumbai in 1982 using cord blood TSH and subsequently in 1984 using postnatal DBS T4 [7, 8]. The prevalence was 1:2481 and 1:2804 respectively, higher than reported worldwide (1:3000–4000). Subsequent studies from various parts of India have reported higher prevalence (Supplementary Table 2) [1, 9,10,11,12], which may be related to variations in ethnicity, consanguinity, nutritional and environmental factors including iodine deficiency, and improved hormone assays. Due to increased awareness, many pediatricians now screen newborns at private institutions and a few state governments support screening of newborns in government facilities.
Recommendation
-
NBS for CH should be done for every newborn in India. (1 ⊕ ⊕⊕)
The Sample for NBS
Cord Blood vs. Postnatal Sample
The crucial point to remember when screening for CH is the neonatal surge of TSH and T4 [13]. The TSH surge starts 30 min after birth (T4 some hours later), is most marked for the next 24 h, but may persist for 48 to 72 h. Thus, cord blood is largely spared of the neonatal surge. If the screen sample is taken during the surge, a false positive result will follow, leading to a large number of infants being recalled for a confirmatory sample (a high “recall rate”). Parents will be made unnecessarily anxious and the system will be overburdened. The screen sample should therefore be taken either from the cord (placental end, immediately after delivery) or postnatally after 72 h of life. If the hospital stay is shorter, it may be taken after 48 h of life [14]. The advantages and disadvantages of cord vs. postnatal sample are highlighted in Table 1. Pediatricians should decide what kind of NBS is most suited to their needs. Cord blood NBS for CH is an effective strategy to reduce missed opportunities for screening due to early discharge [10, 15]. On the other hand, postnatal samples offer the advantage of screening for other treatable inborn errors of metabolism like congenital adrenal hyperplasia, biotidinase deficiency and those dependent on feeding (galactosemia, phenyketonuria), and should be used wherever feasible and economically viable.
Recommendation
-
Either cord blood or postnatal day 3-day 5 samples should be used for screening for CH. (1 ⊕ ⊕O)
In-House Lab Testing vs. Central Network, and Filter Paper vs. Serum Sample
A cord blood or postnatal heel prick filter paper DBS sample can be transported easily to a central NBS laboratory. However, filter paper sample requires special equipment and kits for assay, as only a small amount of serum is available from a filter paper punch. If these facilities are not available, the cord blood or venous postnatal sample (Supplementary material, Appendix 1 for the method of taking a cord sample) may be sent to a routine laboratory in a plain vacutainer.
Recommendation
-
Either serum or filter paper thyroid hormone assays should be used for screening for CH, the choice of method depends on local facilities. (1 ⊕ ⊕O)
Sample Collection and Transport
A good sample and a properly filled demographic form are essential for the success of any NBS programme (Supplementary Appendices 2 and 3). For mothers discharged within 24 h of delivery, sampling may be performed early and age-related TSH cut-offs employed for recall.
The Test: Biochemical Assay for NBS for CH
TSH vs. T4 Based Screening
The ideal screening test should have high sensitivity and specificity so as not to miss any case of CH (no false negatives) and, at the same time, have an acceptable recall rate for confirmatory sampling (low false positives). There are two main screening strategies for CH: primary T4 testing (with backup TSH) or primary TSH testing (Supplementary Table 3).
Primary T4 testing helps to identify patients with primary and secondary (central) CH. However, it misses neonates with compensated forms of CH (normal T4 with high TSH, which is commonly seen in ectopic thyroid, the most frequent cause of CH) [16]. Moreover, there is a high rate of false positive results, whether done from cord blood or postnatal day 3–5 sample [17, 18]. These include infants with thyroid-binding globulin (TBG) deficiency and preterm and sick neonates [19]. Hence, low T4 values must be followed by backup TSH on the same DBS [17, 18]. TSH is measured in the samples with the lowest percentiles of T4 (from 3 to 20%, the range varying between different programs) [19]. Neonates are recalled for confirmatory venous sampling if TSH is greater than the cut-off. The recall rate with primary T4 testing with backup TSH is around 0.1–1% [20]. Primary T4 screening is followed in some states in the US and in Israel [2]. Netherlands employs a T4 with backup TSH and TBG, however, this entails higher cost and retesting rates [21]. Some US states screen for T4 and TSH simultaneously, which may not be cost-effective for all countries [2].
Primary TSH screen is more sensitive and specific for the diagnosis of primary CH compared to T4 screen [16, 17, 22]. Primary TSH screening may miss infants with delayed rise of TSH most often seen in preterm babies due to immaturity of the hypothalamic-pituitary-thyroid (HPT) axis [7]. It also fails to detect cases of central CH; however, detection of this rare disorder is not the target of mass NBS [23].
Overall, primary TSH-based CH screening is more practical and cost-effective [16, 24]. It is followed in most parts of the world. Many states in the USA have shifted from T4 to TSH testing and most newly developed CH screening programmes in different parts of the world have adopted the primary TSH strategy [23].
Recommendation
-
Primary TSH assay is recommended for NBS. (1 ⊕ ⊕⊕)
Conduct of a TSH Based Screening Program [24]
Measurement of TSH on DBS is done using an immunofluorescence or colorimetric neonatal TSH kit (e.g., Perkin Elmer® or Biorad®) at a centralized NBS laboratory. Alternatively, the serum sample can be analysed by ELISA or chemiluminescence methods at routine laboratories. The TSH measured from a DBS is expressed in whole blood units. Serum units may be derived by multiplying the whole blood units value by 2.2 (to adjust for the hematocrit). The expression of serum or whole blood units for TSH results should be clearly specified and uniform. The authors will be using serum units in this Guideline, to express filter paper as well as serum TSH results.
Recommendation
-
Since reporting units for TSH should be uniform in the country, the authors recommend the uniform use of serum units for all, rather than whole blood units. (1 ⊕ OO)
TSH Cut-Offs to be Used for the Screening Test
Only 1 in a few babies with an abnormal screen TSH value will finally have true CH. To identify the true positives, confirmatory test must be done for all babies whose screen TSH is above a chosen cut-off. Various cut-offs have been used in different studies across the world. A TSH cut-off of >20 mIU/L for recall has been shown to be associated with reasonable specificity and recall rate [25]. Studies which examined the outcome of shifting to lower cut-offs for recall showed substantial increase in recall rates to diagnose a few additional cases of mild CH; for example, decreasing the cut-off from 20 mIU/L to 12 mIU/L serum units in a study from UK led to a 126% increase in recall rate to detect 3 additional mild cases in a screen sample of 65,000 newborns [26].
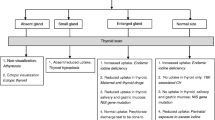
Therefore, the authors recommend a screen TSH cut-off of >20 mIU/L for recall. Mildly elevated screen TSH (between 20 and 40 mIU/L) dictates recall early in the second week of life for a repeat screening TSH (most of the mildly high TSH reports due to unresolved neonatal TSH surge or other reasons would have normalized in a few days). However a clear-cut high screen TSH >40 mIU/L necessitates immediate recall (after 72 h of age) for a confirmatory venous sample [27, 28] (Fig. 1). The recall rates are high when cord blood samples are used because of its high standard deviation, therefore these guidelines may need to be revised in future when false negative rates are available using this cut-off level.
In centres where the second TSH screen is done using a venous sample rather than a heel prick DBS, both TSH and T4 may be performed on this sample itself, as it may not be cost-effective to recall the family once again for T4/FT4 if needed.
The use of age related cut-offs for samples taken between 24 and 48 h of age [2, 29] has been shown to drastically reduce false positivity and recall rate in one of the Indian NBS programs [1]. For mothers discharged within 24 h of delivery, some international programs have used the age-related TSH cut-off of 100 mIU/L for recall.
Recommendation
-
1.
TSH >20 mIU/L (serum units) is recommended as the cut-off for recall for cord blood and postnatal screen samples after 48 h of age. (1 ⊕ ⊕O)
-
2.
Screen TSH >40 mIU/L is recommended for defining screen positive cases for immediate recall for venous confirmatory test, whereas those with mildly elevated TSH from 20 to 40 mIU/L should have a second TSH screen at 7 to 10 d of age.(1 ⊕ ⊕O)
-
3.
Age-related TSH cut-off (>34 mIU/L) is suggested for screen samples taken between 24 to 48 h of age. (2 ⊕ ⊕O)
Decision Making for Recalled Infants
For clearly elevated screen positive neonates (TSH >40 mIU/L), parents should be contacted immediately by an appropriate health worker (Supplementary Appendix 4) or doctor for obtaining the confirmatory venous sample, and the baby should be evaluated by a pediatrician or pediatric endocrinologist and levothyroxine therapy initiated if indicated. Measurement of venous serum T4/FT4 and TSH are done by chemiluminescence or ELISA assay. Before 2 wk of age, venous TSH >20 mIU/L and after 2 wk of age, >10 mIU/L, is indicative of primary CH [22, 30, 31]. Serum T4 < 10 μg/dL (<128 nmol/L) or FT4 < 1.17 ng/dL (<15 pmol/L) is considered low in infancy (Table 2) [22, 27, 31] (in contrast to lower levels in older children and adults). The indications for starting treatment on confirmatory venous samples are given in Table 2. Babies with screen TSH >80 mIU/L serum units are highly likely to have low T4 or FT4 levels, therefore commencement of therapy is recommended as soon as the confirmatory sample is taken, without waiting for the results unless these results are available on the same day [22, 32].
For mildy elevated TSH results as defined above, a repeat filter paper sample (for screening under central NBS programme) or a repeat serum sample (for screening by routine laboratory) should be obtained as early as possible, in the second week of life. This will ensure treatment initiation within two weeks of life for better neurodevelopmental outcome [27, 33]. The reason for not taking the repeat sample immediately is to allow the neonatal factors causing a false positive result to settle down. If the second screen TSH is high (>20 mIU/L for age < 2 wk and >10 mIU/L for age > 2 wk), immediate confirmatory venous sample for T4/FT4 and TSH measurement should be taken. Where a venous sample has been taken for the second screen, the TSH and T4/FT4 in that sample will serve as the confirmatory venous sample. The causes of deranged thyroid function tests in the newborn are given in supplementary Table 4.
Recommendation
-
1.
Venous TSH > 20 mIU/L before age 2 wk and, >10 mIU/L after age 2 wk with serum T4 < 10 μg/dL (< 128 nmol/L) and FT4 < 1.17 ng/dL (< 15 pmol/L) is indicative of primary CH and needs treatment initiation. (1 ⊕ ⊕O)
-
2.
For babies with screen TSH > 80 mIU/L serum units, the authors recommend commencement of therapy as soon as the confirmatory sample is taken, without waiting for the results unless results are available on the same day. (1 ⊕ ⊕O)
Decision Making for Borderline Thyroid Function Reports
For elevated venous TSH and normal FT4 levels, the baby may be retested after 2 wk (treatment may be started immediately if there is any doubt about compliance to instructions). At this point, referral to a pediatric endocrinologist is made where feasible. After 3 wk of age, if TSH remains persistently >10 mIU/L even with normal range of T4/FT4, levothyroxine treatment may be started to avoid insult to the developing brain. One may also encounter low T4/FT4 with normal TSH levels. Table 2 provides guidelines for therapy in this instance, but referral to a pediatric endocrinologist is helpful. Thyroid imaging may also help to get a definitive diagnosis. Re-evaluation is recommended after 3 y of age (see below), by which age the phase of rapid brain development has been achieved [34, 35].
Recommendation
-
1.
Referral to a pediatric endocrinologist is suggested for all babies diagnosed with CH especially those with borderline results of thyroid function. (2OOO)
-
2.
Infants with persistently elevated TSH >10 mIU/L (after 3 wk of age) with normal T4/FT4, or normal confirmatory sample TSH with clearly low T4 (<8 μg/dL) / FT4 (<1.1 ng/dL) may be treated with levothyroxine, and a re-evaluation off therapy should be planned after 3 y of age. (2 ⊕ ⊕O)
Special Situations (Twins, IUGR, Preterm and Sick Neonates)
High risk neonates such as preterm, low birth weight (LBW) (1500–2499 g), very-low birth-weight (VLBW) (1000–1499 g) and sick neonates, multiple births, particularly same sex twins are at increased risk for an inappropriate TSH level at initial screening (both false positive and false negative) [2, 22, 36].
The postnatal TSH surge and rise in thyroid hormones seen in term infants are present in preterm infants as well, but attenuated owing to immaturity of the HPT axis [37]. Moreover, preterm and sick infants often have a fall in serum T4 and T3 in first week of life, which may be due to poor nutrition, decreased hepatic TBG production, immaturity of the HPT axis, use of iodine antisepsis, increased tissue utilization of T4 or sick euthyroidism. Sick euthyroid syndrome (low serum T4 with normal TSH) resulting from associated medical problems, such as respiratory distress syndrome or the consequences of intrauterine growth retardation (IUGR) may persist until the infant recovers from the acute illness or gains weight [22]. No causal relationship has been established between hypothyroxinemia of prematurity and problems in neurodevelopment and intellectual disability; current evidence does not indicate benefit from therapy of hypothyroxinemia of prematurity in the absence of raised TSH [38]. In the majority of preterm infants, T4 rises into the normal range when a repeat screening test is performed at two to four weeks of age, as the HPT function matures [39, 40]. Occasionally, recovery from sick euthyroidism may lead to mild elevation of TSH and give rise to false positive screen results [2].
Conversely, preterm infants with true CH may not be able to mount an appropriate TSH response in the first 2 wk of life due to immaturity of the HPT axis or treatment with glucocorticoids or dopamine, leading to false negative initial screen [22]. Various studies have reported a higher incidence of CH (both transient and permanent) in preterm, VLBW and LBW infants [41, 42], who may be missed in the first screen. A second screen done after 2 wk of age will pick up the delayed rise of TSH. On the contrary, the cases of CH detected by a second screen often represent mild or transient CH, and many question the policy of re-screening at 2 wk [42].
Another group at high risk of CH consists of newborns with Down syndrome - not only do these infants have a higher incidence of CH picked up by NBS, but they may also have mildly elevated TSH levels that can be missed by screening and therefore, require careful followup and re-testing before 6 mo of age [43].
As for all newborns, preterm and LBW/VLBW infants should undergo routine screening for CH only at 48–72 h postnatal age, not earlier [36]. Only in instances of acute hemorrhage or hemolysis, when transfusion is warranted, they may be screened before 24–48 h of birth [36]. With sick infants in NICUs, screening should be performed at least by 7 d of postnatal life [27].
It is suggested to do a second screening test at 2–4 wk of age for high-risk babies, though not at the expense of missing infants with severe CH in an already overburdened system [44, 45].
The final TSH cut-offs for preterm, LBW/VLBW infants and twins remain the same as for term infants.
Recommendation
-
1.
As for all newborns, preterm and LBW/VLBW infants should undergo routine screening for CH at 48–72 h postnatal age. (1 ⊕ ⊕⊕)
-
2.
Sick neonates should be screened at least by 7 d of age. (1 ⊕ ⊕⊕)
-
3.
High risk neonates such as preterm, LBW and VLBW babies, ill neonates admitted to NICU and multiple births (particularly same sex twins) may have a second screen at 4 wk of age (or at 2 wk of age if discharged early). (2 ⊕ ⊕O)
Conclusions
NBS for CH is one of the most successful interventions in preventive pediatrics worldwide. Till such time as NBS is integrated in national health programmes in India, pediatricians and health authorities should make concerted efforts to initiate NBS for CH for all newborns in their care. These guidelines make available broad principles for health care providers to implement NBS for CH in their settings that can be adapted to their local conditions. Summary of Recommendations are also given in Supplementary Table 5.
Abbreviations
- TSH :
-
Thyroid stimulating hormone
- T4 :
-
Thyroxine
- FT4 :
-
Free thyroxine
- CH :
-
Congenital hypothyroidism
- NBS :
-
Newborn screening
- DBS :
-
Dried blood spot
- LBW :
-
Low birth weight
- VLBW :
-
Very low birth weight
- IUGR :
-
Intrauterine growth retardation
- TBG :
-
Thyroxine binding globulin
References
Gopalakrishnan V, Joshi K, Phadke SR, et al. Newborn screening for congenital hypothyroidism, galactosemia and biotinidase deficiency in Uttar Pradesh, India. Indian Pediatr. 2014;51:701–5.
LaFranchi SH. Newborn screening strategies for congenital hypothyroidism: an update. J Inherit Metab Dis. 2010;33:S225–33.
Swiglo BA, Murad MH, Schunemann HJ, et al. A case for clarity, consistency and helpfulness: state-of-the-art clinical practice guidelines in endocrinology using the grading of recommendations, assessment, development, and evaluation system. J Clin Endocrinol Metab. 2008;93:666–73.
Guthrie R. Screening for inborn errors of metabolism in the newborn infant- a multiple test program. Birth Defects Original Article, Series IV 1962. p. 92–8.
Dussault JH, Coulombe P, Laberge C, Letarte J, Guyda H, Khoury K. Preliminary report on a mass screening program for neonatal hypothyroidism. J Pediatr. 1975;86:670–4.
Fisher DA, Dussault JH, Foley TP Jr, et al. Screening for congenital hypothyroidism: results of screening one million North American infants. J Pediatr. 1979;94:700–5.
Colaco MP, Desai MP, Ajgaonkar AR, et al. Neonatal screening for hypothyroidism. Indian Pediatr. 1984;21:695–700.
Desai MP, Upadhye P, Colaco MP, et al. Neonatal screening for congenital hypothyroidism using the filter paper thyroxine technique. Indian J Med Res. 1994;100:36–42.
Kaur G, Srivastav J, Jain S, et al. Preliminary report on neonatal screening for congenital hypothyroidism, congenital adrenal hyperplasia and glucose-6-phosphate dehydrogenase deficiency: a Chandigarh experience. Indian J Pediatr. 2010;77:969–73.
Mathai S. Newborn screening for congenital hypothyroidism- experience from India. Abstract presented at 8th Asia Pacific Regional Meeting of the International Society for Neonatal Screening. New Delhi, Sep 2013.
Rama Devi AR. Newborn screening in India, experience from pilot initiative (ICMR Multicenter Project). Abstract presented at 8th Asia Pacific Regional Meeting of the International Society for Neonatal Screening. New Delhi, Sep 2013.
Kishore KR, Ranieri E, Fletcher J. Newborn screening for congenital hypothyroidism in India - is overdue. J Neonatal Biol. 2014;3:129.
Fisher DA, Klein AH. Thyroid development and disorders of thyroid function in the newborn. N Engl J Med. 1981;304:702–12.
Delange F, Camus M, Winkler M, Dodian J, Ermans A. Serum thyrotrophin determination on day 5 of life as screening procedure for congenital hypothyroidism. Arch Dis Child. 1977;52:89–96.
Vela M, Gamboa S, Loera-Luna A, Aguirre BA, Pérez-Palacios G, Velázquez A. Neonatal screening for congenital hypothyroidism in Mexico: experience, obstacles, and strategies. J Med Screen. 1999;6:77–9.
Delange F. Neonatal screening for congenital hypothyroidism: results and perspectives. Horm Res. 1997;48:51–61.
Walfish PG. Evaluation of three thyroid-function screening tests for detecting neonatal hypothyroidism. Lancet. 1976;1:1208–10.
Dussault JH, Morissette J, Letarte J, Guyda H, Laberge C. Modification of a screening program for neonatal hypothyroidism. J Pediatr. 1978;92:274–7.
Willi SM, Moshang T Jr. Diagnostic dilemmas. Results of screening tests for congenital hypothyroidism. Pediatr Clin North Am. 1991;38:555–66.
Fisher DA. Effectiveness of newborn screening programs for congenital hypothyroidism: prevalence of missed cases. Pediatr Clin North Am. 1987;34:881–90.
Kempers MJ, Lanting CI, van Heijst AF, et al. Neonatal screening for congenital hypothyroidism based on thyroxine, thyrotropin, and thyroxine-binding globulin measurement: potentials and pitfalls. J Clin Endocrinol Metab. 2006;91:3370–6.
Léger J, Olivieri A, Donaldson M, et al; ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE; Congenital Hypothyroidism Consensus Conference Group. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 2014;99:363–84.
Ford G, LaFranchi SH. Screening for congenital hypothyroidism: a worldwide view of strategies. Best Pract Res Clin Endocrinol Metab. 2014;28:175–87.
International Atomic Energy Agency (IAEA). Screening of newborns for congenital hypothyroidism-Guidance for developing programmes. Vienna, 2005.
Corbetta C, Weber G, Cortinovis F, et al. A 7-year experience with low blood TSH cutoff levels for neonatal screening reveals an unsuspected frequency of congenital hypothyroidism (CH). Clin Endocrinol. 2009;71:739–45.
Korada SM, Pearce M, Platt MPW, et al. Difficulties in selecting an appropriate neonatal thyroid stimulating hormone (TSH) screening threshold. Arch Dis Child. 2010;95:169–73.
Rose SR, Brown RS, Foley T, et al. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006;117:2290–303.
Saleh DS, Lawrence S, Geraghty MT, et al. Prediction of congenital hypothyroidism based on initial screening thyroid stimulating hormone. BMC Pediatr. 2016;16:24.
Lott JA, Sardovia-Iyer M, Speakman KS, Lee KK. Age-dependent cut off values in screening newborns for hypothyroidism. Clin Biochem. 2004;37:791–7.
Fisher DA. Disorders of the thyroid in the newborn and infant. In: Sperling MA, editor. Clinical Pediatric and Adolescent Endocrinology, 3rd ed. Philadelphia: Saunders; 2008. p. 214.
Mutlu M, Karagüzel G, Alıyaziciolu Y, Eyüpolu I, Okten A, Aslan Y. Reference intervals for thyrotrophin and thyroid hormones and ultrasonographic thyroid volume during the neonatal period. J Matern Fetal Neonatal Med. 2012;25:120–4.
Pokrovska T, Jones J, Shaikh MG, Smith S, Donaldson MDC. How well does the capillary thyroid-stimulating hormone test for newborn thyroid screening predict the venous free thyroxine level? Arch Dis Child. 2016;101:539–45.
Dimitropoulos A, Molinari L, Etter K, et al. Children with congenital hypothyroidism: long-term intellectual outcome after early high-dose treatment. Pediatr Res. 2009;65:242–8.
Eugster EA, LeMay D, Zerin JM, Pescovitz OH. Definitive diagnosis in children with congenital hypothyroidism. J Pediatr. 2004;144:643–7.
Nair PS, Sobhakumar S, Kailas L. Diagnostic re-evaluation of children with congenital hypothyroidism. Indian Pediatr. 2010;47:757–60.
Slaughter JL, Meinzen-Derr J, Rose SR, et al. The effects of gestational age and birth weight on false-positive newborn-screening rates. Pediatrics. 2010;126:910–6.
LaFranchi S. Thyroid function in the preterm infant. Thyroid. 1999;9:71–80.
Hollanders JJ, Israëls J, Van der Pal SM, et al. No association between transient hypothyroxinemia of prematurity and neurodevelopmental outcome in young adulthood. J Clin Endocrinol Metab. 2015;100:4648–53.
Williams FL, Simpson J, Delahunty C, et al. Developmental trends in cord and postpartum serum thyroid hormones in preterm infants. J Clin Endocrinol Metab. 2004;89:5314–20.
Carrascosa A, Ruiz-Cuevas P, Potau N, et al. Thyroid function in seventy-five healthy preterm infants thirty to thirty-five weeks of gestational age: a prospective and longitudinal study during the first year of life. Thyroid. 2004;14:435–42.
Mandel SJ, Hermos RJ, Larson CA, Prigozhin AB, Rogas DA, Mitchell ML. Atypical hypothyroidism and the very low birth weight infant. Thyroid. 2000;10:693–5.
Woo HC, Lizarda A, Tucker R, et al. Congenital hypothyroidism with a delayed thyroid-stimulating hormone elevation in very premature infants: incidence and growth and developmental outcomes. J Pediatr. 2011;158:538–42.
Purdy IB, Singh N, Brown WL, Vangala S, Devaskar UP. Revisiting early hypothyroidism screening in infants with Down syndrome. J Perinatol. 2014;34:936–40.
Tylek-Lemanska D, Kumorowicz-Kopiec M, Starzyk J. Screening for congenital hypothyroidism: the value of retesting after four weeks in neonates with low and very low birth weight. J Med Screen. 2005;12:166–9.
Krude H, Blankenstein O. Treating patients not numbers: the benefit and burden of lowering TSH newborn screening cut-offs. Arch Dis Child. 2011;96:121–2.
Author information
Authors and Affiliations
Contributions
MD, RS, SS, RP, RI, VB reviewed the literature, drafted the manuscript, obtained extensive inputs from the editorial team, and finalised the manuscript incorporating these inputs. VB will act as the guarantor of the study.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Writing Committee: MP Desai, R Sharma, I Riaz, S Sudhanshu, R Parikh, V Bhatia
Editorial Committee: G Gupta, G Jevalikar, S Mathai, PSN Menon, P Raghupathy, S Rao, A Seth, A Simon, M Vijayakumar, A Virmani
Electronic supplementary material
ESM 1
(DOC 87.5 kb)
Rights and permissions
About this article
Cite this article
Desai, M.P., Sharma, R., Riaz, I. et al. Newborn Screening Guidelines for Congenital Hypothyroidism in India: Recommendations of the Indian Society for Pediatric and Adolescent Endocrinology (ISPAE) – Part I: Screening and Confirmation of Diagnosis. Indian J Pediatr 85, 440–447 (2018). https://doi.org/10.1007/s12098-017-2575-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-017-2575-y