Abstract
Objective
To find out correlation between serum anti-tissue transglutaminase immunoglobulin-A (tTGA) levels and Marsh grading on duodenal histopathology in Celiac disease (CD).
Methods
In a prospective cohort study, a total of 52 symptomatic patients between age group of 2–18 y were enroled. All enroled patients were subjected to upper GI endoscopy by an experienced endoscopist. Two biopsies each from the bulb (D1) and second part (D2) of the duodenum were taken and Marsh grading was performed by a single experienced pathologist. Serum tTGA levels were also performed to find out correlation between serum tTGA levels and Marsh grading.
Results
The mean age of the patients was 8.21 ± 3.45 y (Range: 2–16 y). Anemia was the most common non-gastrointestinal (GI) sign and was present in 73% of the cases. However the authors could not find out any significant association between Marsh grading and hemoglobin levels (r = 0.32, p > 0.05). Serum tTGA levels were found to be positively correlated with Marsh grading (Spearmen correlation coefficient ρ = 0.74, p 0.000). Significant differences were found in tTGA levels between different Marsh gradings (ANOVA test) (p 0.000). Receiver-operator curve (ROC) analysis cut-off value of serum tTGA for predicting villous atrophy was 178.8 (nine times of cut-off value) with sensitivity of 100% and specificity of 85.7%.
Conclusions
Serum tTGA levels can be used to predict villous atrophy and biopsy may be avoided in strongly suspected cases with more than 9 times of cut-offs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Celiac disease (CD) is a chronic immunologically determined form of enteropathy affecting the small intestine in genetically predisposed children and adults. The ingestion of gluten-containing foods precipitates it [1, 2]. It is considered as an autoimmune condition because of the presence of serum anti-tissue transglutaminase immunoglobulin-A (tTGA) and its association with other autoimmune diseases (thyroid, liver, diabetes, adrenal) [3].
As per the current practice, diagnosis of CD hinges on a diagnostic intestinal biopsy and the concomitant presence of a positive CD specific serology [4–8]. With increasing number of cases of CD there are certain limiting factors for histological diagnosis of CD specially in resource limited countries like India; some of these are limited number of pediatric endoscopic centers, lack of expert pathologists, risk and inconvenience associated with procedure, high cost and it may not lead to firm conclusions because of inadequate quality or patchy disease or orientation of the biopsy specimen and thus research work now-a-days is shifting towards finding out non-invasive markers for diagnosis of CD [9–11]. Indeed, new ESPHAGEN guidelines for the diagnosis of CD state that a confirmatory small intestine biopsy is no longer a requirement in case of individuals who are genetically predisposed and symptomatic and who have a tTGA of atleast 10 times the upper limit of normal range, who have a positive endomysial antibodies (EMA) and those exhibiting a positive response to gluten free diet [12]. However, it is essential to mention here that said observations are exposed to the risk of selection bias as these recent guidelines for children are based upon retrospective data [13, 14]. A CD diagnosis simply implies lifelong gluten free diet for the patient and thus diagnosis of CD must be based upon serology only when chance of a false positive result is almost zero. Thus this study has been planned to evaluate prospective correlation between serum tTGA and modified Marsh grading and to determine that whether relatively high serum tTGA levels can be used for predicting villous atrophy or not. The secondary aim was to determine the correlation of clinical profile with the Marsh grading and to find out the role of abdominal ultrasonography in CD, if any.
Material and Methods
The present study was a prospective cohort study carried out in the Department of Pediatrics, Umaid Hospital for Women & Children, Dr. S.N. Medical College, Jodhpur, over a period of twelve months. Sample size was calculated at power 80%, confidence interval (CI) 95%, precision error 3% and assuming prevalence of Celiac disease 1% as observed in a previous study by Makharia et al. in 2011 [15]. The resulting value of sample size came out to be 42. A total of 81 symptomatic children (anemia, diarrhea, bloating, abdominal pain, weight loss, short stature, anorexia, vomiting, constipation) between age group of 2–18 y were enroled from the outdoor and indoor wards of this tertiary care center as potential participants (Fig. 1). Patients with stool positive for Giardiasis (n = 5), IgA deficiency (n = 2) and who were already diagnosed with CD and were on gluten free diet (n = 7) were excluded from the study. A total of 67 patients (58 patients exhibiting positive tTGA serology and 9 patients showing negative serology but with consistent clinical suspicion fulfilled the eligible criteria for biopsy. Out of them 15 patients did not give consent for biopsy and were excluded from the study. Finally, a total of 52 cases were subjected to upper GI endoscopy by an experienced endoscopist, using a predefined endoscopy protocol [16]. Two biopsies each from the bulb (D1) and second part (D2) of the duodenum were taken [17]. Both specimens were processed and stained with H&E, and mucosal morphology was determined under light microscopy by a single trained pathologist without knowledge of serologic or clinical findings. Poorly oriented sections were dissected again until they were of good quality. Furthermore, the mucosal specimens were graded independently according to the Marsh–Oberhuber classification as follows: Marsh 0 denotes normal villi and crypt depth with no excess of intraepithelial lymphocytes (IEL), Marsh I–II denotes normal villi but intraepithelial lymphocytosis without (I) or with (II) hyperplastic crypts, and Marsh IIIa–c denotes varying villous atrophy with hyperplastic crypts [18]. Type 3 specimens (any degree of villous atrophy) were considered characteristic of CD. Marsh 1 and 2 lesions were considered nonspecific but consistent with CD diagnosis with positive serology. Anti-tTG IgA antibodies were tested in all patients using an enzyme-linked immunosorbent assay (ELISA) technique by a commercially available kit (Oscar EA 1910–9601 A). Antibody levels above 20 Au/ml were considered positive, as per the manufacturers’ recommended tTG cut-off value. Laboratory data including complete blood count (CBC), liver, and thyroid function tests, ferritin, iron, Total iron binding capacity (TIBC), and abdominal ultrasonography along with demographic and anthropometric data, clinical symptoms (general, gastrointestinal, extra-intestinal manifestations) and medical history of all the patients were recorded. Serum samples were collected at the time of endoscopic biopsy in most cases and always within one month before biopsy.
Approval for the study was obtained from the S. N. Medical College’s Institutional Research Review Board. Written informed consent was obtained from the parents or guardians. The nature, purpose and possible risks of the study were explained to the parents in detail before consent was obtained.
SPSS version 20.0 was used for analysis of the data. Numbers and percentages were used to denote categorical variables and mean + SD for denoting quantitative variables. Chi-square test was used for the analysis of categorical variables; similarly, student’s t test or ANOVA was used to assess quantitative variables. Spearman rank correlation test was used to assess associations between quantitative variables. Two-tailed test was used to reject null hypothesis using P < 0.05. For evaluating cut-off points for tTG antibodies as a predictor of Marsh scores, an analysis of receiver operating characteristics (ROC) curve was used.
Results
Twenty-seven (51.9%) boys and 25 (48.1%) girls were included in the study population. The mean age of the patients was 8.21 ± 3.45 y (Range: 2–16 y). The demographic characteristics of the patients are summarized in Table 1.
As shown in Table 1, the overall prevalence of anemia was 73.08% and anemia was the most common non-gastrointestinal (GI) sign. However, the authors could not find any significant association between Marsh grading and hemoglobin levels (r = 0.32, p > 0.05). Statistically no significant difference was noticed in GI symptoms between different Marsh gradings (p > 0.05). Serum tTGA levels were found positively correlated with Marsh grading (Spearmen correlation coefficient ρ = 0.74, p 0.000). Total seven patients had discordance between the serology and biopsy findings. Six patients had high tTGA titers (>100) and low biopsy grade (grade I & II) and one patient had low tTGA titers (<100) and higher biopsy grade (grade III). Significant differences were found in serum tTGA levels between different Marsh gradings (ANOVA test) (p 0.000). However when post hoc test was applied, significant differences were found between grade I and II (mean difference − 41.4, 95% CI:-66.6–16.28, p 0.002), grade II and IIIa (mean difference − 50.6, 95% CI:-93.44–7.7, p 0.02) and grade IIIa and IIIb (mean difference − 60.7, 95% CI:-119.6–1.83, p 0.04) while lost between grade IIIb and IIIc (mean difference − 34.45, 95% CI:-87.66 + 8.26, p 0.19). Figure 2 shows the increasing trend of mean tTG antibody titers from normal duodenal histology to complete atrophy.
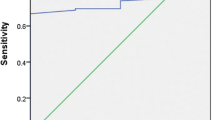
Receiver-operator curve (ROC) analysis was used to find a cut-off point for tTGA to discriminate, absence (Marsh ≤ III) vs. presence (Marsh IIIa-c) of villous atrophy (Fig. 3). Area under the curve (AUC) for graph was; AUC = 0.94 (Std. error 0.046 and 95% CI 0.84–0.98). Optimal cut-off points and corresponding sensitivity and specificities were calculated according to Youden index (J) = maximum {sensitivity – specificity - 1}. Cut-off value of serum tTG for predicting villous atrophy was 178.8 (nine times of cut-off value) with sensitivity of 100% and specificity of 85.7%.
The most common finding on abdominal ultrasound was distended bowel loops which were present in fifteen (28.84%) cases. Among co-morbidities, diabetes mellitus was the most common (11.3%), followed by hypothyroidism (7.69%).
Discussion
An increasing evidence has been found in support of the fact that serum tTGA level positively correlates with duodenal damage and a small intestinal biopsy has not been a requirement for diagnosis of CD, as these increased levels are highly suggestive of the disease. Further as per recent ESPGHAN guidelines for the diagnosis of CD in pediatric population, for those symptomatic individuals who show tTGA levels of at least 10 times the upper limit of normal range and who respond positively to the gluten free diet, histological confirmation is not needed [12]. As the guidelines are based on retrospective data, the possibility of selection bias cannot be completely overruled and thus there was a need for a prospective study to evaluate whether relatively high serum tTGA levels can be used to predict villous atrophy in clinical practice.
A prospective clinical CD diagnosis was the base for the study population in the present study and a positive correlation was found between tTGA levels and degrees of duodenal damage. Cut-off value of serum tTGA for predicting villous atrophy was 178.8 (nine times of cut-off value) with sensitivity of 100% and specificity of 85.7%. The complete reliance on serology for the diagnosis of CD is a correct method only if the positive predictive value (PPV) is near to 100%. It was prospectively shown in the study that all 14 out of 14 patients with a tTGA greater than 178.8 U/ml did indeed suffer from CD, giving a PPV of 100%.
A study by Mubarak and co-workers also confirms similar diagnostic approach that a small intestinal biopsy can be avoided in symptomatic pediatric patients with tTGA ≥100 U/ml. However, it can be questioned whether this perfect PPV will also be observed in asymptomatic patients due to the underrepresentation of asymptomatic patients in this cohort [19].
Concomitant to this, reference is made to other prospective study which was performed in a mix of pediatric and adult population. This study shows that 1 of the 72 patients, with a tTGA of at least 11.4 times the upper limit of normal, had a normal small intestinal biopsy, yielding a PPV of 98.6%, which has been considered by the author as insufficient for omission of biopsy. However, in this study, instead of the presence of symptoms, positive tTGA serology was taken as inclusion criteria for study population and which may have influenced the PPV [20]. Data were also prospectively collected by Onyeador and co-workers in 126 symptomatic children and findings were that 98.3% children with tTG > 100 U/ml (>10ULN) had histologically confirmed CD, however, total villous atrophy was associated more frequently with tTG level of >200 U/ml [21].
In an another prospective cohort study by Vivas and colleagues a comparison was made between adult and pediatric CD population. Their results show less severe histopathology (26% vs. 63%, P < 0.0001) and lower tTG antibody titers in adults than children. Strongly positive tTG antibody titres >30 U/ml might be sufficient for CD diagnosis in children without biopsy, however, it cannot be avoided in adults because of difference in disease presentation and monitoring [22].
In previous retrospective studies, similar comparable results have been noticed, showing that high tTGA levels are associated with histological lesions compatible with CD [13, 14]. Barker et al. showed that 48 of 49 mostly symptomatic children with a tTGA level ≥ 100 U/ml had positive biopsy results with 98% sensitivity and 97% specificity [23]. Comparably, Donaldson et al. showed that 38 of the 38 pediatric patients with tTGA ≥100 U/ml had Marsh III histopathology [24]. A subsequent retrospective study, also in a pediatric population, showed that all symptomatic patients with tTGA of at least 100 U/ml, in whom symptoms improve upon consuming a gluten free diet may not need a small intestine biopsy to confirm CD (n = 111), thereby reaching a PPV of 100% [25]. Recently a retrospective study in pediatric population showed that, a biopsy sparing protocol seems applicable to both symptomatic and asymptomatic patients with anti tTG titre ≥10 times ULN [26].
The current study has several methodological strengths. First, it is one of the few which implements a prospective, longitudinal design and therefore it is more likely that the results presented in this study are more easily generalized to other populations than those based on retrospective laboratory results. Second, in this study all patients were studied according to the same clinical, laboratory and histological protocol.
A limitation was that the authors had no information on those factors that may have affected the severity of histological lesions in CD, the genetic background, and the amount of gluten in the diet. Another possible limitation of index study is that it is likely to give false high sensitivities because of ascertainment bias, the possibility of previous serological testing performed by outside physicians to identify whom to refer for biopsy. In authors’ opinion, these two points deserve specific testing in future research activities.
Conclusions
The results of the present study show that serum tTGA level can be used to predict villous atrophy and biopsy may be avoided in strongly suspected cases with more than 9 times of cut-offs which will not only reduce the stress and inconveniences associated with the diagnosis, but will also save time and costs.
References
Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for celiac disease-related terms. Gut. 2013;62:43–52.
Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13–25.
Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–92.
Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001;120:636–51.
Ciclitira PJ. Celiac disease: a technical review. Gastroenterology. 2001;120:1526–40.
Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43.
Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med. 2010;123:691–3.
Lindfors K, Koskinen O, Kaukinen K. An update on the diagnostics of celiac disease. Int Rev Immunol. 2011;30:185–96.
Mubarak A, Nikkels P, Houwen R, Ten Kate F. Reproducibility of the histological diagnosis of celiac disease. Scand J Gastroenterol. 2011;46:1065–73.
Ravelli A, Villanacci V, Monfredini C, Martinazzi S, Grassi V, Manenti S. How patchy is patchy villous atrophy? Distribution pattern of histological lesions in the duodenum of children with celiac disease. Am J Gastroenterol. 2010;105:2103–10.
Pais WP, Duerksen DR, Pettigrew NM, Bernstein CN. How many duodenal biopsy specimens are required to make a diagnosis of celiac disease? Gastrointest Endosc. 2008;67:1082–7.
Husby S, Koletzko S, Korponay-Szabó IR, et al; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–60.
Hill PG, Holmes GK. Coeliac disease: a biopsy is not always necessary for diagnosis. Aliment Pharmacol Ther. 2008;27:572–7.
Dahlbom I, Korponay-Szabó IR, Kovács JB, Szalai Z, Mäki M, Hansson T. Prediction of clinical and mucosal severity of coeliac disease and dermatitis herpetiformis by quantification of IgA/IgG serum antibodies to tissue transglutaminase. J Pediatr Gastroenterol Nutr. 2010;50:140–6.
Makharia GK, Verma AK, Amarchand R, et al. Prevalence of celiac disease in the northern part of India: a community based study. J Gastroenterol Hepatol. 2011;26:894–900.
Aro P, Ronkainen J, Storskrubb T, et al. Valid symptom reporting at upper endoscopy in a random sample of the Swedish adult general population: the Kalixanda study. Scand J Gastroenterol. 2004;39:1280–8.
Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–81.
Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–94.
Mubarak A, Wolters VM, Gmelig-Meyling FH, Ten Kate FJ, Houwen RH. Tissue transglutaminase levels above 100 U/mL and celiac disease: a prospective study. World J Gastroenterol. 2012;18:4399–403.
Fernández-Bañares F, Alsina M, Modolell I, et al. Are positive serum-IgA-tissue-transglutaminase antibodies enough to diagnose coeliac disease without a small bowel biopsy? Post-test probability of celiac disease. J Crohns Colitis. 2012;6:861–6.
Onyeador N, Jennings N, Paul SP, Ramani P, Sandhu BK. The relationship between tissue transglutaminase antibody titers and histological classification in celiac disease. Arch Dis Child. 2014;99:A34.
Vivas S, Ruiz de Morales JG, Riestra S, et al. Duodenal biopsy may be avoided when high transglutaminase antibody titers are present. World J Gastroenterol. 2009;15:4775–80.
Barker CC, Mitton C, Jevon G, Mock T. Can tissue transglutaminase antibody titers replace small-bowel biopsy to diagnose celiac disease in select pediatric populations? Pediatrics. 2005;115:1341–6.
Donaldson MR, Firth SD, Wimpee H, et al. Correlation of duodenal histology with tissue transglutaminase and endomysial antibody levels in pediatric celiac disease. Clin Gastroenterol Hepatol. 2007;5:567–73.
Mubarak A, Wolters VM, Gerritsen SA, Gmelig-Meyling FH, Ten Kate FJ, Houwen RH. A biopsy is not always necessary to diagnose celiac disease. J Pediatr Gastroenterol Nutr. 2011;52:554–7.
Trovato CM, Montuori M, Anania C, et al. Are ESPGHAN “biopsy sparing” guidelines for celiac disease also suitable for asymptomatic patients? Am J Gastroenterol. 2015;110:1485–9.
Contributions
RJ, VP and PS: Conceptualized the study, developed search strategy; VR: Did the study, analyzed the data and wrote the manuscript; SKV: Helped in preparing the manuscript. All authors approved the final version of manuscript. RJ will act as guarantor for the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Source of Funding
None.
Rights and permissions
About this article
Cite this article
Jora, R., Raghuvanshi, V., Payal, V. et al. Correlation of Tissue Transglutaminase with Modified Marsh Grading in Celiac Disease: A Prospective Cohort Study. Indian J Pediatr 84, 515–520 (2017). https://doi.org/10.1007/s12098-017-2323-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-017-2323-3