Abstract
Background
A celiac disease (CD) diagnosis is based on duodenal histology, with the exception of children showing anti-tissue transglutaminase (anti-tTG) serum levels exceeding ten times the cut-off. Our aim was to reproduce this simplified approach in adults, identifying an anti-tTG threshold value useful to diagnose CD without endoscopic procedures.
Methods
A total of 671 adult CD patients were subjected to blood sampling to determine anti-tTG serum levels, as well as to endoscopy with biopsy to perform duodenal histology. The anti-tTG serum levels/cut-off ratio was compared with the degree of duodenal lesions.
Results
Anti-tTG serum levels/cut-off ratio determined in patients with type IIIc was significantly higher than that measured in patients with type IIIb (p < 0.001), IIIa (p < 0.001), II (p < 0.05) and 0 (p < 0.001) of Marsh–Oberhuber histological classification. A significant correlation (r = 0.297, p < 0.0001) was found between the anti-tTG serum levels/cut-off ratio and the degree of duodenal lesions. The anti-tTG serum levels/cut-off ratio was classified as an accurate parameter (AUC = 0.715, p < 0.0001), with the best diagnostic performance obtained considering the threshold value >3.6 (sensitivity = 76.8 %, PPV = 97.2 %).
Conclusions
The anti-tTG serum levels/cut-off ratio correlates with the degree of duodenal lesions and, if used with the threshold value >3.6, could avoid endoscopy with biopsy in about 75 % of seropositive adults waiting for CD diagnosis. However, since this procedure could also imply CD diagnosis in almost 3 % of seropositive patients with normal villous architecture, a consensus opinion is needed to suggest its use in the diagnosis of adult CD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Celiac disease (CD) is a chronic, immune-mediated small bowel disorder triggered by the ingestion of wheat gluten (gliadins and glutenins) and related proteins of rye and barley in human leukocyte antigen (HLA) DQ2- and/or DQ8-positive individuals [1].
The gluten-induced duodenal mucosa alterations consist of intraepithelial lymphocytosis, crypt hyperplasia and villous atrophy that usually recover after gluten withdrawal from the diet [2]. Moreover, CD patients develop antibodies against tissue transglutaminase (anti-tTG) and endomysium (EMA), suggesting an autoimmune mechanism [3], as well as antibodies against deamidated gliadin peptides (anti-DGP) [4, 5]. Despite that it was previously thought that CD occurred mainly in white European children, up to today, new highly sensitive immunological assays have identified an increasing number of CD patients of all ages in many different countries (approximately 0.5–1 % in Western European and North American populations) [2, 6].
The diagnostic criteria for CD proposed by the European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) in 1990 were based on clinical case identification, detection of specific circulating antibodies, and consequent histological finding of duodenal mucosa damage (type III according to Marsh–Oberhuber classification) [7, 8]. On the basis of current knowledge and diagnostic tools, the new ESPGHAN guidelines for CD stress the crucial role that serological and genetic tests can play in symptomatic and asymptomatic subjects. In detail, children and adolescents with signs and symptoms suggestive of CD can avoid duodenal biopsy for diagnostic purposes if presenting anti-tTG serum levels ten times above the upper limit of normal (10× ULN), supported by EMA detection and HLA typing. Conversely, symptomatic patients with positive anti-tTG antibody levels lower than 10× ULN should undergo upper endoscopy. IgA anti-tTG and total IgA serum determination is recommended in asymptomatic children and adolescents in case of HLA-DQ2- and/or DQ8-positive results. If positive anti-tTG antibody levels are lower than 3× ULN, patients should undergo EMA confirmation and upper endoscopy. Conversely, if anti-tTG are positive with levels higher than 3× ULN, patients should undergo upper endoscopy [9].
Since the new ESPGHAN guidelines are focused on the crucial role of serological tests in the pediatric population, our aim was to reproduce this simplified diagnostic approach in adults. For this purpose, we compared the anti-tTG serum levels with the degree of duodenal lesions to identify an anti-tTG threshold value useful to diagnose CD in adults avoiding endoscopic procedures.
Materials and methods
Patients
Six hundred seventy-one consecutive CD patients [149 male/522 female (ratio 1:3.5), median age 34, range 18–65 years], who were referred to our Gastroenterology Unit from February 2009 to October 2014, were evaluated in this retrospective study. In agreement with the British Society of Gastroenterology (BSG) diagnostic guidelines [10], patients before CD diagnosis were on a gluten-containing diet, were clinically evaluated and subjected to blood collection in order to detect serum antibodies and, in those showing discordant results, to type HLA. All patients also underwent upper endoscopy with biopsy sampling to perform duodenal histology.
All procedures followed in this study were made for diagnostic purposes and, therefore, were in accordance with the ethical standards of the institutional committee responsible for human experimentation. Furthermore, informed consent was obtained from each participant being studied.
Anti-tissue transglutaminase antibody determination
IgA anti-tTG antibodies were measured in serum samples diluted 1:101 by enzyme-linked immunosorbent assay (ELISA) on microtiter-plate wells coated with recombinant human tTG (QUANTA Lite R h-tTG IgA; INOVA Diagnostics, San Diego, CA; distributed by Instrumentation Laboratory, Milan, Italy). Results, firstly quantified by an ELISA plate reader at 450 nm (A 450nm), were compared with a standard reference curve (1.23, 3.70, 11.1, 33.3, 100 U/ml) and then expressed in U/ml. Samples with a concentration exceeding the higher standard were further diluted, reanalyzed and their results were multiplied by the dilution factor. According to the manufacturer’s instructions (negative <4 U/ml; weak positive 4–10 U/ml; positive >10 U/ml), the antibody level 4 U/ml was used as a cut-off to identify anti-tTG-positive results. Data were finally expressed as anti-tTG serum levels/cut-off ratio (absolute number).
To investigate the reproducibility of this ELISA test, we calculated the intra- and inter-assay coefficient of variation (CV). For the intra-assay variation, 20 reference samples were tested four times in the same run; CV (range) was 0.7–3.2 %. For the inter-assay variation, the same 20 reference samples were tested one time in three different runs; CV (range) was 1.8–7.9 %.
Endomysium antibody detection
IgA EMA were searched in serum samples diluted 1:5 by indirect immunofluorescence analysis (iIFA) on cryostat sections of monkey esophagus (Eurospital, Trieste, Italy). Results, expressed as “positive/negative”, were blindly evaluated by two trained observers (agreement rate 99.6 %). EMA-positive results were identified by the typical honeycomb-like staining pattern along muscularis mucosae that marks the collagenous matrix of type 3 connective tissue surrounding the smooth muscle fibers (endomysium) of the primate esophagus.
DNA extraction and HLA typing
Genomic DNA was extracted from whole blood samples using a salting-out procedure [11]. Patients showing anti-tTG-positive but EMA-negative results were typed for DQA1 and DQB1 genes by sequence-specific primer-polymerase chain reaction (SSP-PCR) using commercial kits according to the manufacturer’s instructions (Dynal Biotech, Bromborough, UK). Results were expressed as HLA-DQ2-positive to indicate patients carrying both DQA1*05 and DQB1*02 alleles encoding a complete DQ2 molecule in a single or double dose; HLA-DQ8-positive for patients carrying both DQA1*03 and DQB1*0302 alleles encoding a complete DQ8 molecule in a single or double dose; HLA-DQ2/DQ8-positive to indicate patients carrying DQA1*05 and DQB1*02 alleles in heterozygosis with DQA1*03 and DQB1*0302 alleles overall encoding complete DQ2 and DQ8 molecules; HLA-DQ2/DQ8-negative for patients who do not carry the alleles mentioned above.
Upper endoscopy with biopsy sampling
All patients underwent upper endoscopy after fasting for at least 8 h. In most cases, conscious sedation was obtained using pharyngeal anesthesia with lidocaine spray 1 % and midazolam 2 mg i.v. In a few cases, deep sedation using propofol had to be carried out. At least four biopsy specimens were obtained, including a duodenal bulb biopsy from each patient using a standard 5-mm biopsy forceps, correctly oriented on acetate cellulose filters (Bio-Optica, Milan, Italy), embedded in 10 % formaldehyde, and subjected to histological analysis.
Histological analysis
Duodenal biopsy samples were fixed overnight in 4 % formalin. After orientation, inclusion in paraffin and cutting, morphologic analysis was performed by hematoxylin and eosin (HE) staining technique. To better evaluate the small intestine mucosal architecture, villous height/crypt depth ratio was measured and values <3:1 were considered indicative of morphologic alteration. The number of intraepithelial lymphocytes (IELs) per 100 intestinal epithelial cells (IECs) was evaluated by the immunohistochemical staining for CD3 lymphocyte surface marker. The upper limit of the reference interval was 25 IELs/100 IECs. The histological pattern was blindly evaluated by a trained pathologist according to Marsh–Oberhuber classification: type 0 indicates a normal histology; type I (infiltrative) is characterized by an increased number of IELs; type II (hyperplastic) also indicates crypt hyperplasia; type III (destructive) is also characterized by a partial (IIIa), subtotal (IIIb) or total (IIIc) villous atrophy; type IV is represented by villous atrophy alone [8].
Statistical analysis
Data achieved in this study were firstly analyzed by the D’Agostino–Pearson omnibus test to verify the normal distribution hypothesis within each statistical sample. Given that some resulting p values were significant (p < 0.05), it is reasonable to assume that not all data obtained from every group of patients fall into Gaussian distributions and therefore, were synthesized as median value and quartile cut-points (first and third quartile) or range (maximum and minimum value). For the same reason, data were processed by means of non-parametric tests.
Comparisons among the anti-tTG serum levels/cut-off ratio obtained from patients grouped according to the degree of duodenal lesions were performed by using the Kruskal–Wallis test where, for overall p values <0.05, the Dunn multiple comparison was used as post test. The relationship between the anti-tTG serum levels/cut-off ratio and the degree of duodenal lesions obtained from all patients in the study was assessed by the Spearman rank correlation. The analysis of receiver operating characteristic (ROC) curve was carried out to determine the optimal threshold value of the anti-tTG serum levels/cut-off ratio, as well as to evaluate its diagnostic performance through the assessment of both area under curve (AUC) and Youden index calculated as (sensitivity + specificity) − 1. In detail, the optimal threshold value was identified as the point of the ROC curve where the Youden index assumes the highest possible value, while the anti-tTG serum levels/cut-off ratio was classified as not informative if AUC = 0.50, inaccurate for 0.51 ≤ AUC ≤ 0.70, moderately accurate for 0.71 ≤ AUC ≤ 0.90, highly accurate for 0.91 ≤ AUC ≤ 0.99, perfect if AUC = 1.00. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), diagnostic accuracy, and their 95 % confidence interval (95 % CI) were also calculated.
In all tests applied, the p values <0.05 were considered significant. The statistical evaluations were performed by using the GraphPad Prism package version 5.2 (GraphPad Software Inc., San Diego, CA, USA) for the D’Agostino–Pearson omnibus test, Kruskal–Wallis test, Dunn multiple comparison and Spearman rank correlation, as well as the MedCalc Software version 14.8.1 (MedCalc Software bvba, Ostend, Belgium) for the ROC curve analysis.
Results
Four hundred seventy-eight out of 671 (71.2 %) CD patients enrolled in the study mainly complained intestinal symptoms including abdominal pain, swelling, diarrhea, and dyspepsia (typical presentation). The remaining 193 (28.8 %) patients presented systemic manifestations, such as iron deficiency anemia, weight loss, osteopenia/osteoporosis, and dental enamel hypoplasia (atypical presentation).
All 671 (100 %) CD patients presented IgA anti-tTG serum levels over the cut-off, while only 635 of them (94.6 %) showed IgA EMA-positive results. Of the 36 (5.4 %) patients showing anti-tTG-positive but EMA-negative results, 33 (91.7 %) tested positive for HLA-DQ2, 2 (5.5 %) for HLA-DQ8 and one (2.8 %) for HLA-DQ2/DQ8.
Six hundred thirty-three out of 671 (94.3 %) CD patients showed intestinal villous atrophy (98 patients type IIIa, 199 type IIIb, 333 type IIIc, and three type IV according to Marsh–Oberhuber classification). The remaining 38 (5.7 %) patients presented normal villous architecture and, accordingly, were classified as having potential CD (12 patients type 0) or mild enteropathy CD (11 patients type I and 15 type II). Of the 36 symptomatic patients showing anti-tTG and HLA-positive but EMA-negative results, 30 (83.3 %) presented intestinal villous atrophy (nine patients type IIIa, 14 type IIIb and seven type IIIc) and only six (16.7 %) had normal villous architecture (four patients type 0 and two type I).
All demographic, clinical, serological, and histological data obtained from CD patients in study are summarized in Table 1.
Moreover, the anti-tTG serum levels were compared with the degree of duodenal lesions. As result, the anti-tTG serum levels/cut-off ratio determined in patients with type IIIc was significantly higher than that measured in patients with type IIIb (p < 0.001), IIIa (p < 0.001), II (p < 0.05), and 0 (p < 0.001) (Fig. 1). A significant correlation (r = 0.297, p < 0.0001) was found between the anti-tTG serum levels/cut-off ratio and the degree of duodenal lesions obtained from all patients studied (Fig. 2).
Anti-tTG serum levels/cut-off ratio in CD patients grouped according to the degree of duodenal lesions. Data are reported as median value and quartile cut-points (first and third quartile). The degree of duodenal lesions is expressed according to Marsh–Oberhuber classification with types 0, I, and II indicating normal villous architecture; types IIIa, IIIb, IIIc, and IV representing intestinal villous atrophy. The p values refer to Dunn multiple comparison applied as Kruskal–Wallis post-test between each groups’ pair. Anti-tTG anti-tissue transglutaminase, CD celiac disease
Correlation between the anti-tTG serum levels/cut-off ratio and the degree of duodenal lesions obtained from all CD patients. The degree of duodenal lesions is expressed according to Marsh–Oberhuber classification with types 0, I, and II indicating normal villous architecture; types IIIa, IIIb, IIIc, and IV representing intestinal villous atrophy. The r and p values refer to Spearman rank correlation applied between the parameters above mentioned. The best-fit line (solid line) and its 95 % confidence bands (dashed lines) are plotted in the graph. Anti-tTG anti-tissue transglutaminase, CD celiac disease
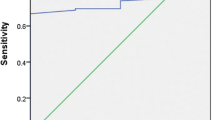
According to the ROC curve analysis (Fig. 3), the anti-tTG serum levels/cut-off ratio was classified as a moderately accurate parameter to identify CD patients with intestinal villous atrophy (AUC = 0.715, p < 0.0001), with the best diagnostic performance achieved by considering the threshold value >3.6 as optimal (sensitivity = 76.8 %, specificity = 63.2 %, Youden index = 0.399, PPV = 97.2 %, NPV = 14.0 %, diagnostic accuracy = 76.0 %). These data are summarized in Table 2, in comparison with the diagnostic performance obtained using the threshold value >10 as the ESPGHAN guidelines indicate for pediatric population.
ROC curve analysis of anti-tTG serum levels/cut-off ratio determined in all CD patients. The solid line corresponds to the ROC curve with the optimal threshold value marked by an empty circle, while the dashed line refers to the worst achievable curve (AUC = 0.50). The optimal threshold value, sensitivity, specificity, Youden index, AUC and relative p value are reported in the graph. Anti-tTG anti-tissue transglutaminase, AUC, area under curve, CD celiac disease, J Youden index, ROC receiver operating characteristic
Considering the threshold value >3.6, the anti-tTG serum levels/cut-off ratio was positive in 500 out of 671 (74.5 %) CD patients. Four hundred eighty-six out of these 500 (97.2 %) patients showed intestinal villous atrophy, whereas the remaining 14 (2.8 %) had normal villous architecture. On the other hand, using the threshold value >10, the anti-tTG serum levels/cut-off ratio was positive in only 216 out of 671 (32.2 %) CD patients, of whom 212 (98.1 %) showed intestinal villous atrophy and the remaining four (1.9 %) had normal villous architecture (Fig. 4).
Overall results of anti-tTG serum levels/cut-off ratio obtained using different threshold values. Data are reported as absolute and relative frequency of patients showing positive or negative results for anti-tTG serum levels/cut-off ratio and, within these, as relative frequency of cases presenting positive or negative histology. The threshold value >3.6 was identified by the ROC curve analysis, while the threshold value >10 is currently used in the pediatric population. Anti-tTG anti-tissue transglutaminase, ROC receiver operating characteristic
Discussion
Unlike in the past, CD is universally recognized as a common condition that involves not only children, but patients at any age presenting both intestinal and extra-intestinal manifestations [6]. According to ESPGHAN guidelines established in 1970 [12], a correct diagnosis of CD required a first duodenal biopsy showing villous atrophy, a second biopsy with histological recovery after gluten-free diet, and a third biopsy showing the histological relapse after gluten challenge. These criteria have been modified over time, until serial biopsies and gluten challenge have been excluded by new guidelines established in 1990. CD diagnosis was indeed based on a combination of clinical findings, serum-positive results, and histological demonstration of intestinal villous atrophy [7]. However, the latest advances in the field of serology have further revolutionized the diagnosis and monitoring of CD. An international, multicenter pediatric study showed that high anti-tTG serum levels are indicative of severe intestinal lesions [13]. Even Diamanti and colleagues found a strong correspondence between anti-tTG serum levels and the extent of mucosal injury. In particular, anti-tTG values >5× ULN seemed to be highly predictive of villous atrophy [14]. A retrospective study showed that pediatric patients with both symptoms suggestive of CD and very high anti-tTG serum titers could be considered affected by CD, even in the absence of a small-bowel biopsy. In detail, anti-tTG values >5× ULN had a sensitivity and a specificity of 98 and 97.2 %, respectively [15]. Two other studies confirmed that strongly positive anti-tTG serum levels might be sufficient for CD diagnosis in children, with anti-tTG values >3× ULN or >10× ULN as highly predictive of villous atrophy, respectively [16, 17]. On the basis of a systematic literature review, in 2012, 17 ESPGHAN experts produced new guidelines reflecting the rising role and relevance of serum antibodies, able to provide CD diagnosis without the need of duodenal biopsy. Therefore, children and adolescents with signs and symptoms suggestive of CD and anti-tTG serum levels >10× ULN, confirmed by EMA detection and HLA typing, can get CD diagnosis [9].
Recently, 21 BSG experts recently developed diagnostic guidelines for adult CD in which duodenal biopsy remains essential and cannot be replaced by serology. Patients with coagulopathies and pregnant women are exceptions in whom duodenal biopsy may not be feasible or should be postponed until postpartum [10]. However, these guidelines are not universally accepted [18] and recent studies showed that an appropriate cut-off for anti-tTG antibodies can allow CD diagnosis in a high proportion of adults with a PPV of 100 % [19] and that, a combination of serological tests can avoid duodenal biopsy in 78 % of cases [20]. Although these studies report excellent predictive values for CD, a standardized anti-tTG threshold value to avoid duodenal biopsy is still lacking.
On the basis of these considerations, our aim was to compare the anti-tTG serum levels with the degree of duodenal lesions to identify an anti-tTG threshold value able to diagnose CD in adults avoiding endoscopic procedures. For this purpose, we evaluated 671 seropositive CD patients with intestinal symptoms and/or systemic manifestations, who completed the current diagnostic protocol. The patients’ stratification according to histological classification of Marsh–Oberhuber was fairly homogeneous among types 0, I, and II, growing from type IIIa to IIIc, and poor for type IV which is probably associated with refractory CD that can evolve in enteropathy-associated T cell lymphoma [21]. In patients with type IIIc, the anti-tTG serum levels/cut-off ratio was significantly higher than in patients with type IIIb, IIIa, II, and 0. The absence of other significant differences is attributable to the variability among anti-tTG serum levels found by the omnibus test of D’Agostino–Pearson (p < 0.05), as well as to the small number of patients especially belonging to types 0, I, II, and IV. A growing trend of the anti-tTG serum levels/cut-off ratio was however evident in patients with duodenal lesions from type 0 to IV. Consistently, the anti-tTG serum levels/cut-off ratio was significantly correlated with the degree of duodenal lesions, although with a modest correlation index (r = 0.297). Also, this finding is likely due to the variability found among anti-tTG serum levels. Our data thus confirm previous studies showing that anti-tTG serum levels themselves correlate with the degree of duodenal lesions [22, 23].
The ROC curve analysis allowed to classify the anti-tTG serum levels/cut-off ratio as an accurate parameter in predicting the intestinal villous atrophy in adult CD patients (AUC = 0.715, p < 0.0001), with the best diagnostic performance achieved by considering the threshold value >3.6 as optimal, mainly in terms of sensitivity (76.8 %) and PPV (97.2 %). Using the threshold value >10 as proposed for the pediatric population [9], the sensitivity decreased to 33.5 % and the PPV remained almost unchanged (98.1 %), confirming data recently obtained by Sugai and co-workers [24]. This finding is probably due to the higher variability and the lower levels of serum anti-tTG in adults than in children [13, 16], suggesting that two different anti-tTG threshold values should be chosen for adult and pediatric populations.
In this scenario, our data suggest a possible simplification in the diagnostic protocol of CD according to the flow chart reported in Fig. 5. Briefly, all adult patients suspected for CD should be screened by means of serum IgA anti-tTG antibody determination. Patients showing anti-tTG negative results should be excluded from the diagnostic protocol or be screened for selective IgA deficiency in case of strong clinical suspicion [25, 26].
Those patients presenting anti-tTG-positive results with levels >3.6× ULN should be subjected to serum IgA EMA detection as confirmation. In these circumstances, if EMA test shows positive results, CD can be diagnosed without duodenal biopsy, so invasive, expensive, and unpleasant endoscopic procedures can be avoided [18]. In case of EMA-negative results, HLA typing is mandatory. Patients showing HLA-negative results in these conditions should undergo differential diagnosis with illnesses known to be associated to anti-tTG false-positive results, such as arthritic diseases [27], inflammatory bowel disease [28], and cardiovascular disorders [29]. Conversely, if HLA test is positive, upper endoscopy with duodenal biopsy should be performed.
Those patients showing anti-tTG-positive results with levels <3.6× ULN should follow the same above-mentioned protocol, except in the case of EMA-positive results in which endoscopic procedures and histological examination have to be performed. Moreover, the organ culture system may support histology in CD diagnosis in case of patchy atrophy, mild enteropathy, or normal histological finding as observed in potential CD [30–33].
The major limitation of our study concerns the absence of asymptomatic patients from serological screening or at-risk groups (e.g., first-degree relatives). Further studies also evaluating these patients are needed because the ESPGHAN diagnostic guidelines describe different algorithms for symptomatic and symptomless individuals [9]. Moreover, the anti-tTG serum levels were measured using only a commercial kit. Since it has previously been shown that the different anti-tTG commercial kits have a high variability rate [9], further studies evaluating this aspect would be welcome.
In conclusion, our data show that the anti-tTG serum levels/cut-off ratio correlates significantly with the degree of duodenal lesions and accordingly, the anti-tTG threshold value >3.6× ULN could avoid the upper endoscopy with biopsy sampling in 74.5 % of seropositive adults waiting for a diagnosis of CD. According to this procedure, 2.8 % of them could be, however, diagnosed as CD although presenting normal villous architecture. Our data also show that the anti-tTG threshold value >10× ULN, currently used in the pediatric population, could avoid endoscopic procedures in only 32.2 % of seropositive adults, with a slightly lower risk of diagnosing CD in patients with negative histology (1.9 %). This finding confirms that two different anti-tTG threshold values should be chosen for adult and pediatric populations. However, further investigations are needed to confirm our data and obtain a consensus opinion before suggesting this procedure in the diagnostic work-up of adult CD.
Abbreviations
- 95 % CI:
-
95 % confidence interval
- Anti-DGP:
-
Anti-deamidated gliadin peptides
- Anti-tTG:
-
Anti-tissue transglutaminase
- AUC:
-
Area under curve
- BSG:
-
British Society of Gastroenterology
- CD:
-
Celiac disease
- CV:
-
Coefficient of variation
- ELISA:
-
Enzyme-linked immunosorbent assay
- EMA:
-
Endomysium antibodies
- ESPGHAN:
-
European Society of Pediatric Gastroenterology, Hepatology and Nutrition
- HE:
-
Hematoxylin and eosin
- HLA:
-
Human leukocyte antigen
- IECs:
-
Intestinal epithelial cells
- IELs:
-
Intraepithelial lymphocytes
- iIFA:
-
Indirect immunofluorescence analysis
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristic
- SSP-PCR:
-
Sequence-specific primer-polymerase chain reaction
- ULN:
-
Upper limit of normal
References
Green PH, Lebwohl B, Greywoode R. Celiac disease. J Allergy Clin Immunol. 2015;135:1099–106.
Abadie V, Sollid LM, Barreiro LB, et al. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu Rev Immunol. 2011;29:493–525.
Alaedini A, Green PH. Autoantibodies in celiac disease. Autoimmunity. 2008;41:19–26.
Volta U, Granito A, Parisi C, et al. Deamidated gliadin peptide antibodies as a routine test for celiac disease: a prospective analysis. J Clin Gastroenterol. 2010;44:186–90.
Di Sabatino A, Vanoli A, Giuffrida P, et al. The function of tissue transglutaminase in celiac disease. Autoimmun Rev. 2012;11:746–53.
Kang JY, Kang AH, Green A, et al. Systematic review: worldwide variation in the frequency of celiac disease and changes over time. Aliment Pharmacol Ther. 2013;38:226–45.
Walker-Smith JA, Guandalini S, Schmitz J. Revised criteria for diagnosis of celiac disease. Arch Dis Child. 1990;65:909–11.
Oberhuber G, Granditsch G, Vogelsang H. The histopathology of celiac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–94.
Husby S, Koletzko S, Korponay-Szabó IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of celiac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–60.
Ludvigsson JF, Bai JC, Biagi F, et al. Diagnosis and management of adult celiac disease: guidelines from the British Society of Gastroenterology. Gut. 2014;63:1210–28.
Miller SA, Dykes DD, Polesky HF. A simple salting-out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215.
Meeuwisse GW. Diagnostic criteria in celiac disease. Acta Paediatr Scand. 1970;58:461–3.
Fabiani E. Catassi C; International Working Group. The serum IgA class anti-tissue transglutaminase antibodies in the diagnosis and follow up of celiac disease. Results of an international multi-centre study. International Working Group on Eu-tTG. Eur J Gastroenterol Hepatol. 2001;13:659–65.
Diamanti A, Colistro F, Calce A, et al. Clinical value of immunoglobulin A antitransglutaminase assay in the diagnosis of celiac disease. Pediatrics. 2006;118:e1696–700.
Barker CC, Mitton C, Jevon G, et al. Can tissue transglutaminase antibody titers replace small-bowel biopsy to diagnose celiac disease in select pediatric populations? Pediatrics. 2005;115:1341–6.
Vivas S, Ruiz de Morales JG, Riestra S, et al. Duodenal biopsy may be avoided when high transglutaminase antibody titers are present. World J Gastroenterol. 2009;15:4775–80.
Mubarak A, Wolters VM, Gerritsen SA, et al. A biopsy is not always necessary to diagnose celiac disease. J Pediatr Gastroenterol Nutr. 2011;52:554–7.
Hill P, Austin A, Forsyth J, et al. British Society of Gastroenterology guidelines on the diagnosis and management of celiac disease. Gut. 2015;64:691–2.
Tortora R, Imperatore N, Capone P, et al. The presence of anti-endomysial antibodies and the level of anti-tissue transglutaminases can be used to diagnose adult celiac disease without duodenal biopsy. Aliment Pharmacol Ther. 2014;40:1223–9.
Bürgin-Wolff A, Mauro B, Faruk H. Intestinal biopsy is not always required to diagnose celiac disease: a retrospective analysis of combined antibody tests. BMC Gastroenterol. 2013;13:19.
Malamut G, Cellier C. Complications of celiac disease. Best Pract Res Clin Gastroenterol. 2015;29:451–8.
Taavela J, Kurppa K, Collin P, et al. Degree of damage to the small bowel and serum antibody titers correlate with clinical presentation of patients with celiac disease. Clin Gastroenterol Hepatol. 2013;11:166–71.
Singh P, Kurray L, Agnihotri A, et al. Titers of anti-tissue transglutaminase antibody correlate well with severity of villous abnormalities in celiac disease. J Clin Gastroenterol. 2015;49:212–7.
Sugai E, Hwang HJ, Vázquez H, et al. Should ESPGHAN guidelines for serologic diagnosis of celiac disease be used in adults? A prospective analysis in an adult patient cohort with high pretest probability. Am J Gastroenterol. 2015;110:1504–5.
Picarelli A, Di Tola M, Sabbatella L, et al. Identification of a new celiac disease subgroup: antiendomysial and anti-transglutaminase antibodies of IgG class in the absence of selective IgA deficiency. J Intern Med. 2001;249:181–8.
Wang N, Truedsson L, Elvin K, et al. Serological assessment for celiac disease in IgA deficient adults. PLoS One. 2014;9:e93180.
Picarelli A, Di Tola M, Sabbatella L, et al. Anti-tissue transglutaminase antibodies in arthritic patients: a disease-specific finding? Clin Chem. 2003;49:2091–4.
Di Tola M, Sabbatella L, Anania MC, et al. Anti-tissue transglutaminase antibodies in inflammatory bowel disease: new evidence. Clin Chem Lab Med. 2004;42:1092–7.
Di Tola M, Barillà F, Trappolini M, et al. Antitissue transglutaminase antibodies in acute coronary syndrome: an alert signal of myocardial tissue lesion? J Intern Med. 2008;263:43–51.
Picarelli A, Di Tola M, Sabbatella L, et al. Usefulness of the organ culture system in the in vitro diagnosis of celiac disease: a multicentre study. Scand J Gastroenterol. 2006;41:186–90.
Santaolalla R, Fernández-Bañares F, Rodríguez R, et al. Diagnostic value of duodenal antitissue transglutaminase antibodies in gluten-sensitive enteropathy. Aliment Pharmacol Ther. 2008;27:820–9.
Tosco A, Aitoro R, Auricchio R, et al. Intestinal anti-tissue transglutaminase antibodies in potential celiac disease. Clin Exp Immunol. 2013;171:69–75.
Picarelli A, Di Tola M, Marino M, et al. Usefulness of the organ culture system when villous height/crypt depth ratio, intraepithelial lymphocyte count, or serum antibody tests are not diagnostic for celiac disease. Transl Res. 2013;161:172–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Di Tola, M., Marino, M., Goetze, S. et al. Identification of a serum transglutaminase threshold value for the noninvasive diagnosis of symptomatic adult celiac disease patients: a retrospective study. J Gastroenterol 51, 1031–1039 (2016). https://doi.org/10.1007/s00535-016-1188-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-016-1188-y