Abstract
Gliomas are primary intracranial tumors with defined molecular markers available for precise diagnosis. The prognosis of glioma is bleak as there is an overlook of the dynamic crosstalk between tumor cells and components of the microenvironment. Herein, different phases of gliomagenesis are presented with reference to the role and involvement of secreted proteomic markers at various stages of tumor initiation and development. The secreted markers of inflammatory response, namely interleukin-6, tumor necrosis factor-α, interferon-ϒ, and kynurenine, proliferation markers human telomerase reverse transcriptase and microtubule-associated-protein-Tau, and stemness marker human-mobility-group-AThook-1 are involved in glial tumor initiation and growth. Further, hypoxia and angiogenic factors, heat-shock-protein-70, endothelial-growth-factor-receptor-1 and vascular endothelial growth factor play a major role in promoting vascularization and tumor volume expansion. Eventually, molecules such as matrix-metalloprotease-7 and intercellular adhesion molecule-1 contribute to the degradation and remodeling of the extracellular matrix, ultimately leading to glioma progression. Our study delineates the roadmap to develop and evaluate a non-invasive panel of secreted biomarkers using liquid biopsy for precisely evaluating disease progression, to accomplish a clinical translation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioma remains a dreaded brain malignancy marked by a short-life expectancy, increased invasiveness, and a high relapse rate. More than 100,000 cases of central nervous system (CNS) cancers are diagnosed each year worldwide, and gliomas represent 35–40% of all brain tumors [1]. The currently practiced multimodal-clinical protocol for treatment entails surgical excision followed by adjuvant chemotherapy and/or radiotherapy [2]. Still, significant issues of treatment failure remain, namely, inaccessible location of the tumor [3], incomplete resection [4], and development of chemo-radio-resistance [5], apart from deferment of adjuvant therapy by the patient in some cases. Achieving a total surgical resection is generally difficult as the glial tumor lacks precise edges thereby leading to high recurrence and mortality levels. In this backdrop, the median survival of glioma patients has remained mostly unchanged over the past decade; even in low-grade glioma, it is documented to lie between 5 to 10 years [6], while glioblastoma (GB) has a worse survival rate of just 12–15 months [7].
Current scenario of molecular diagnosis and prognosis
The molecular stratification of brain tumors released by the World Health Organization (WHO 2021) has become an integral part of clinical diagnosis, prognosis, and therapy decision-making. The initial diagnosis of glioma is based on screening using imaging techniques followed by tissue biopsy [8]. However, computerized tomography scans or magnetic resonance imaging cannot reliably differentiate non-neoplastic lesions, the extent of actual tissue infiltration by the tumor, and glial tumor sub-types [9, 10]; hence tissue biopsy becomes unavoidable. In the last decade, the WHO classification of CNS tumors [11] laid down specific molecular markers to add value to histopathology and define the tumor entities. In accordance, several studies validated tissue-based diagnostic markers, which are now used in the clinical setup. Isocitrate dehydrogenase-1/2 (IDH-1/2), alpha-thalassemia/mental retardation X-linked gene (ATRX), epidermal growth factor receptor (EGFR), phosphatase and tensin homolog (PTEN), glial fibrillary acidic protein (GFAP), O-6-methylguanine-DNA methyltransferase (MGMT), BRAFV600E (B-Raf), human telomerase reverse transcriptase (hTERT), tumor protein 53 (TP53), histone H3F3A have been validated as diagnostic makers in tissue biopsies [12, 13]. Some of these tissue-based diagnostic markers have been studied for their expression in blood samples. Kiviniemi et al. reported high levels of serum GFAP determined by enzyme-linked immune sorbent assay (ELISA) in primary and recurrent high-grade gliomas (HGG). They correlated this expression to the lack of IDH-1 mutation [14]. Manda and his co-workers detected EGFRvIII positivity in serum of HGG patients by semiquantitative polymerase chain reaction technique and stated that it can serve as a non-invasive marker for diagnosis of this grade [15].
In addition to the molecular and histological typing for diagnosis, assessing a glial tumor’s progression is equally essential, to define the likely outcome in terms of disease recurrence and overall survival (OS) of the patient. According to a few citations available in the literature, the molecular markers commonly tested for prognosis in tissue biopsies as a part of the routine clinical investigation include IDH-1/2, EGFR, PTEN, MGMT, TP53, hTERT, platelet-derived growth factor receptor (PDGFR); however, the studies are based only on GB samples and not glioma per se [16, 17]. More importantly, tissue-based prognostic biomarkers are less convenient in the clinical setup for several reasons, viz., repeated biopsy by a surgical procedure is invasive and involves high cost. Sometimes, it cannot be performed when the patient’s clinical condition has worsened or when a tumor is surgically inaccessible. Looking at the heterogeneity of gliomas, circulating markers in the blood (liquid biopsy) might better represent the entire tumor cell population than a classical tissue biopsy [18] Liquid biopsy can thus serve as a promising alternative to standard tissue-based testing for patient prognosis. The technique offers high specificity and sensitivity for a molecular marker, allowing the collection of robust and reproducible data repetitively, in a simple and non-invasive way using a peripheral blood sample [19].
The tri-phasic pattern of glioma development
It is well established that tumor initiation and progression are complex and multistep processes in which various tumor microenvironment factors play a part. The initial phase of tumor initiation has inflammation playing a key role in cell proliferation and growth promotion [20]. The secretion of inflammatory molecules accentuates the growth of the glial tumor via signaling pathway(s) involved in proliferation [21]. Further, the glial tumor's stemness is also triggered by these inflammatory molecules; simultaneously, the freshly recruited inflammatory cells and newly sprouting blood vessels support its growth by promoting extensive angiogenesis [22], thus marking the phase of tumor promotion. The unlimited proliferation of cells results in oxygen depletion in the cell mass and surrounding environment and the release of various growth factors to initiate survival mechanisms, which together drive more inflammation and angiogenesis in the tumor stroma. Thus, hypoxia is created in the tumor core, which strongly stimulates further angiogenesis [23] and is directly linked with persistent inflammation [24]. This cascade of events culminates into a phase representing tumor progression which involves damage to the extracellular matrix (ECM) by the activated inflammatory signals [25] via the release of degrading enzymes, namely matrix metalloproteinases (MMPs). Due to the degradation of the ECM as well as to escape hypoxia, glioma cells start to migrate and reach oxygen-rich areas adjacent to blood vessels [26]. This crosstalk between the components of different phases of the tumor’s existence is a crucial aspect of glioma initiation, progression, and response to therapy. This review provides a unique insight into the three phases of gliomagenesis defined with reference to the mechanism (Fig. 1) as well as the role and involvement of secreted proteomic markers at various stages of disease development. The chosen biomarkers of all three phases are secretory, and can cross the blood–brain barrier (BBB), they are also highly sensitive, most specific, and stable in the systemic circulation of glioma patients. A comprehensive analysis of the available liquid biopsy-based studies for the past 10 years (Table 1) indicates that it is imperative to lay the blueprint for more clinical studies based on a non-invasive biomarker panel that can be sequentially assessed for precise prognosis and post-therapy disease monitoring in glioma. This review highlights how the chosen secretory biomarkers can be used to non-invasively analyze the changes that arise in a phased manner during glio-oncogenesis for better prognostication and therapeutic targeting using liquid biopsy technique.
A crosstalk exists between the tumor and its surrounding microenvironment during the three phases of development of a glioma. The initial phase is marked by tumor initiation; triggered by inflammation, followed by uncontrolled cell proliferation and stemness in the tumor. The second phase consists of tumor growth promoted by hypoxia and aggressive angiogenesis. The last phase of tumor progression involves remodeling of ECM leading to grade progression, recurrence or metastasis
As a template of methodology, we utilized the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for this systematic review. Queries were completed in four databases: MEDLINE, Cochrane library, Google scholar and Web of Science. The keywords were human glioma, tumor microenvironment, molecular markers, blood, non-invasive, liquid biopsy, prognostic, and survival to find related publications published from the beginning of molecular era in 2000 till 2022. Results of this two‐section search were merged using “AND” Boolean. Subsequently, the outcomes were combined and later, the duplicates were removed. All titles and abstracts were screened for relevance by two authors based on predefined in- and exclusion criteria. Subsequently, the authors evaluated all full-text articles. Disagreement on eligibility was addressed by discussion and consensus.
Studies were found to be eligible when reporting on the predictive and prognostic value of blood-based molecular markers in glioma. Measurement of markers in plasma or serum of patient was considered. Articles reporting diagnostic markers and languages other than English were excluded. References of included articles were crosschecked, and relevant studies were included if appropriate.
The microenvironment (ME) partakers
Inflammation: the cause or consequence
Oncogenic mutations, injury, and/or ME alterations can trigger the secretion of inflammatory signals, specifically cytokines, which have a significant role in glial tumor initiation and development. Cytokines are critical autocrine/paracrine factors released into the tumor ME to recruit and activate various inflammatory cells [27] during the initial event of an injury/alteration in the ME. In turn, these educated inflammatory cells initiate the inflammatory cascade, creating a persistent (meta)inflamed ME leading to aberrant glial cell proliferation by evading immune surveillance. Finally, this inflammatory stimulus contributes to tumor growth and progression [28]. The precise relation between inflammation and glial tumor growth is still not worked out in terms of molecular markers of disease prognostication. The magnitude of activation of the inflammatory response can be measured in terms of specific pro-inflammatory molecules interleukin-6 (IL-6), tumor necrosis factor-α (TNFα), interferon-γ (IFN-γ), and circulating inflammatory metabolite like kynurenine (KYN) because these markers are stable and detectable levels are present in the systemic circulation. Most importantly, all these molecules can cross the BBB.
The IL-6 is a pleiotropic cytokine of 25 kDa and has been attributed with an important role in the inflammation-linked initiation of solid tumors. During gliomagenesis, IL-6 executes a prominent part from among other interleukins since it predominately triggers the pro-inflammatory cascade and controls various cellular processes [29, 30]. An investigation by Shan and group measured IL-6 in glioma serum samples and showed that it could predict the prognosis and serve as a therapeutic target for the treatment of glioma patients [31]. Similarly, in another blood-based study, Li et al. [32] revealed that IL-6 concentrations were higher in GB patients and significantly associated with progression-free survival (PFS) and OS, indicating IL-6 to be an independent prognostic feature for GB. In a case report of a GB patient, lower circulating IL-6 levels were noted by the investigators than the GB reference group, and the observed low expression correlated with the patient's extended PFS [33]. Another group of scientists worked on a cohort of 69 high-grade glioma patients and indicated that elevated blood IL-6 concentrations were associated with shorter survival [34] (Table 1 A1).
In addition to IL-6, some studies suggest that a panel of secreted cytokines inclusive of TNF-α and IFN-γ could add to the speed and accuracy of making a more precise prognosis and thereby early initiation of therapy. In the previous decade, Albulescu et al. [35] had conducted a serum profiling for pro-inflammatory cytokines IL-6, IFN-γ, and TNF-α to assess the potential prognostic and therapeutic application in GB patients. Their results indicated significant deregulation in cytokine levels with a threefold up-regulation of IL-6, IFN-γ, and TNF-α, suggesting that these cytokines were involved in tumor progression and its aggressiveness. A couple of years later, Nijaguna and his co-workers [36] developed a serum cytokine signature by profiling 48 cytokines, including IL-6, IFN-γ, and TNF-α, in different tumor grades of glioma. They created a training set for prediction analysis from the microarray data and identified a panel of 18 cytokines that could discriminate GB from normal controls with an accuracy of 95.40%. Interestingly, the 18-cytokine signature also differentiated grade-II/diffuse astrocytoma and Grade-III/anaplastic astrocytoma from normal sera very efficiently. In 2018, Deniz and his team examined the effect of chemo-radiotherapy (CRT) on a GB cohort and prospectively evaluated pre and post-CRT levels of IFN-γ, IL-6, and TNF-α. Among the three markers, post-CRT, TNF-α, and IFN-γ levels were significantly lower than pre-CRT levels indicating a favorable prognosis [37]. In the same year, Zhenjiang et al. [38] evaluated serum cytokine profiles in treatment-naive glioma patients to check the levels of a combination of cytokine networks, i.e., IL-4/IL-5/IL-6 and IFN-γ/TNF-α/IL-17A. Their findings presented that among the two panels of cytokine networks, IL-6, IFN-γ, and TNF-α helped in predicting and correlated positively with improved patient survival suggesting that the cytokine-marker-signature can be taken up as a primary screen to predict glioma prognosis non-invasively. Shamshdin and his group measured a single marker TNF-α in the serum of glioma patients of different grades. They too found an increased expression with increasing grades and stated that the marker could serve as a critical prognosticator in glioma [39] (Table 1 A2, A3).
Altered KYN metabolism
In the event of the generation of a chronic inflammatory response within the tumor, the overexpression of IL-6 also launches the kynurenine pathway proteins via activation of IFNγ and TNF-α cytokines in the glial tumor ME eliciting its secretion from the immediately surrounding tissues [40]. An important circulating metabolic protein of the KYN pathway is KYN. The generated inflammation leads to KYN being transduced across the BBB to be detected in the systemic circulation. A study on systemic KYN by Zhai and co-authors compared the marker levels in a small group of 10 GB patients before surgical resection of the tumor. Their data suggested that the value of KYN along with tryptophan can be set as a clinical point of reference for determining the prognosis of GB patients [41]. The findings of Lenzen et al. [42] also indicated that the metabolite KYN was significantly lower in the plasma of heat shock protein-peptide complex-96 (HSPPC-96) vaccinated GB patients compared to controls. There was a significant association between lower KYN levels and longer OS suggesting that KYN could be an important molecule to determine GB patients’ prognosis after immunotherapy. Recently, our lab-based study established the utility of an inflammatory marker panel including KYN in estimating systemic inflammation in treatment-naive glioma patients. The panel could discriminate between WHO grades, and IDH-mutant/wildtype and define differential survival between astrocytic IDH-mutant/wildtype tumors [43] (Table 1 A4). All the inflammatory markers discussed above are stable and can be easily detected in blood circulation due to their permeation through BBB. But more studies in a large cohort are required to establish their utility as prognostic biomarkers before use in the clinic.
Proliferation of the tumor
In the continuance of chronic inflammation in the ME, the association between increased inflammation and telomerase activity is an aspect of tumor growth that also needs to be understood and investigated. The human telomerase reverse transcriptase (hTERT) is an enzyme that executes many vital functions independent of its telomere maintenance, including regulation of inflammation; however, its activity beyond a critical limit initiates excessive cell proliferation [44]. Several studies suggest that hyper-TERT activity promotes over-secretion of pro-inflammatory molecules such as interleukin IL-6, leading to persistent chronic inflammation and tumor progression [45,46,47].
Labussiere et al. sequenced blood DNA samples by RT-PCR for TERTp-mut in glioma and identified it in 60.8% of the samples. It was seen to be associated with a more unsatisfactory outcome, thus indicating that a TERTp-mut status could provide prognostic stratification and therapeutic target for glial tumors [48]. Three studies published from our lab also support the prognostic role of hTERT. The first one was a case report where low plasma hTERT levels in a GB patient were linked to PFS. The next extended study established with experimental evidence, the association of higher plasma levels of hTERT with a poor OS of both low and high-grade patients, presenting hTERT as an independent prognostic marker. The third study proposed crosstalk between markers of tumor proliferation (hTERT), angiogenesis (YKL-40), and extracellular matrix (ECM) degradation (TIMP-1) in GB patients recording hTERT to be related to poor survival [33, 49, 50]. Recently, Muralidharan and his co-worker [51] demonstrated a trend of higher TERT-MAFmut in patients with enhancing tumors and the outcome of the study presented the feasibility of detecting circulating cfDNA TERT promoter mutations in glioma patients using liquid biopsy. This test also enhanced the ability to diagnose, monitor, and assess responses to therapy (Table 1 B1).
During the rapid growth of the glial tumor, the human microtubule-associated protein tau (MAP tau) binds specifically to tubulin and modulates the stability of microtubules, thereby blocking mitosis. By binding and stabilizing polymerized microtubule filaments, MAP tau-based fusion proteins skew microtubule dynamics towards cell cycle arrest and apoptosis. This biological activity can earmark rapidly proliferating cells for MAP tau-based targeted treatments [52]. Two recent studies have proposed that TAU protein might serve as an important hallmark of tumor pathology, especially in gliomas. The research groups determined the association of Tau expression with survival in low-grade glioma (LGG) by retrieving the RNASeq data from the Cancer Genome Atlas (TCGA) that made up their observation cohort. Their analysis suggested that higher Tau expression was related to the less aggressive behavior of brain tumors. These studies indicated that TAU protein might play an important role in the diagnosis and prognosis of gliomas [53, 54]. A single blood-based study in literature by Darlix et al. [55] recorded the prognostic value of Tau in brain metastasis (BM) patients. Their work suggested that elevated serum levels of Tau were independently associated with a poor outcome in patients with and without BM (Table 1 B2). Thus, Tau can be a novel non-invasive biomarker of interest and needs to be worked out in multicentric cohorts of glioma patients for further validation.
Stemness of the tumor
The comprehensive findings of many scientific groups reviewed by Ahmed et al. specified a small population of therapy-resistant and slow-dividing malignant cells inside the main tumor bulk, which are responsible for tumor maintenance, invasiveness, and recurrence [56]. These glioma stem cells (GSCs) are a heterogeneous population of multipotent undifferentiated cells with self-renewal capacity [57]. Experimental evidence suggests that activated GSCs play a crucial role in tumor progression and therapy resistance through multiple mechanisms [58]. Therefore, it is an important parameter to be monitored for prognostication. In the case of glioma, several studies on stemness markers detected in blood circulation such as CD133, CD15/SSEA, CD44, and CD117 are referenced in the scientific databases [59, 60], yet no single marker has been diagnostically established for CSC detection.
The active involvement of proliferation (hTERT) in maintaining the stem-like property in human cancers cell is also well documented [61]. The unlimited proliferation of tumor cells leads to the secretion of some intracellular proteins into the extracellular spaces, which represent the actual stage of the existing tumor [62]. One of these proteins is the High Mobility Group AT-Hook 1 (HMGA1) protein, a member of the HMGA family, which is involved in stem-cell self-renewal, proliferation, and differentiation. Its expression was documented to closely relate to malignant proliferation, invasion, and differentiation of the tumor from glial stem cells [63]. To date, there is only a single blood-based report, which recorded a lower value of circulating HMGA1 in the serum of a GB patient as compared to the median value of the GB reference group and correlated it with an extended PFS of the patient [33] (Table 1 C1). This stemness marker of the glioma tumor offers a new tool that could be used for monitoring the malignancy and predicting the recurrence of the tumor.
Hypoxia
The malignant gliomas are aggressive CNS tumors with limited therapeutic options, and improvements in treatment outcomes require a deeper molecular understanding of this disease’s multiple partakers contributing to progression. Hypoxia is a dynamic state of the glioma microenvironment, as growing tumors frequently exist in hypoxic conditions because of insufficient blood supply. It is one of the main factors responsible for tumor progression and resistance to therapy [64, 65]. As in other cancers, recent studies have identified that glioma stem cells produce nitric oxide via elevated nitric oxide synthase-2 (NOS2) expression, which creates a hypoxic environment within the tumor [66]. According to Wilson and Hay [67], there exists a conflict between the demand of a fast-growing tumor mass and insufficient support of relatively slow neovascularization, regional hypoxia comes to play an important role and becomes a negative prognostic and predictive factor of tumor progression.
The highly malignant phenotypes of glioma are considered hypoxic regions, which are more frequent in GB [68] and play an important role in driving tumor growth. The phenomena of neovascularization, immunomodulation, and metabolic dysfunction, are all tumor-supportive and stimulated by intratumoral hypoxia [69]. In this category, heat shock proteins (HSPs) are an important group of intracellular proteins that maintain and protect cell integrity from lethal damage under hypoxic conditions within the tumor.
Delving into the role of one such molecule is of clinical relevance, the heat shock protein-70 (HSP-70), a major stress-inducible heat shock protein that is secreted into extracellular space in the form of exosomes [70]. In 2014, Breuninger et al. [71] quantified HSP-70 marker in the serum of GB patients and found that HSP-70 serum levels were significantly higher in GB than those of the healthy donors. Another study by Thorsteinsdottir et al. [72] estimated the extracellular HSP-70 in LGG, primary and secondary GB plasma samples. The authors found plasma levels to be significantly increased in primary GB compared to secondary GB and LGG samples and thus noted that HSP-70 might serve as a screening factor for clinical and therapeutic response (Table 1 D1). Thus, HSP-70 can prove to be a valuable marker for screening primary GB and also a progression from LGG to GB.
Angiogenesis
Brain hypoxia is a critical parameter in the TM known to be linked to tumor progression, an altered pattern of angiogenesis [73, 74]. A hypoxic environment stimulates endothelial progenitor cells and mature endothelium to generate new blood vessels around the tumor in the presence of deregulated angiogenic growth factors [75]. The brain tumor exhibit a marked and aberrant blood vessels formation (angiogenesis) which is a key feature of an increase in tumor volume. Complex multiple processes follow involving the recruitment, proliferation, and alignment of the endothelial blood vessel cells for the delivery of nutrients and oxygen for tumor growth [76]. Experimental evidence suggests that one of the important hallmarks, stemness, is activated in the tumor via uncontrolled proliferation and hypoxia which plays a crucial role in tumor progression through angiogenesis [77]. The most worked out and stable angiogenic regulators in glioma are EGFR and vascular endothelial growth factor (VEGF) (Table 1 E1 and E2).
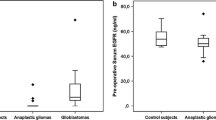
EGFR is a transmembrane cell surface receptor and a member of the ERBB1 family of tyrosine kinases, known to be overexpressed in various human malignant tumors, including glioma [78]. Aberrant EGFR alterations promote proliferation, survival, angiogenesis, and invasion, which can impact the process of gliomagenesis, but their prognostic, and therapeutic relevance remains controversial [79]. According to the WHO classification of 2016, EGFR is designated as a marker for the diagnosis of GB [11]. However, there are only a couple of investigations on this marker using liquid biopsy where a correlation with prognosis has been worked out. In 2007, Quaranta et al. [80] took to quantify serum EGFR levels in glioma and found them to be significantly elevated in patients compared with healthy controls, higher EGFR levels were consistent with a reduced OS of patients. Subsequently, Bieńkowski et al. [81] analyzed EGFR amplification in serum of GB patients and observed the association of EGFR with survival, stating it to be also useful for monitoring disease progression in response to adjuvant therapy after surgery. When they performed a multivariate analysis, it confirmed that EGFR amplification was significantly associated with better survival in younger patients. Later, Spath and co-workers [82] assessed the association between genetic risk and serum concentrations of EGFR in a pre-diagnostic cohort of glioma. Their observation was that higher serum EGFR levels were associated with the risk of developing glioma (Table 1 E2). These blood-based studies suggest that EGFR can be a potentially useful biological indicator to predict prognosis and even treatment outcome in glioma.
The other angiogenic factor, VEGF, is a family of four structurally related proteins and is essential for regulating the critical steps of cell proliferation and migration. Based on their in vitro experiments, Chengshi et al. [83] illustrated that VEGF is secreted by endothelial cells, and hypoxia can promote the secretion of VEGF. VEGF synthesis can also be triggered by inflammation, consequently, its overexpression enhances tumor vascularization and angiogenesis [84]. Using liquid biopsy, Rafat et al. [85] calculated concentrations of serum VEGF in 22 patients with GB and metastatic tumors, which indicated that significantly higher values were positively correlated with tumor angiogenic activity. Later, Nowacka et al. [86] studied a cohort of intracranial tumors including HGG, LGG, meningiomas, metastatic tumors, and others and determined the serum concentrations of VEGF in the cohort. The evaluation showed the highest serum VEGF-A concentrations to be present in primary intracranial tumors compared to metastatic tumors, indicating that higher serum VEGF-A concentrations may be associated with active neoangiogenesis within the tumor. According to the latest investigation carried out by Shamsdin et al., [39] hypoxic tumor cells in glioma release angiogenic cytokine VEGF, which further stimulates neovascularization. They measured serum VEGF levels in patients diagnosed with different grades of glioma and observed significantly higher levels in glioma than controls, with a positive association with grade progression. The authors concluded that VEGF could be an important molecular parameter for histological diagnosis and vascularization-based progression prediction (Table 1 E1). In the same year, Seyedmirzaei and the group conducted a meta-analysis to determine the alterations in VEGF levels in different grades of glioma. Out of a total number of 3,612 studies, 12 studies revealed that serum levels of VEGF in patients were higher compared to healthy controls and the levels varied significantly in glioma grades; therefore, the results of this systematic review and meta-analysis demonstrated that VEGF concentrations could be potentially considered as a prognostic biomarker for this CNS tumor [87]. However, more studies on the non-invasive evaluation of the neoangiogenesis process using factors are required for them to be clinically relevant in determining prognosis.
Extracellular matrix
The ECM is a major structural component of the tumor microenvironment, and it undergoes extensive reorganization during glioma progression [88]. Although the glial tumor is initiated in the native ECM of the primary site, this matrix changes throughout tumor progression. Tumor cells modify their phenotype for interacting with the surrounding ME thereby disrupting the ECM of the brain, partly through glioma-secreted ECM proteins or matrix remodeling enzymes, such as matrix metalloproteinases-7 [MMPs] and adhesion molecule; intercellular adhesion molecule-1 (ICAM-1) to promote pro-invasive ECM remodeling. While considered a significant contributor to glioma invasion, members of the MMP family are also linked with other pathological hallmarks of glioma. MMPs are zinc-dependent, calcium-containing endopeptidases accountable for various oncological events, including proliferation, angiogenesis, migration, and cell survival [89]. Dimitrova and his team [90] evaluated the role of serum MMP-7 in controls, and patients with benign tumors, GB, and metastasis. The results showed equivalent serum levels of MMP-7 in GB and controls but significantly differential levels in patients with benign tumors and metastases. This finding suggests that serum MMP-7 levels might be used as a differential marker for benign brain tumors and brain metastases (Table 1 F1). Further detailed investigations using liquid biopsy are required to better understand its role in ME.
ICAM-1, an adhesion transmembrane glycoprotein, is a vital regulator of inflammation-dependent ECM remodeling, leading to tumor progression [91]. The experimental data on this marker’s circulatory levels were first published by Salmaggi et al. [92] wherein the ICAM-1 levels were comparatively higher in GB patients than healthy controls (Table 1 F2). A few years later Nano et al. [93] quantified ICAM-1 in the serum of glioma patients and compared it with control samples. Their results were negative, sICAM-1 serum levels were not significantly increased in GB and astrocytoma patients. Deviating from this trend, Burim et al. investigated the polymorphism in codon 469 of ICAM-1 in astrocytoma blood samples using PCR. Results showed that ICAM-1 genotype had the highest frequency in grade II astrocytomas compared to controls and other astrocytoma grades, suggesting that this adhesion molecule could be involved in the initial stages and progression of grade II astrocytomas [94].
Conclusion
This is the first initiative to delineate the crosstalk between the glioma tumor and its multiple ME components (Fig. 2). It also highlights the sequential role of the defined circulatory ME partakers in glioma initiation, promotion, and progression; their plausible function as prognostic markers, and their detection using liquid biopsy for potential use in the clinical setting. The analysis of reviewed literature indicates that the markers of inflammatory response IL-6, TNF-α, IFN-ϒ and KYN, proliferation markers hTERT and TAU, and stemness marker HMGA1 are important contributors to glial tumor initiation and growth. Molecules HSP-70, EGFR, and VEGF representing hypoxia and angiogenic factors play a key role in promoting vascularization and therefore progression. At a later stage biomarkers MMP-7 and ICAM-1 are involved in the degradation and remodeling of the ECM, ultimately leading to tumor grade progression, recurrence, and/or metastasis In our opinion biomarkers of inflammation (IL-6, KYN) and proliferation (hTERT and HMGA1) along with VEGF can serve as promising markers for glioma staging and prognosis.
A schematic representation delineating the molecular markers secreted during different phases of glioma development. The interplay between expressions of biomarkers in different stages of glioma progression is elucidated. Markers highlighted in white color represent the initiation phase of the tumor, while yellow color represents the promotion phase and the red color represents the tumor progression phase. IL-6 interleukin-6, TNFα tumor necrosis factor-α, IFN-γ interferon-γ, KYN kynurenine, hTERT human telomerase reverse transcriptase, TAU microtubule-associated protein tau, HMGA1 High Mobility Group AT-Hook 1, HSP-70 heat shock protein-70, EGFR endothelial growth factor receptor, VEGF vascular endothelial growth factor, MMP-7 matrix metalloproteinase-7, ICAM-1 intercellular adhesion molecule-1
In its totality, this review provides a road map for the development of a blood-based, non-invasive biomarker panel and its sequential evaluation as it has enormous potential and contributes to clinical decision-making to predict prognosis (Fig. 3).
Proposed roadmap of non-invasive panel of biomarkers presenting the key phases of glioma progression and the sequence of biomarker evaluation at each stage. IL-6 interleukin-6, TNFα tumor necrosis factor-α, IFN-γ interferon-γ, KYN kynurenine, hTERT human telomerase reverse transcriptase, TAU microtubule-associated protein tau, HMGA1 High Mobility Group AT-Hook 1, HSP-70 heat shock protein-70, EGFR endothelial growth factor receptor, VEGF vascular endothelial growth factor, MMP-7 matrix metalloproteinase-7, ICAM-1 intercellular adhesion molecule-1
Data availability
NA.
References
Ostrom QT, Cote DJ, Ascha M, Kruchko C, Barnholtz-Sloan JS. Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol. 2018;4:1254–62. https://doi.org/10.1001/jamaoncol.2018.1789.
Fernandes C, Costa A, Osório L, et al. Current Standards of Care in Glioblastoma Therapy. In: De Vleeschouwer S, editor. Glioblastoma [Internet]. Brisbane (AU): Codon Publications; 2017. Chapter11. Available from: https://www.ncbi.nlm.nih.gov/books/NBK469987/https://doi.org/10.15586/codon.glioblastoma.2017.ch11
Wang Y, Liu S, Fan X, Li S, Wang R, Wang L, Ma J, Jiang T, Ma W. Age-associated brain regions in gliomas: a volumetric analysis. J Neurooncol. 2015;123:299–306. https://doi.org/10.1007/s11060-015-1798-x.
Jungk C, Chatziaslanidou D, Ahmadi R, Capper D, Bermejo JL, Exner J, von Deimling A, Herold-Mende C, Unterberg A. Chemotherapy with BCNU in recurrent glioma: Analysis of clinical outcome and side effects in chemotherapy-naïve patients. BMC Cancer. 2016;16:81. https://doi.org/10.1186/s12885-016-2131-6].
Alphandéry E. Glioblastoma treatments: an account of recent industrial developments. Front Pharmacol. 2018;9:879. https://doi.org/10.3389/fphar.2018.00879].
Xia L, Fang C, Chen G, Sun C. Relationship between the extent of resection and the survival of patients with low-grade gliomas: a systematic review and meta-analysis. BMC Cancer. 2018;1:48. https://doi.org/10.1186/s12885-017-3909-x].
Liang J, Lv X, Lu C, Ye X, Chen X, Fu J, Luo C, Zhao Y. Prognostic factors of patients with Gliomas – an analysis on 335 patients with Glioblastoma and other forms of Gliomas. BMC Cancer. 2020; 20:35 https://doi.org/10.1186/s12885-019-6511-6
Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G. ESMO guidelines working group. high-grade glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:93–101. https://doi.org/10.1093/annonc/mdu050.
Guo L, Wang G, Feng Y, Yu T, Guo Y, Bai X, Ye Z. Diffusion and perfusion weighted magnetic resonance imaging for tumor volume definition in radiotherapy of brain tumors. Radiat Oncol. 2016;11:123. https://doi.org/10.1186/s13014-016-0702-y].
Sawlani V, Patel MD, Davies N, Flintham R, Wesolowski R, Ughratdar I, Pohl U, Nagaraju S, Petrik V, Kay A, Jacob S, Sanghera P, Wykes V, Watts C, Poptani H. Multiparametric MRI: practical approach and pictorial review of a useful tool in the evaluation of brain tumours and tumour-like lesions. Insights Imaging. 2020;11:84. https://doi.org/10.1186/s13244-020-00888-1].
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–20. https://doi.org/10.1007/s00401-016-1545-1.
DeWeerdt S. The genomics of brain cancer. Nature. 2018;561:S54–5. https://doi.org/10.1038/d41586-018-06711-8].
Ramkissoon LA, Britt N, Guevara A, Whitt E, Severson E, Sathyan P, Gay L, Elvin J, Ross JS, Brown C, Stogner-Underwood K, Mott R, Kram D, Strowd R, Lesser GJ, Ramkissoon SH. Precision neuro-oncology: the role of genomic testing in the management of adult and pediatric gliomas. Curr Treat Options Oncol. 2018;19:41. https://doi.org/10.1007/s11864-018-0559-4].
Kiviniemi A, Gardberg M, Frantzén J, Parkkola R, Vuorinen V, Pesola M, Minn H. Serum levels of GFAP and EGFR in primary and recurrent high-grade gliomas: correlation to tumor volume, molecular markers, and progression-free survival. J Neurooncol. 2015;124:237–45. https://doi.org/10.1007/s11060-015-1829-7.
Manda SV, Kataria Y, Tatireddy BR, Ramakrishnan B, Ratnam BG, Lath R, Ranjan A, Ray A. Exosomes as a biomarker platform for detecting epidermal growth factor receptor-positive high-grade gliomas. J Neurosurg. 2018;128:1091–101. https://doi.org/10.3171/2016.11.JNS161187.
Karsy M, Neil JA, Guan J, Mahan MA, Colman H, Jensen RL. A practical review of prognostic correlations of molecular biomarkers in glioblastoma. Neurosurg Focus. 2015;38:E4. https://doi.org/10.3171/2015.1.FOCUS14755.].
Silantyev AS, Falzone L, Libra M, Gurina OI, Kardashova KS, Nikolouzakis TK, Nosyrev AE, Sutton CW, Mitsias PD, Tsatsakis A. Current and future trends on diagnosis and prognosis of glioblastoma: from molecular biology to proteomics. Cells. 2019;8:863. https://doi.org/10.3390/cells8080863].
Figueroa JM, Carter BS. Detection of glioblastoma in biofluids. J Neurosurg. 2018;129:334–40. https://doi.org/10.3171/2017.3.JNS162280].
Chen D, Xu T, Wang S, Chang H, Yu T, Zhu Y, Chen J. Correction to: liquid biopsy applications in the clinic. Mol Diagn Ther. 2020;24:125–33. https://doi.org/10.1007/s40291-020-00449-8].
Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. https://doi.org/10.1016/j.immuni.2019.06.025.
Marinari E, Allard M, Gustave R, Widmer V, Philippin G, Merkler D, Tsantoulis P, Dutoit V, Dietrich PY. Inflammation and lymphocyte infiltration are associated with shorter survival in patients with high-grade glioma. Oncoimmunology. 2020;9:1779990. https://doi.org/10.1080/2162402X.2020.1779990].
Aguilar-Cazares D, Chavez-Dominguez R, Carlos-Reyes A, Lopez-Camarillo C, Hernadez de la Cruz ON, Lopez-Gonzalez JS. Contribution of Angiogenesis to Inflammation and Cancer. Front Oncol. 2019; 9: 1399. https://doi.org/10.3389/fonc.2019.01399
Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. https://doi.org/10.1016/j.molcel.2010.09.022.
Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–17. https://doi.org/10.1038/nri2607].
Konnecke H, Bechmann I. The role of microglia and matrix metalloproteinases involvement in neuroinflammation and gliomas. Clin Dev Immunol. 2013;2013:914104. https://doi.org/10.1155/2013/914104].
Armento A, Ehlers J, Schötterl S, Naumann U. Molecular Mechanisms of Glioma Cell Motility. In: De Vleeschouwer S, editor. Glioblastoma [Internet]. Brisbane (AU): Codon Publications; 2017. Chapter 5. [https://www.ncbi.nlm.nih.gov/books/NBK470001/https://doi.org/10.15586/codon.glioblastoma.2017.ch5
West AJ, Tsui V, Stylli SS, Nguyen HP, Morokoff AP, Kaye AH, Luwor RB. The role of interleukin 6 STAT3 signalling in glioblastoma (Review). Oncol Lett. 2018;16:4095–104. https://doi.org/10.3892/ol.2018.9227.
Ha ET, Antonios JP, Soto H, Prins RM, Yang I, Kasahara N, Liau LM, Kruse CA. Chronic inflammation drives glioma growth: cellular and molecular factors responsible for an immunosuppressive microenvironment. NeuroimmunolNeuroinflamm. 2014;1:66–76. https://doi.org/10.4103/2347-8659.139717.
Mauer J, Denson JL, Brüning JC. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 2015;36:92–101. https://doi.org/10.1016/j.it.2014.12.008.].
Luo Y, Zheng SG. Hall of fame among pro-inflammatory cytokines: interleukin-6 gene and its transcriptional regulation mechanisms. Front Immunol. 2016;7:604. https://doi.org/10.3389/fimmu.2016.00604].
Shan Y, He X, Song W, Han D, Niu J, Wang J. Role of IL-6 in the invasiveness and prognosis of glioma. Int J Clin Exp Med. 2015;8:9114–20.
Li Z, Huang Q, Chen H, Lin Z, Zhao M, Jiang Z. Interferon regulatory factor 7 promoted glioblastoma progression and stemness by modulating IL-6 expression in microglia. J Cancer. 2017;8:207–19. https://doi.org/10.7150/jca.16415.
Gandhi P, Khare R, Garg N, Sorte S. Immunophenotypic signature of primary glioblastoma multiforme: A case of extended progression free survival. World J Clin Cases. 2017; 5: 247–253. https://doi.org/10.12998/wjcc.v5.i6.247
Bunevicius A, Radziunas A, Tamasauskas S, Tamasauskas A, Laws ER, Iervasi G, Bunevicius R, Deltuva V. Prognostic role of high sensitivity C-reactive protein and interleukin-6 in glioma and meningioma patients. J Neurooncol. 2018;138:351–8. https://doi.org/10.1007/s11060-018-2803-y].
Albulescu R, Codrici E, Popescu ID, Mihai S, Necula LG, Petrescu D, Teodoru M, Tanase CP. Cytokine patterns in brain tumour progression. Mediators Inflamm. 2013;2013:979748. https://doi.org/10.1155/2013/979748.
Nijaguna MB, Patil V, Hegde AS, Chandramouli BA, Arivazhagan A, Santosh V, Somasundaram K. An eighteen serum cytokine signature for discriminating glioma from normal healthy individuals. PLoS One. 2015;10: e0137524. https://doi.org/10.1371/journal.pone.0137524.
Deniz CD, Gürbilek M, Koç M. Prognostic value of interferon-gamma, interleukin-6, and tumor necrosis factor-alpha in the radiation response of patients diagnosed with locally advanced non-small-cell lung cancer and glioblastoma multiforme. Turk J Med Sci. 2018;48:117–23. https://doi.org/10.3906/sag-1611-77].
Zhenjiang L, Rao M, Luo X, Valentini D, von Landenberg A, Meng Q, Sinclair G, Hoffmann N, Karbach J, Altmannsberger HM, Jäger E, Peredo IH, Dodoo E, Maeurer M. Cytokine networks and survivin peptide-specific cellular immune responses predict improved survival in patients with glioblastoma multiforme. EBioMedicine. 2018;33:49–56. https://doi.org/10.1016/j.ebiom.2018.06.014].
Shamsdin SA, Mehrafshan A, Rakei SM, Mehrabani D. Evaluation of VEGF, FGF and PDGF and Serum Levels of Inflammatory Cytokines in Patients with Glioma and Meningioma in Southern Iran. Asian Pac J Cancer Prev. 2019; 20: 2883–2890. https://doi.org/10.31557/APJCP.2019.20.10.2883
Adams S, Teo C, McDonald KL, Zinger A, Bustamante S, Lim CK, Sundaram G, Braidy N, Brew BJ, Guillemin GJ. Involvement of the kynurenine pathway in human glioma pathophysiology. PLoS One. 2014;9: e112945. https://doi.org/10.1371/journal.pone.0112945].
Zhai L, Dey M, Lauing KL, Gritsina G, Kaur R, Lukas RV, Nicholas MK, Rademaker AW, Dostal CR, McCusker RH, Raizer JJ, Parsa AT, Bloch O, Wainwright DA. The kynurenine to tryptophan ratio as a prognostic tool for glioblastoma patients enrolling in immunotherapy. J Clin Neurosci. 2015;22:1964–8. https://doi.org/10.1016/j.jocn.2015.06.018.
Lenzen A, Zhai L, Lauing KL, Gritsina G, Ladomersky E, Genet M, James CD, Bloch O, Wainwright DA. The Kynurenine/tryptophan ratio and glioblastoma patients treated with hsppc-96 vaccine. Immunotherapy (Los Angel). 2016;2:125. https://doi.org/10.4172/2471-9552.1000125.
Gandhi P, Shrivastava R, Garg N, Sorte SK. Novel molecular panel for evaluating systemic inflammation and survival in therapy naïve glioma patients. World J Clin Oncol 2021; 12(10): 0–0
El-Badawy A, Ghoneim NI, Nasr MA, Elkhenany H, Ahmed TA, Ahmed SM, El-Badri N. Telomerase reverse transcriptase coordinates with the epithelial-to-mesenchymal transition through a feedback loop to define properties of breast cancer stem cells. Biol Open. 2018;7: bio034181. https://doi.org/10.1242/bio.034181
Ghosh A, Saginc G, Leow SC, Khattar E, Shin EM, Yan TD, Wong M, Zhang Z, Li G, Sung WK, Zhou J, Chng WJ, Li S, Liu E, Tergaonkar V. Telomerase directly regulates NF-κB-dependent transcription. Nat Cell Biol. 2012;14:1270–81. https://doi.org/10.1038/ncb2621].
Chen R, Zhang K, Chen H, Zhao X, Wang J, Li L, Cong Y, Ju Z, Xu D, Williams BR, Jia J, Liu JP. Telomerase deficiency causes alveolar stem cell senescence-associated low-grade inflammation in lungs. J Biol Chem. 2015;290:30813–29. https://doi.org/10.1074/jbc.M115.681619.
Liu H, Yang Y, Ge Y, Liu J, Zhao Y. TERC promotes cellular inflammatory response independent of telomerase. Nucleic Acids Res. 2019;47:8084–95. https://doi.org/10.1093/nar/gkz584.
Labussière M, Di Stefano AL, Gleize V, Boisselier B, Giry M, Mangesius S, Bruno A, Paterra R, Marie Y, Rahimian A, Finocchiaro G, Houlston RS, Hoang-Xuan K, Idbaih A, Delattre JY, Mokhtari K, Sanson M. TERT promoter mutations in gliomas, genetic associations and clinico-pathological correlations. Br J Cancer. 2014;111:2024–32. https://doi.org/10.1038/bjc.2014.538.
Gandhi P, Khare R, Garg N. Evaluating the potential of circulating hTERT levels in glioma: can plasma levels serve as an independent prognostic marker? J Neurooncol. 2017;135:255–61. https://doi.org/10.1007/s11060-017-2578-6.
Gandhi P, Khare R, Garg N, Sorte Sk. Interlinked expression of tumor attributes: Can their evaluation serve as a noninvasive paradigm for prognosis in glioblastoma? Int J Neurooncol. 2020; 3:32–7. https://doi.org/10.4103/IJNO.IJNO_22_19
Muralidharan K, Yekula A, Small JL, Rosh ZS, Kang KM, Wang L, Lau S, Zhang H, Lee H, Bettegowda C, Chicoine MR, Kalkanis SN, Shankar GM, Nahed BV, Curry WT, Jones PS, Cahill DP, Balaj L, Carter BS. TERT promoter mutation analysis for blood-based diagnosis and monitoring of gliomas. Clin Cancer Res. 2021;27:169–78. https://doi.org/10.1158/1078-0432.CCR-20-3083.
Akinrinmade OA, Jordaan S, Hristodorov D, Mladenov R, Mungra N, Chetty S, Barth S. Human MAP Tau based targeted cytolytic fusion proteins. Biomedicines. 2017;5:36. https://doi.org/10.3390/biomedicines5030036.
Gargini R, Segura-Collar B, Sánchez-Gómez P. Novel functions of the neurodegenerative-related gene Tau in cancer. Front Aging Neurosci. 2019;11:231. https://doi.org/10.3389/fnagi.2019.00231.
Zaman S, Chobrutskiy BI, Sikaria D, Blanck G. MAPT (Tau) expression is a biomarker for an increased rate of survival for low grade glioma. Oncol Rep. 2019;41:1359–66. https://doi.org/10.3892/or.2018.6896.
Darlix A, Hirtz C, Thezenas S, Maceski A, Gabelle A, Lopez-Crapez E, De Forges H, Firmin N, Guiu S, Jacot W, Lehmann S. The prognostic value of the Tau protein serum level in metastatic breast cancer patients and its correlation with brain metastases. BMC Cancer. 2019;19:110. https://doi.org/10.1186/s12885-019-5287-z.
Ahmed AU, Auffinger B, Lesniak MS. Understanding glioma stem cells: rationale, clinical relevance and therapeutic strategies. Expert Rev Neurother. 2013;13:545–55. https://doi.org/10.1586/ern.13.42.
Brown DV, Stylli SS, Kaye AH, Mantamadiotis T. Multilayered heterogeneity of glioblastoma stem cells: biological and clinical significance. Adv Exp Med Biol. 2019;1139:1–21. https://doi.org/10.1007/978-3-030-14366-4_1.
Liebelt BD, Shingu T, Zhou X, Ren J, Shin SA, Hu J. Glioma stem cells: signaling, microenvironment, and therapy. Stem Cells Int. 2016;2016:7849890. https://doi.org/10.1155/2016/7849890].
Chen R, Nishimura MC, Bumbaca SM, Kharbanda S, Forrest WF, Kasman IM, Greve JM, Soriano RH, Gilmour LL, Rivers CS, Modrusan Z, Nacu S, Guerrero S, Edgar KA, Wallin JJ, Lamszus K, Westphal M, Heim S, James CD, VandenBerg SR, Costello JF, Moorefield S, Cowdrey CJ, Prados M, Phillips HS. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell. 2010;17:362–75. https://doi.org/10.1016/j.ccr.2009.12.049.
Mahzouni P, Jafari M. The study of CD117 expression in glial tumors and its relationship with the tumor-type and grade. J Res Med Sci. 2012;17:159–63.
Hannen R, Bartsch JW. Essential roles of telomerase reverse transcriptase hTERT in cancer stemness and metastasis. FEBS Lett. 2018;592:2023–31. https://doi.org/10.1002/1873-3468.13084.
Mendez O, Pérez J, Soberino J, Racca F, Cortés J, Villanueva J. Clinical implications of extracellular HMGA1 in breast cancer. Int J Mol Sci. 2019;20:5950. https://doi.org/10.3390/ijms20235950.
Fan H, Guo H, Zhang IY, Liu B, Luan L, Xu S, Hou X, Liu W, Zhang R, Wang X, Pang Q. The different HMGA1 expression of total population of glioblastoma cell line U251 and glioma stem cells isolated from U251. Brain Res. 2011;1384:9–14. https://doi.org/10.1016/j.brainres.2011.01.105.
Oliver KM, Garvey JF, Ng CT, Veale DJ, Fearon U, Cummins EP, Taylor CT. Hypoxia activates NF-kappaB-dependent gene expression through the canonical signaling pathway. Antioxid Redox Signal. 2009;11:2057–64. https://doi.org/10.1089/ars.2008.2400.
Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7:10. https://doi.org/10.1038/s41389-017-0011-9.
Eyler CE, Wu Q, Yan K, MacSwords JM, Chandler-Militello D, Misuraca KL, Lathia JD, Forrester MT, Lee J, Stamler JS, Goldman SA, Bredel M, McLendon RE, Sloan AE, Hjelmeland AB, Rich JN. Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell. 2011;146:53–66. https://doi.org/10.1016/j.cell.2011.06.006.
Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393–410. https://doi.org/10.1038/nrc3064.
Huang WJ, Chen WW, Zhang X. Glioblastoma multiforme: Effect of hypoxia and hypoxia inducible factors on therapeutic approaches. Oncol Lett. 2016;12:2283–8. https://doi.org/10.3892/ol.2016.4952].
Colwell N, Larion M, Giles AJ, Seldomridge AN, Sizdahkhani S, Gilbert MR, Park DM. Hypoxia in the glioblastoma microenvironment: shaping the phenotype of cancer stem-like cells. Neuro Oncol. 2017;19:887–96. https://doi.org/10.1093/neuonc/now258.
Iglesia RP, Fernandes CFL, Coelho BP, Prado MB, Melo Escobar MI, Almeida GHDR, Lopes MH. Heat shock proteins in glioblastoma biology: where do we stand? Int J Mol Sci. 2019;20:5794. https://doi.org/10.3390/ijms20225794.
Breuninger S, Erl J, Knape C, Gunther S, Regel I, Rodel F, Gaip US, Thorsteinsdottir J, Giannitrapani L, Dickinson AM, Multhoff G. Quantitative analysis of liposomal heat shock protein 70 (Hsp70) in the blood of tumor patients using a novel lipHsp70 Elisa. J Clin Cell Immunol. 2014;5:264. https://doi.org/10.4172/2155-9899.1000264.
Thorsteinsdottir J, Stangl S, Fu P, Guo K, Albrecht V, Eigenbrod S, Erl J, Gehrmann M, Tonn JC, Multhoff G, Schichor C. Overexpression of cytosolic, plasma membrane bound and extracellular heat shock protein 70 (Hsp70) in primary glioblastomas. J Neurooncol. 2017;135:443–52. https://doi.org/10.1007/s11060-017-2600-z.
Peitzsch C, Perrin R, Hill RP, Dubrovska A, Kurth I. Hypoxia as a biomarker for radioresistant cancer stem cells. Int J Radiat Biol. 2014;90:636–52. https://doi.org/10.3109/09553002.2014.916841.
Fidoamore A, Cristiano L, Antonosante A, d’Angelo M, Di Giacomo E, Astarita C, Giordano A, Ippoliti R, Benedetti E, Cimini A. Glioblastoma stem cells microenvironment: the paracrine roles of the niche in drug and radioresistance. Stem Cells Int. 2016;2016:6809105. https://doi.org/10.1155/2016/6809105.
Ahir BK, Engelhard HH, Lakka SS. Tumor development and angiogenesis in adult brain tumor: glioblastoma. Mol Neurobiol. 2020;57:2461–78. https://doi.org/10.1007/s12035-020-01892-8.
Wurdinger T, Tannous BA. Glioma angiogenesis: Towards novel RNA therapeutics. Cell Adh Migr. 2009;3:230–5. https://doi.org/10.4161/cam.3.2.7910.
Treps L, Perret R, Edmond S, Ricard D, Gavard J. Glioblastoma stem-like cells secrete the pro-angiogenic VEGF-A factor in extracellular vesicles. J Extracell Vesicles. 2017;6:1359479. https://doi.org/10.1080/20013078.2017.1359479.
Xu H, Zong H, Ma C, Ming X, Shang M, Li K, He X, Du H, Cao L. Epidermal growth factor receptor in glioblastoma. Oncol Lett. 2017;14:512–6. https://doi.org/10.3892/ol.2017.6221.
Saadeh FS, Mahfouz R, Assi HI. EGFR as a clinical marker in glioblastomas and other gliomas. Int J Biol Markers. 2018; 33: 22–32 https://doi.org/10.5301/ijbm.5000301
Quaranta M, Divella R, Daniele A, Di Tardo S, Venneri MT, Lolli I, Troccoli G. Epidermal growth factor receptor serum levels and prognostic value in malignant gliomas. Tumori. 2007;93:275–80.
Bienkowski M, Piaskowski S, Stoczyńska-Fidelus E, Szybka M, Banaszczyk M, Witusik-Perkowska M, Jesień-Lewandowicz E, Jaskólski DJ, Radomiak-Załuska A, Jesionek-Kupnicka D, Sikorska B, Papierz W, Rieske P, Liberski PP. Screening for EGFR amplifications with a novel method and their significance for the outcome of glioblastoma patients. PLoS One. 2013; 8: e65444. https://doi.org/10.1371/journal.pone.0065444
Spath F, Andersson U, Dahlin AM, Langseth H, Hovig E, Johannesen TB, Grankvist K, Björkblom B, Wibom C, Melin B. Pre-diagnostic serum levels of EGFR and ErbB2 and genetic glioma risk variants: a nested case-control study. Tumour Biol. 2016;37:11065–72. https://doi.org/10.1007/s13277-015-4742-y.
Chengshi Xu, Xing Wu, Zhu J. VEGF promotes proliferation of human glioblastoma multiforme stem-like cells through VEGF receptor 2. Scient World J. 2013. https://doi.org/10.1155/2013/417413.
Setrerrahmane S, Xu H. Tumor-related interleukins: old validated targets for new anti-cancer drug development. Mol Cancer. 2017;16:153. https://doi.org/10.1186/s12943-017-0721-9.
Rafat N, Beck GCh, Schulte J, Tuettenberg J, Vajkoczy P. Circulating endothelial progenitor cells in malignant gliomas. J Neurosurg. 2010;112:43–9. https://doi.org/10.3171/2009.5.JNS081074.
Nowacka A, Smuczyński W, Rość D, Woźniak-Dąbrowska K, Śniegocki M. Serum VEGF-A concentrations in patients with central nervous system (CNS) tumors. PLoS One. 2018;13: e0192395. https://doi.org/10.1371/journal.pone.0192395.
Seyedmirzaei H, Shobeiri P, Turgut M, Hanaei S, Rezaei N. VEGF levels in patients with glioma: a systematic review and meta-analysis. Rev Neurosci. 2020;32:191–202. https://doi.org/10.1515/revneuro-2020-0062.
Popova NV, Jucker M. The functional role of extracellular matrix proteins in cancer. Cancers. 2022;14:238. https://doi.org/10.3390/cancers14010238.
Pullen NA, Pickford AR, Perry MM, Jaworski DM, Loveson KF, Arthur DJ, Holliday JR, Van Meter TE, Peckham R, Younas W, Briggs SEJ, MacDonald S, Butterfield T, Constantinou M, Fillmore HL. Current insights into matrix metalloproteinases and glioma progression: transcending the degradation boundary. Metalloprotein Med. 2018;5:13–30. https://doi.org/10.2147/MNM.S105123.
Dimitrova I, Tacheva T, Mindo I, Petrov B, Aleksandrova E, Valkanov S, Gulubova M, Vlaykova T. Serum levels of MMP-7 in primary brain cancers and brain metastases. Biotechnol Equip. 2019;33:881–5. https://doi.org/10.1080/13102818.2019.1626282.
Bonan S, Albrengues J, Grasset E, Kuzet SE, Nottet N, Bourget I, Bertero T, Mari B, Meneguzzi G, Gaggioli C. Membrane-bound ICAM-1 contributes to the onset of proinvasivetumor stroma by controlling acto-myosin contractility in carcinoma-associated fibroblasts. Oncotarget. 2017; 8: 1304–1320. https://doi.org/10.18632/oncotarget.13610
Salmaggi A, Eoli M, Frigerio S, Ciusani E, Silvani A, Boiardi A. Circulating intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and plasma thrombomodulin levels in glioblastoma patients. Cancer Lett. 1999;146:169–72. https://doi.org/10.1016/s0304-3835(99)00255-4.
Nano R, Capelli E, Argentina F, Facoetti A, Gerzeli G. Evaluation of serum levels of cytokines and intercellular adhesion molecule-1 (ICAM-1) in astrocytic tumours. Cellular and Molecular Biology (Noisy-le-Grand, France). 2003; 49: 525–528
Burim RV, Teixeira SA, Colli BO, Peria FM, Tirapelli LF, Marie SK, Malheiros SM, Oba-Shinjo SM, Gabbai AA, Lotufo PA, Carlotti-Júnior CG. ICAM-1 (Lys469Glu) and PECAM-1 (Leu125Val) polymorphisms in diffuse astrocytomas. Clin Exp Med. 2009;9:157–63. https://doi.org/10.1007/s10238-009-0040-6.
Acknowledgements
All the authors are thankful to Department of science and technology, New Delhi (WOS-A/LS-684/2016) for financial assistance and Bhopal Memorial Hospital and Research Centre for infrastructural facilities.
Funding
The author RS has received research support from Department of Science and Technology, New Delhi Grant numbers WOS-A/LS-684/2016.
Author information
Authors and Affiliations
Contributions
RS and PG contributed to the study conception and design. Material preparation, data collection and analysis were performed by RS and PG. The first draft of the manuscript was written by RS and PG, and RG commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
NA.
Consent to participate
NA.
Consent to publish
Yes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shrivastava, R., Gandhi, P. & Gothalwal, R. The road-map for establishment of a prognostic molecular marker panel in glioma using liquid biopsy: current status and future directions. Clin Transl Oncol 24, 1702–1714 (2022). https://doi.org/10.1007/s12094-022-02833-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02833-8