Abstract
Background
To demonstrate whether extensive intraoperative peritoneal lavage (EIPL) could yield better results in overall survival and less recurrence, regardless of peritoneal cytology, compared to standard peritoneal lavage (SPL).
Methods
A prospective randomised multicenter study including 94 patients (47 per arm) to detect a 20% difference in 3-year overall survival in patients with locally advanced tumours without peritoneal carcinomatosis. Three samples of peritoneal fluid were obtained (at the beginning, the end of procedure and after the assigned peritoneal lavage). Clinicopathological and surgical data were analysed by group. Postoperative complications, location of recurrence and surgical approach were evaluated. Overall survival was calculated by the Kaplan–Meier method and the uni/multivariate analysis for prognostic factors was carried out using Cox regression analysis.
Results
A total of 86 patients were analysed (4 excluded per group). No statistical differences were observed in clinicopathological or surgical data between groups, considering both groups well-balanced for analysis. Overall survival at 3 years was 64.3% for SPL vs. 62.3% for EIPL (p 0.421). Only three patients had at least one positive peritoneal cytology (1:2). There were no differences regarding postoperative complications (SPL: 37.2% vs. EIPL: 32.5%, p 0.65) or between location of recurrence and number of recurrences. The number of recurrences did not differ between surgical approaches, but locoregional and peritoneal recurrences were fewer with the laparoscopic approach (p 0.048).
Conclusions
The regular use of extensive peritoneal lavage in patients with locally advanced gastric cancer, regardless of peritoneal cytology, has not been effective as prophylaxis of peritoneal recurrence or better survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advanced gastric cancer (AGC) is the most common form of presentation at the moment of diagnosis in Western countries and Europe. T3–T4 gastric wall invasion cancer has a poor prognosis with a 5-year survival of less than 35%, even after curative resection [1]. The most frequent cause of treatment failure after surgery for gastric cancer is peritoneal metastasis that is reported as the most frequent pattern of recurrence in advanced gastric cancer (32–54%) and the most frequent cause of death (> 60%) during the first 2 years after surgery [1,2,3]. Both the Japanese Gastric Cancer Association (since 1998) and the American Joint Committee on Cancer (since 2010, 7th TNM) established positive peritoneal cytology as a metastatic disease [4].
Free cancer cells in the peritoneal cavity may be the result of the exfoliation of cells from the serosa surface of the primary tumour or after manipulation during surgery (gastrectomy plus lymphadenectomy) through blood vessels and perigastric lymphatic channels and posterior growth and dissemination in peritoneal surface.
A relevant study published by Kuramoto et al. demonstrated that patients with AGC and positive peritoneal lavage cytology (CY1) without carcinomatosis (P0) had important benefits in 5-year overall survival with intraperitoneal chemotherapy (IPC) plus extended intraperitoneal lavage (EIPL) with 10 L of physiologic serum by the “limiting dilution method” (1 L diluted by tenfold) (43.8%) compared to intraperitoneal chemotherapy alone (4.6%) [5]. This observation suggests that EIP, but not IPC, was key to the significant results obtained, proposing this method as a possible standard prophylactic strategy for peritoneal recurrence in patients with gastric cancer [6]. Some trials have tried to demonstrate the effect of extensive intraoperative lavage (EIPL) in cases of positive peritoneal cytology and P0 after potentially curative surgery for gastric cancer [7, 8].
Makino et al. in a prospective series of 113 patients with invaded serosa gastric tumour without peritoneal metastasis, reported 31% of positive cytologies in a preoperative peritoneal lavage [9]. However, the sensitivity of peritoneal cytology and molecular methods for the detection of free cancer cells in the peritoneal cavity may vary among centres and pathologists [1, 10,11,12]. For this reason, the aim of the present study was to investigate the role of EIPL as a standard prophylactic strategy for peritoneal recurrence and its impact on survival in cases with clinically local advanced gastric cancer and potentially curative surgery regardless of the peritoneal cytology.
This procedure has several advantages due to its simplicity, easy reproducibility, low cost and minimal complications. For this reason, the authors believe that EIPL may be used as a standard prophylactic strategy for peritoneal recurrence and survival in cases with suspected macroscopic serosa invasion or lymph node infiltration observed during surgery, cT3–4 or cN+ (by preoperative staging CT, EUS) independently from a perioperative positive peritoneal cytology. The aim of this trial is to demonstrate this hypothesis.
Materials and methods
This is a prospective randomised multi-institutional phase III trial. Patients were enrolled from five institutions that form part of the Spanish EURECCA Esophagogastric Cancer Project. The study was approved by the Clinical Trial Committee of the reference hospital (Hospital Mútua de Terrassa) and the ethic committees of each participating hospital. All candidates who accepted to participate provided written informed consent.
Outcomes
The primary outcome was overall survival and the secondary outcomes were incidence of adverse events and type of recurrence.
Inclusion criteria
Two types of inclusion criteria were applied (preoperative or intraoperative).
-
(a)
Preoperative: cT3–4 and/or cN+ , M0, P0 (by previous staging CT, EUS), histologically–confirmed gastric adenocarcinoma (biopsy), planned total or distal gastrectomy plus D2 lymphadenectomy (or D1 in total spleen-preserving gastrectomy) according to the Japanese Classification of Gastric carcinoma, Fourth English edition.
-
(b)
Intraoperative (depending on surgeon criteria): Macroscopic infiltration of gastric serosa previously unsuspected, suspicion of macroscopic lymph nodes involvement without macroscopic serosa infiltration, no evidence of carcinomatosis (P0) and curative surgery (R0).
All patients with neoadjuvant chemotherapy ± radiotherapy were excluded if staging laparoscopy was not performed before the treatment to rule out peritoneal carcinomatosis.
Study variables
Age, sex, tumour location, Lauren classification, surgical approach (open or laparoscopy), type of gastrectomy (total or subtotal), lymphadenectomy type, surgical resection (R0,R1,R2), reconstruction (Roux-en-Y, Billroth I or II), combined resection of other organs, clinical and pathological stage (according according to the TNM 7th edition), standard lavage vs. EIPL, number of resected nodes, number of invaded nodes, peritoneal cytology (before and after surgery and after lavage), postoperative morbidity (Clavien-Dindo classification), reintervention, hospital stay (days), neoadjuvant therapy, adjuvant therapy, recurrence and site of recurrence.
Sample size
The number of patients was determined comparing the difference of expected survival with the log-rank test with a two-sided alpha level of 0.05 and a power of 90%. A previous study showed that overall 3-year survival for patients of a cohort of 115 patients T3–T4, M0 (TNM 6th edition) with R0 and surgical D2 lymphadenectomy in the University Hospital Mútua de Terrassa was 50.3%. A total of 94 patients (47 patients in each group) were needed to detect a 20% difference in 3-year OS of 50% for the surgery alone group and 70% for the surgery plus EIPL group.
Patients were randomised to surgery alone or surgery plus EIPL group in a 1:1 ratio stratified by every participating hospital (with a block size of four) using a computer program. This decision was taken because one of the important biases could be the results of the peritoneal cytology due to the different sensitivity between participating centres or pathologists. Allocation was communicated to the surgeon after the end of the surgery to ensure the same procedure for every group. Patients were blinded to the procedure assignment.
Procedure
All patients were proposed to be treated with open or laparoscopic curative D2 gastrectomy (R0) depending of the hospital and according the Guidelines of the Japanese Research Society for the study of gastric cancer (4th English version) [13]. A total or subtotal gastrectomy was performed depending of the location of the tumour. In cases of total gastrectomy, the preservation of splenic nodes (group 10) was considered D1 but these patients were also included in the essay.
Three peritoneal cytology samples were collected from every patient. The first at the beginning of surgery with a wash in all abdominal cavity of 100 cc physiological serum, or a direct sample was taken in cases with ascites. The second at the end of the surgery with a wash of 100 cc physiological serum. And the third after a standard lavage (< 2 L) or EIPL (10 L). All the samples were identified separately and collected in different tubes. All the samples were analysed in each respective hospital by centrifugation 1000 × 10 min with Papanicolau and Giemsa staining.
Adjuvant chemotherapy was recommended for patients with locally advanced tumours and curative resection by the multidisciplinary tumour board of each centre.
Follow-up
Physical examination and analysis with tumours markers were done postoperatively at 3 months and every 6 months up to 3 years. Abdominal and chest CT was performed every 6 months for the first 3 years after surgery and once a year thereafter. Upper endoscopy was carried out every year. However, in cases of suspected recurrence by clinical symptoms during this period, additional imaging examination (CT or PET) was performed for confirmation.
Statistical analysis
The data were analysed on an intention-to-treat basis for all patients that fulfilled the inclusion criteria. The difference of overall survival was compared using the Kaplan–Meier method (Log-rank). Uni/multivariate analysis of survival was done using the Cox regression method. Exploratory subgroup analysis was also conducted to evaluate the effects of perioperative factors on between-group differences in OS using the Cox regression model including interaction term of treatment group and each perioperative factor. Qualitative or quantitative variables were compared using a χ2 test or t test. All p < 0.05 values were considered statistically significant. The analysis was performed using the SPSS software, version 25 (IBM Corporation).
Results
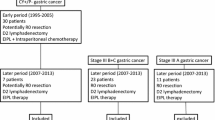
From January 2015 to December 2017, 94 patients were randomised to surgery plus EIPL or surgery plus standard lavage (47 patients in each group). Eight patients were excluded from the analysis after randomisation: Two due to postoperative Clavien V complications (1:1), three for R1 resection (1:2) and three for incomplete data (2:1). A total of 86 patients were included in the statistical analysis (43 per group) (Fig. 1).
Clinical and surgical outcomes characteristics
The clinical characteristics of patients are shown in Table 1. A good statistical balance between both groups was observed. There were no statistical differences between age, sex, ASA, tumour localisation, histologic type, pTNM classification or stage. The surgical outcomes are shown in Table 2. There were no differences in types of gastrectomy and lymphadenectomy, number of resected lymph nodes, surgical approach (open vs. laparoscopy), postoperative complications, reoperations, hospital stay and adjuvant chemotherapy, between groups. Distal tumours, intestinal type, subtotal gastrectomy and open approach were the most frequent in global series.
Prognostic factors
The characteristics of clinical and pathological data showed that tumour localisation (p < 0.001), type of gastrectomy (p 0.013) and lymph nodes status (pN) (p 0.010) were independent factors by univariate Cox regression for survival in this series. Multivariate Cox regression analysis (by steps) demonstrated that lymph nodes status (pN) was the most important prognostic factor in this series (p 0.006).
In the subgroup analyses, there were no significant interactions between treatment effect and any patient’s characteristics (Table 3).
Overall survival
The median follow-up of survival was 45 months (95% CI 34.6–55.4). The 3-year OS for the standard lavage group was 64.6% and 62.3% for the EIPL group (HR 1.36 (95%CI 0.64–2.91; p 0.421) (Fig. 2).
Peritoneal cytology
Three peritoneal cytology samples were collected from every patient. In the first peritoneal cytology (at the beginning of the surgery), only 1 case was positive in the EIPL group and none in the standard lavage group. In the second peritoneal cytology (at the end of the surgery) only 1 case was positive in each group. In the third peritoneal cytology (after lavage) there were no positive cases in EIPL and only 1 positive in standard lavage.
In summary, four cytologies were positive in three patients. In the SL group, one patient had (first−/second + /third +) and died 15 months after surgery for locoregional recurrence. In EIPL, one patient had (first + /second−/third−) and another patient had (first−/second + /third−).
Complications
No intraoperative complications related to EIPL were observed. There was no difference in postoperative complications between groups (37.2% standard lavage vs. 32.5% EIPL p 0.65). Intraabdominal collection and pancreatic fistula were seen more often in standard than extended lavage. Clavien–Dindo classification of surgical complications did not demonstrate differences between groups (p 0.47). Open vs. laparoscopic approach showed differences in overall complications 39 vs. 29% but not significant (p 0.30).
There were two postoperative in-hospital deaths, one in each group, for anastomotic leakage and another for pneumonia with respiratory failure (Table 2).
Neoadjuvant or postoperative adjuvant chemotherapy
A total of 38.4% of patients received neoadjuvant chemotherapy, 60.5% adjuvant chemotherapy and 30% both, according to the oncological protocols of each institution. There were no differences between groups in neoadjuvant chemotherapy (32.6% standard lavage vs. 44.1% EIPL, p 0.27) or in adjuvant chemotherapy (62% standard lavage vs. 63.4% EIPL, p 0.88).
Site of recurrence/lavage
There were no differences between groups in recurrence (p 0.37) or between site of recurrence (peritoneal, locoregional, hepatic, others) (p 0.79) (Table 4).
Recurrence/surgical approach
No differences in recurrence between open or laparoscopic approach were observed (41.6% open vs. 42.2% laparoscopic approach, p 0.34). Locoregional and peritoneal recurrence were more frequent in open than laparoscopic approach. However, this does not allow to conclude that the laparoscopic approach produces less locoregional recurrence as the present study was not designed to respond this question (Table 4).
Discussion
This study was designed to demonstrate whether EIPL can be effective as a prophylactic approach to prevent recurrence in locally advanced gastric cancer, independently from the presence of a positive peritoneal cytology. The results obtained show that there is no benefit in overall survival, recurrence or type of recurrence. However, some relevant aspects should be highlighted. Intraabdominal collections and pancreatic fistula were not observed in EIPL group comparing to standard lavage and the recurrence site differs between surgical approaches. There were no statistical differences between the number of cases with recurrence but locoregional and peritoneal site was most frequent in the open than in the laparoscopic approach (p 0.048) and the only patient with three positive cytologies was in the SL group. OS was chosen as the primary outcome rather than disease-free survival because of the difficulty to detect early recurrence or peritoneal dissemination by imaging studies or clinical symptoms.
Since Kuramoto and colleges demonstrated that EIPL plus peritoneal chemotherapy could play a special role in CY1/P0 cases, some authors have tried to demonstrate the contribution of EIPL alone in the good results obtained in their study [7]. Moreover, in Western countries, free peritoneal tumour cells are detected only sporadically in the staging cytology and sensitivity could differ between pathologists and institutions [11,12,13]. For these reasons, we designed this trial to evaluate the EIPL in patients with clinical suspicion of locally advanced cancer but independently from the results of peritoneal cytology. The results obtained could be very promising due to the simplicity, easy reproducibility, low cost and time-saving aspect of the technique. One of the most interesting aspects was the concept of “limiting dilution theory” that consists in a peritoneal wash of one litre of physiological saline followed total aspiration repeated 10 times expecting to lead to a logarithmic reduction of cancer cells to zero [14, 15].
To date, there are three prospective trials, from different eastern countries, that try to analyse whether EIPL could be a standard procedure in locally advanced gastric cancer to prevent recurrence and improve survival. The CCOG 1102 included 314 patients (Japan), EXPEL with a total of 800 subjects (Singapore, Malaysia, Korea, China and Japan) and SEIPLUS with 662 patients (China) [7, 16, 17]. Of these trials, CCOG 1102 trial results has not shown an improvement in survival or peritoneal recurrence in the EIPL group [18]. SEIPLUS trial recently published the results of short-term postoperative complications and mortality, showing a significant difference in postoperative complications (17 vs. 11.1%), reduced mortality (1.9 vs. 0%) and less postoperative pain (17.7 vs. 10.8%) favouring EIPL. The results of survival and recurrence are pending [17]. During the editorial revision of this manuscript, the EXPEL trial concluded that EIPL did not have a survival benefit and cannot be recommended for standardised use [19].
To our knowledge, this is the first randomised trial performed in a Western country and the results could be different from the East experience. The percentage of postoperative complications is significantly higher than in Eastern countries. However, the difference between both groups were similar (37.2 vs. 32.5%, p 0.65) and all types of complications were included (even Clavien–Dindo I–II grade). Another possible justification may be the influence of different surgical approaches (open vs. laparoscopic) but we could not demonstrate statistical differences between postoperative complication and the approach (p 0.30).
In the univariate analysis for prognostic factors, tumour localisation, gastrectomy type and pN have been shown as independent factors related to survival. In this study, pT and type of lymphadenectomy were not so relevant because the majority of patients were pT3–4 (locally advanced) and D2 lymphadenectomies. In the multivariate analysis, pN was the only independent prognostic factor. These results are in line with those previously published. We have observed that pT3–4 in SL was 70 vs. 74.4% in EIPL and it could seem strange because one of the inclusion criteria was cT3–4. However, the majority of these understaged patients received neoadjuvant chemotherapy (according to the oncologic protocol of the institution) or have enlarged or pathological lymph nodes by CT in the preoperative staging. The CGOG 1102 trial, with similar ethodology, also showed pT3–4 in 77.9% in EIPL and 86% in SL [17].
Peritoneal washing cytology has a high specificity but a questionable sensitivity (11.1 vs. 80%) [9]. The sensitivity (12.3–67%) and specificity (94–100%) of immunochemistry does not show a clear benefit compared to conventional Papanicolau staining. Molecular methods for the detection of intraperitoneal free cancer cells such as mature messenger ribonucleic acid of carcinoembryonic antigen (CEA mRNA) and cytokeratin 20 (CK-20 mRNA) have expressed a sensitivity and specificity of 38–100% and 7.3–100% and 25–64% and 80.3–94%, respectively. Due to the limitations of each method, the use of both cytology and molecular examination could be mandatory [20]. However, lack of immediate intraoperative results, relatively high false-positive results, higher cost and lack of this technology in each centre are some of the limitations for the standard use [20, 21].
An analysis of the number of cases with positive cytology and the number of cases with peritoneal or locoregional recurrence in both groups may account for the scepticism of their use in the habitual practice. The sensitivity of peritoneal cytology in this study was very low: only 1 case in EIPL and 0 in standard lavage at the beginning of the surgery. After resection there was 1 positive cytology in each group (both patients were previously negative and it may be explained by exfoliation during surgery). No patient remained positive after EIPL and 1 patient after SL. This patient died of loco-regional recurrence 15 months after surgery. The authors consider that the cytology may have been negative if EIPL had been performed. Another aspect that requires a special mention is that the number of patients with positive peritoneal cytologies (3.5%) does not match with the number of recurrences observed in this study (41.6% SL and 42.2% EIPL). The Misawa K. study showed a similar number of positive cytologies in 3.4% with EIPL group and 8% with SL with recurrence in 33.1 vs. 40%, respectively without statistical significance, in contrast with the study of Kuramoto et al. [5].
The results of the present study are in concordance with those of the CCOG 1102. However, our sample size is significantly minor and may be underpowered. Although a longer follow-up may produce more solid results, we believe that outcomes are very unlikely to change after 3 years. Large studies with a greater number of patients, EXPEL and SEIPLUS could demonstrate if a subgroup of patients could benefit of EIPL as prophylactic strategy for recurrence [15,16,17,18].
In conclusion, our findings suggest that EIPL, regardless of the peritoneal cytology, cannot be supported as a prophylactic standard method to reduce peritoneal recurrence or to increase OS in locally advanced gastric cancer.
References
Fujiwara Y, Doki Y, Taniguchi H, et al. Genetic detection of free cancer cells in the peritoneal cavity of the patient with gastric cancer: present status and future perspectives. Gastric Cancer. 2007;10:197–2014.
Han TS, Kong SH, Lee HJ, et al. Dissemination of free cancer cells from the gastric lumen and from perigastric lymphovascular pedicles during radical gastric cancer surgery. Ann Surg Oncol. 2011;18:2818–25.
Okumitsu Y, Yoshino S, Lida M, et al. Intraoperative dissemination during gastrectomy for gastric cáncer associated with serosal invasion. Surg Today. 2015;45:746–51.
Sobin LH, Gospodarowicz MK, Witteking Ch. TNM. In: Leslie HS, editor. Classification of malignant tumours. Wiley; 2010.
Kuramoto M, Shimada S, Ikeshima S, et al. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy of peritoneal recurrence in patients with gastric carcinoma. Ann Surg. 2009;250:242–6.
Kuramoto M, Shimada S, Ikeshima S, et al. A proposal of a practical and optimal prophylactic strategy for peritoneal recurrence. J Oncol. 2012. https://doi.org/10.1155/2012/340380.
Misawa K, Mochizuli Y, Oshashi N, et al. A randomized phase III exploring the prognostic of extensive intraoperative lavage in addition to standard treatment for resectable advanced gastric cancer: CCOG 1102. Jpn J Clin Oncol. 2014;44:101–3.
Masuda T, Kuramoto M, Shimada S, et al. The effect of extensive intraoperative peritoneal lavage therapy (EIPL) on stage IIIB+C and cytology-positive gastric cancer patients. Int J Clin Oncol. 2016;21:289–94.
Makino T, Fujiwara Y, Takiguchi S, et al. The utility of pre-operative peritoneal lavage examination in serosa-invading gastric cancer patients. Surgery. 2010;148:96–102.
Leake PA, Cardoso R, Seevaratnam R, et al. A systematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gastric Cancer. 2012;15(Suppl 1):S27–37.
Ronellenfitsch U, Ernst K, Mertens C, et al. Extensive intraperitoneal lavage to eliminate intraperitoneal tumor cells in gastrectomy with D2 lymphadenectomy for gastric cancer. Tumori J. 2018;104:361–8.
Yepuri N, Bahary N, Jain A, et al. Review and update on the role of peritoneal cytology in the treatment of gastric cancer. J Surg Res. 2018;235:607–14.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20(1): 1–9. 1 Jan 2017
Shimada S, Tanaka E, Maratsuka T, et al. Extensive intraperative lavage and chemotherapy for gastric cancer patients with peritoneal free cancer cells. Gastric Cancer. 2002;5(3):168–72.
Marutsuka T, Shimada S, Shimori K, et al. Mechanisms of peritoneal metastasis after operation for non-serosa-invasive gastric cancer: an ultrarapid detection system for intraperitoneal free cancer cell and a prophylactic strategy for peritoneal metastasis. Clin Cancer Res. 2003;9:678–85.
Kim G, Chen E, Tay AY, et al. Extensive peritoneal lavage after curative gastrectomy for gastric cancer (EXPEL): study protocol of an international multicenter randomized controlled trial. Jpn J Clin Oncol. 2017;47:179–84.
Guo J, Xu A, Sun X, et al. Combined surgery and extensive intraoperative peritoneal lavage vs surgery alone for treatment of locally gastric cancer. The SEIPLUS randomized clinical trial. JAMA Surg. 2019;154:610–6.
Misawa K, Mochizuki Y, Sakai M, et al. Randomized clinical trial of extensive intraoperative peritoneal lavage versus standard treatment for resecable advanced gastric cancer (CCOG 1102 trial). BJS. 2019;106:1602–10.
Yang HK, Han SU, Terashima M, et al. Extensive peritoneal lavage with saline after curative gastrectomy for gastric cancer (EXPEL): a multicenter randomized controlled trial. Lancet Gastroenterol Hepatol. 2021;6(2):120–7.
Virgilio E, Giarnieri E, Giovagnoli M, et al. Gastric Cancer cells in peritoneal lavage fluid: a systematic review comparing cytological with molecular detection for diagnosis of peritoneal metastases and prediction of peritoneal recurrences. Anticancer Res. 2018;38:1255–62.
Hasbahceci M, Akcakaya A, Guler B, et al. Use of peritoneal washing cytology for the detection of free peritoneal cancer cells before and after surgical treatment of gastric adenocarcinoma. J Can Res Ther. 2018;14:1225–9.
Acknowledgements
We wish to express our gratitude to Dr. Salvador Quintana for statistical support and Sylva-Astrik Torossian for her editorial contribution.
Funding
No grant support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interest.
Ethical approval
The study was approved by the Clinical Trial Commitee of the the reference hospital (Hospital Universitary Mutua de Terrassa) and the ethics committees of each participating hospital.
Informed consent
All candidates accepted to participate provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rodríguez-Santiago, J., Luna, A., Garsot, E. et al. Extended intraoperative peritoneal lavage as prophylactic peritoneal recurrence for locally advanced gastric cancer: a prospective randomized trial. Clin Transl Oncol 23, 1857–1865 (2021). https://doi.org/10.1007/s12094-021-02596-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02596-8