Abstract
Background
The aim of this study was to investigate the effects of extensive intraoperative peritoneal lavage (EIPL) therapy on stage III B + C and CY1/P0 gastric cancer patients after potentially curative surgery.
Methods
The study included 37 patients with CY1/P0 and 23 patients with stage III B + C gastric cancer who were treated with potentially curative gastrectomy and EIPL therapy between March 1995 and May 2013. D2 lymphadenectomy, R0 resection, and EIPL therapy were performed for all cases.
Results
Multivariate analysis revealed that male gender (P = 0.01) and lymph node metastasis (P = 0.03) were independent prognostic factors, while positive cytology was not (P = 0.21). There was no significant difference in overall survival rates between the CY1/P0 and stage III B + C groups (P = 0.93). There was also no significant difference in peritoneal recurrence rates, i.e., 13 (35.1%) in the CY1/P0 group and 5 (21.7%) in the stage III B + C group (P = 0.39).
Conclusions
EIPL therapy combined with complete resection and sufficient (D2) lymphadenectomy could improve the prognosis of CY1/P0 gastric cancer and, to a similar extent, that of stage III B + C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 60 % of gastric cancer patients are reported to die from peritoneal carcinomatosis [1] and the prognosis for advanced gastric cancer patients with peritoneal metastasis is extremely poor [2]. Patients with intraperitoneal free cancer cells without overt peritoneal metastasis (CY1/P0) are categorized as stage IV [3, 4], and their 5-year overall survival rate after potentially curative surgery has been reported to be 0–35 % [5]. Furthermore, the strategy of surgical treatment itself for CY1/P0 gastric cancer still remains controversial [6].
As it is already generally accepted that peritoneal metastasis is completed by the implantation of peritoneal free cancer cells, the status of CY1/P0 can be regarded as a pre-stage of peritoneal metastasis. Therefore, employing some effective measures to remove the peritoneal free cancer cells before the actual completion of peritoneal metastasis is justifiably considered to be prudent. From this point of view we have been advocating extensive intraoperative peritoneal lavage (EIPL) as a reliable prophylactic strategy for peritoneal recurrence after curative surgery for patients with advanced gastric or pancreatic cancer [7–9]. EIPL is a very simple treatment that can be performed anywhere and at any time. The peritoneal cavity is extensively washed and completely aspirated 10 times using 1 L of physiological saline based on the ‘limiting dilution theory’. EIPL is also a powerful means for reducing the number of free cancer cells to potentially zero [10–12]. We have previously reported that EIPL therapy remarkably improved the survival rate of CY1/P0 advanced gastric cancer patients after curative surgery [12]. The effectiveness of EIPL therapy in cyto-reduction in the abdominal cavity proved so remarkable and promising that we have recently extended its application to advanced gastric cancer patients without CY1/P0.
The aim of this study was to investigate the clinical effects of EIPL therapy accompanied by potentially curative surgery on the prognosis of advanced gastric cancers, mainly focusing on stage III B or III C (stage III B + C) and CY1/P0, which are considered a pre-stage of peritoneal carcinomatosis.
Materials and methods
Patients
In the period from March 1995 to July 2005 (the early period), we applied EIPL therapy accompanied by intraperitoneal chemotherapy (IPC) only to patients with CY1/P0 gastric cancer at the following medical institutions—the Department of Surgery, Yatsushiro Social Insurance General Hospital; the Department of Surgery, Kumamoto Regional Hospital; the Department of Surgery, Kumamoto City Hospital; and the Department of Gastrointestinal Surgery, Graduate School of Medical Sciences, Kumamoto University, Japan [12]. In this period, EIPL therapy was not applied to patients with stage III advanced gastric cancer. In the period from August 2005 to May 2013 (the late period), EIPL therapy was performed on all patients who had been preoperatively diagnosed with advanced gastric cancer, including 34 stage III gastric cancer patients at the Department of Surgery, Kumamoto General Hospital, Japan Community Health Care Organization (former Yatsushiro Social Insurance General Hospital), Japan. Of these 34 stage III patients, 11 were stage III A and 23 were stage III B + C. These patients did not receive IPC.
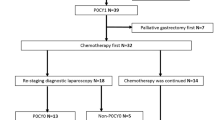
In total, we collected and analyzed data from 60 patients who received EIPL therapy after potentially curative surgery—37 patients with CY1/P0 (30 from the early period and 7 from the late period) and 23 patients with stage III B + C (late period) (Fig. 1).
Surgery, EIPL therapy and adjuvant therapy
For all 60 patients, conventional intraoperative peritoneal lavage cytology was performed immediately after laparotomy. The type of gastrectomy, such as distal or total gastrectomy, was based on the location or the status of the primary tumor extension. D2 lymphadenectomy, gross R0 resection, and EIPL therapy were performed for all cases [11]. The procedure for EIPL therapy was as follows. After potentially curative surgery, the peritoneal cavity was extensively washed, followed by complete aspiration of the fluid. This procedure was performed 10 times using 1 L of physiological saline. In the early period, all 30 patients received IPC therapy. As the protocol of IPC therapy, after EIPL treatment, cisplatin (CDDP) at a dose of 100 mg/body in one-half of normal saline solution was administered into the peritoneal cavity, and the intraperitoneal solution was drained 1 h after administration [12]. All patients received adjuvant chemotherapy after surgical treatment, i.e., oral administration of 5-fluorouracil derivatives for 2 years. In the late period, IPC was not performed for the 7 patients with CY1/P0 or the 23 patients classified as stage III B + C; however, all 30 patients received adjuvant chemotherapy. The choice of regimen to be administered as adjuvant chemotherapy for a particular patient (S-1, S-1 plus docetaxel, or S-1 plus cisplatin) was left to the discretion of the attending doctor. All patients were followed up until August 2013 or for up to 5 years or death.
Evaluations
Prognostic factors were investigated using 9 parameters—age, gender, type of gastrectomy, histologic type, depth of tumor invasion, lymph node metastasis, lymphovascular invasion (ly and v), and cytology. The status of lymph node metastasis was evaluated according to the 2nd edition of the ‘Japanese Classification of Gastric Carcinoma’ [13]. Multivariate analysis was performed using cytology and factors of P < 0.1 in univariate analysis. The 5-year overall survival and recurrence rates and sites were compared between the CY1/P0 and stage III B + C groups.
Statistics
All values are expressed as the mean ± standard deviation. Univariate analyses were performed using chi-squared test or Fisher’s exact probability test for categorical values and the Mann–Whitney U test for continuous variables. Overall survival rates were calculated and compared with the Kaplan–Meier method and log rank test or the Cox regression. Multivariate analyses were performed using the Cox proportional hazards regression model for overall survival. Differences with a P value of <0.05 were considered to be significant. All statistical analyses were performed using StatView ver. 5.0 software (SAS Institute, Cary, NC, USA).
Results
Clinicopathological characteristics of the CY1/P0 and stage III B + C groups are listed in Table 1. More female patients (P = 0.007) and v2 or 3 vascular invasion (P = 0.0002) were seen in the CY1/P0 group. There was no significant difference regarding histological type, depth of tumor invasion, or lymph node metastasis.
Prognostic factors of all 60 patients were evaluated. Univariate analysis showed that male gender correlates with poor overall survival rate (P = 0.04). Multivariate analysis was performed based on cytology and factors of P < 0.1 in univariate analysis, i.e., gender and lymph node metastasis. Male gender (P = 0.01) and lymph node metastasis (P = 0.03) were independent prognostic factors, while positive cytology was not (P = 0.21) (Table 2).
There was no significant difference in overall survival between the CY1/P0 and stage III B + C groups (P = 0.93). The 5-year overall survival rates were 46.5 % in the CY1/P0 group and 33.9 % in the stage III B + C group (Fig. 2).
The mean observation period was 32.7 ± 19.8 months. Total recurrences were observed in 19 (51.4 %) patients in the CY1/P0 group and 13 (56.5 %) in the stage III B + C group (P = 0.79). There was no significant difference in peritoneal recurrence rates, i.e., 13 (35.1 %) in the CY1/P0 group and 5 (21.7 %) in the stage B + C group (P = 0.39) (Table 3).
Discussion
Despite no apparent existence of abdominal free cancer cells or overt peritoneal metastasis, approximately half of all patients with serosa-involved advanced gastric cancer have been reported to develop peritoneal recurrence after curative surgery [10, 14]. Furthermore, non-serosa-involved gastric cancers have advanced to peritoneal recurrence after curative surgery [10, 15–17]. One of the most postulated suppositions for peritoneal dissemination in CY0/P0 advanced gastric cancer is that lymph node dissection surgery itself might open up lymphatic channels and spread viable cancer cells to the peritoneal cavity [12, 14], and we have validated this hypothesis in our previous studies [12, 18].
Based on solid empirical knowledge from our series of studies and clinical observations, we came to the decision that EIPL therapy should be applied not only to CY1/P0 gastric cancer but also to other advanced gastric cancers without CY1, which might prevent the possibility of lymphatic invasion and the development of peritoneal metastasis. We surmised that EIPL therapy would have the potential to eradicate all the intra-abdominal free cancer cells to nearly zero. Therefore, applying EIPL therapy for all advanced gastric cancer patients could prevent ‘iatrogenic’ peritoneal dissemination after curative surgery.
In the current study, we investigated prognostic factors and survival by analyzing the data of the gastric cancer patients who received EIPL therapy during curative surgery. Of 9 factors, gender and lymph node metastasis were independent prognostic factors. Although the sample size is small, CY1 was not extracted as an adverse prognostic factor. Moreover, the 5-year survival of the CY1/P0 group was not worse than that of the stage III B + C group. These data suggested a possibility that the actual status of CY1 could be disregarded by employing EIPL therapy in the treatment of CY1/P0 gastric cancer patients.
In the present study, a more severe lymphatic invasion of cancer cells was observed in the stage III B + C group than in the CY1/P0 group, although it was not significant, which might have some bearing on the similar survival rates in each group. According to a large-scale clinical study recently completed in Japan, the 5-year overall survival rate of stage III B gastric cancer patients treated with surgery and adjuvant chemotherapy was reported to be 37.6 % [17]. Our data showed 5-year overall survival rates of 46.5 % (CY1/P0 group) and 33.9 % (stage III B + C group), and these results were not thought to be inferior to previous reports. However, this study could not prove the efficacy of EIPL therapy for CY0 patients.
The current study suggests that CY1 might no longer be necessarily regarded as an adverse prognostic factor, since effective and convenient adjuvant treatment like EIPL therapy could exclude the risk of intraperitoneal free cancer cells when combined with potentially curative surgery for advanced gastric cancer. Moreover, no significant difference was observed in the survival rates between the CY1/P0 and stage III B + C groups, suggesting that EIPL therapy might have a down-staging effect of changing the category of CY1/P0 gastric cancer from stage IV to III.
In the current study, IPC was not applied to 30 patients in the late period because IPC often causes considerable side-effects despite having several worthy anticancer benefits [19, 20]. Recently, some newly devised drug delivery systems using gelatin microspheres [15] or hyaluronic acid-based hydrogel [21] have been reported which demonstrated enhanced anticancer remedies with decreased adverse side-effects. EIPL therapy combined with some new applicable IPC system might yield a better outcome in the treatment of cancer patients. Adjuvant chemotherapies were administered to all 60 patients in our study based on the evidence of the advantageous effect of adjuvant therapy and it undoubtedly improved the prognosis of the patients in our study [17]; however, the actual effect of adjuvant chemotherapy could not be proved from the current study.
Recently, the prognosis of CY1/P0 gastric cancer has been reported to be much better than that of P1 gastric cancer [6]. Furthermore, our current study confirmed that gastric cancer patients with CY1/P0 could survive as long as those with stage III B + C when more comprehensive treatments were applied including curative surgery with sufficient lymph node dissection, adjuvant chemotherapy and EIPL therapy as an adjuvant surgical technique. Unfortunately, the EIPL therapy could not show sufficient efficacy in improving the prognosis of advanced gastric cancer with severe lymphatic invasion. A new and more progressive chemotherapy is needed for alleviating the suffering of patients in this population.
There were some limitations to this study. It was a small-sized retrospective study and the patient selection was not well controlled. Due to the small number of operable CY1/P0 gastric cancer patients, it was designed to include patients at two different time periods from multiple hospitals. Moreover, there is little difference in the treatment strategies between the two time periods, such as IPC in the early period.
In conclusion, based on our latest findings, we strongly insist that potentially curative surgery should be performed for all gastric cancer patients who have the possibility of developing peritoneal carcinomatosis, as well as for CY1/P0 gastric cancer patients. EIPL therapy is suggested as an adjuvant surgical technique to prevent peritoneal carcinomatosis because of its remarkable cyto-reduction effects. Further studies will be needed to clarify the usefulness of this newly devised treatment as a leading candidate in the comprehensive strategy for gastric cancer.
References
Yonemura Y, Endou Y, Shinbo M et al (2009) Safety and efficacy of bidirectional chemotherapy for treatment of patients with peritoneal dissemination from gastric cancer: selection for cytoreductive surgery. J Surg Oncol 100:311–316
Harmon RL, Sugarbaker PH (2005) Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol 2:3
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101–112
Sobin L, Gospodarowicz M, Wittekind C (2009) TNM classification of malignant tumours, 7th edn. Wiley, New York
Leake PA, Cardoso R, Seevaratnam R et al (2012) A systematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gastric Cancer 15(Suppl 1):S27–S37
Mezhir JJ, Shah MA, Jacks LM et al (2010) Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol 17:3173–3180
Marutsuka T, Shimada S, Shiomori K et al (2003) Mechanisms of peritoneal metastasis after operation for non-serosa-invasive gastric carcinoma: an ultrarapid detection system for intraperitoneal free cancer cells and a prophylactic strategy for peritoneal metastasis. Clin Cancer Res 9:678–685
Shimada S, Tanaka E, Marutsuka T et al (2002) Extensive intraoperative peritoneal lavage and chemotherapy for gastric cancer patients with peritoneal free cancer cells. Gastric Cancer 5:168–172
Yamamoto K, Shimada S, Hirota M et al (2005) EIPL (extensive intraoperative peritoneal lavage) therapy significantly reduces peritoneal recurrence after pancreatectomy in patients with pancreatic cancer. Int J Oncol 27:1321–1328
Ikeshima S, Kuramoto M, Shimada S et al (2013) Standard prophylactic strategy against peritoneal dissemination metastasis in gastric cancer. J Cancer Ther 4:99–103
Kuramoto M, Shimada S, Ikeshima S et al (2012) A proposal of a practical and optimal prophylactic strategy for peritoneal recurrence. J Oncol 2012:340380
Kuramoto M, Shimada S, Ikeshima S et al (2009) Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg 250:242–246
Japanese Gastric Cancer Association (1998) Japanese classification of gastric carcinoma—2nd English edition. Gastric Cancer 1:10–24
Fujimoto S, Takahashi M, Mutou T et al (1997) Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery. Cancer 79:884–891
Gunji S, Obama K, Matsui M et al (2013) A novel drug delivery system of intraperitoneal chemotherapy for peritoneal carcinomatosis using gelatin microspheres incorporating cisplatin. Surgery 154:991–999
Hall JJ, Loggie BW, Shen P et al (2004) Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointest Surg 8:454–463
Sasako M, Sakuramoto S, Katai H et al (2011) Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 29:4387–4393
Yu X, Ren Z, Xue Y et al (2013) D2 lymphadenectomy can disseminate tumor cells into peritoneal cavity in patients with advanced gastric cancer. Neoplasma 60:174–181
Matharu G, Tucker O, Alderson D (2011) Systematic review of intraperitoneal chemotherapy for gastric cancer. Br J Surg 98:1225–1235
Yan TD, Black D, Sugarbaker PH, Zhu J et al (2007) A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol 14:2702–2713
Emoto S, Yamaguchi H, Kamei T et al (2013) Intraperitoneal administration of cisplatin via an in situ cross-linkable hyaluronic acid-based hydrogel for peritoneal dissemination of gastric cancer. Surg Today 44:919–926
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Masuda, T., Kuramoto, M., Shimada, S. et al. The effect of extensive intraoperative peritoneal lavage therapy (EIPL) on stage III B + C and cytology-positive gastric cancer patients . Int J Clin Oncol 21, 289–294 (2016). https://doi.org/10.1007/s10147-015-0892-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0892-6