Abstract
Objectives
To report outcomes of stereotactic body radiotherapy (SBRT) in metastatic castration-resistant prostate cancer (mCRPC) patients with oligoprogression (≤ 5 metastases) during first-line treatment with androgen receptor-targeted therapy (ARTT).
Patients and methods
Retrospective multi-institutional analysis of mCRPC patients treated with SBRT to oligoprogressive lesions during ARTT. End-points were time to next-line systemic treatment (NEST), radiological progression-free survival (r-PFS) and overall survival (OS). Toxicity was registered according to Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Survival analysis was performed using the Kaplan–Meier method, univariate and multivariate analysis (MVA) were performed.
Results
Data from 34 patients were analyzed. Median NEST-free survival, r-PFS, and OS were 16.97, 13.47, and 38.3 months, respectively. At MVA, factors associated with worse NEST-free survival and r-PFS were polymetastatic burden at diagnosis of metastatic hormone-sensitive disease (hazard ratio [HR] 3.66, p = 0.009; HR 3.03, p = 0.034), PSA ≤ 7 ng/ml at mCRPC diagnosis (HR 0.23, p = 0.017; HR 0.19, p = 0.006) and PSADT ≤ 3 months at mCRPC diagnosis (HR 3.39, p = 0.026; HR 2.79, p = 0.037). Polymetastatic state at mHSPC diagnosis was associated with a decreased OS (HR 4.68, p = 0.029). No patient developed acute or late grade ≥ 2 toxicity.
Conclusion
Our results suggest that SBRT in oligoprogressive mCPRC is safe, effective and seems to prolong the efficacy of the ongoing systemic treatment positively affecting disease progression. Prospective trials are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In metastatic castration-resistant prostate cancer (mCRPC), several agents are now available in the clinical practice, but this state of disease is still associated with a high-mortality rate [1]. Castration resistance is characterized by a biochemical or clinical progression in prostate cancer patients under castration, with a serum testosterone below 50 ng/ml. Based on the results of many studies [2,3,4,5], there is great interest in integrating metastasis-directed therapies (MDT) such as surgery and radiotherapy in the management of metastatic disease. Recently, the phase II SABR-COMET trial showed an overall survival benefit with stereotactic body radiotherapy (SBRT) when used in addition to standard-of-care systemic therapy, in patients with oligometastases from different histologies [5]. In prostate cancer, it has been demonstrated that SBRT is safe and effective in the setting of oligometastatic hormone-sensitive disease [6,7,8,9,10,11,12]. To date, few retrospective studies [13,14,15] have been published on MDT in mCRPC patients, demonstrating the possibility to prolong the time to the start of a new line of systemic treatment, and several prospective trials on MDT in this setting are ongoing [16]. Eventually, only some reports [17,18,19,20] are available on progression-directed therapy in mCRPC during first-line treatment with androgen receptor-targeted therapy (ARTT). With this multi-institutional retrospective study, we sought to add data to the current literature on the role of SBRT as a metastasis-directed therapy in mCRPC patients with oligoprogression during first-line treatment with ARTT.
Patients and methods
Criteria
We analyzed data from 34 patients affected by oligoprogressive mCRPC treated with SBRT from May 2015 to March 2020. All patients provided informed consent for this retrospective multi-institutional analysis. Inclusion criteria were: Eastern Oncology Cooperative Group (ECOG) 0–1; oligoprogression during treatment with androgen deprivation therapy (ADT) in combination with androgen receptor-targeted therapy (ARTT); testosterone level below 50 ng/ml; SBRT to all oligoprogressive lesions. Oligoprogression was defined as the onset of up to five metastases, including the radiological progression of existing lesions, in patients with mCRPC while under ADT in combination with ARTT.
Patients
Patients’ baseline clinical characteristics are reported in Table 1. At diagnosis, 28 (82%) patients were affected by localized prostate cancer, whereas 4 patients were metastatic. Overall, 30 patients (88%) underwent local treatment (surgery or radiotherapy) for their primary tumor. The median time from primary treatment to metastatic hormone-sensitive prostate cancer (mHSPC) was 43.5 months (IQR, 15–73). Choline-PET was the most frequent staging imaging modality in this setting (30 out of 34 patients). At diagnosis of metastatic hormone-sensitive prostate cancer, no patient had visceral metastases, 23 (68%) presented with oligometastatic disease (defined as ≤ 5 sites), and 11 (32%) were polymetastatic (> 5 sites and/or bone involvement beyond the vertebral bodies and pelvis). ADT was prescribed in 33 (97%) patients, of whom 16 underwent radiotherapy; only 1 patient was managed with exclusive SBRT. At the time of mCRPC diagnosis, the median age of the patients was 72 years (IQR, 67–77). The median time from mHSPC to mCRPC was 16 months (IQR, 1–37), and the median PSA doubling-time (PSADT) at diagnosis of mCRPC was 3 months (IQR, 2–6). Patients were staged mainly with choline-PET, and were treated with abiraterone as first-line ARTT in 29 (85%) cases, whereas enzalutamide was prescribed in the remaining 5 patients (15%). At oligoprogressive disease, all patients underwent SBRT, maintained first-line ARTT and were scheduled for serial follow-up with PSA, testosterone and imaging (choline-PET) every 3–6 months or at change in PSA dynamics. More specifically, regarding PSA dynamics, biochemical progression after SBRT on oligoprogressive lesions was defined as follows: (1) an initial decline from baseline PSA was observed followed by a PSA increase of 25% and 2 ng/ml above the nadir, or an increase of 25% and greater than the pretreatment PSA value; (2) no initial decline from baseline if the baseline PSA was ≥ 2 ng/ml, a PSA increase of ≥ 25% and ≥ 2 ng/ml above the baseline, or a PSA increase of ≥ 2 ng/ml if the baseline PSA was < 2 ng/ml; or (3) initiation of new systemic therapy [21].
Regarding SBRT planning for oligoprogressive lesions, all patients underwent a CT scan. Diagnostic PET was used for image registration in 31 (91%) patients to delineate gross tumor volume (GTV). The planning target volume (PTV) was defined as the GTV plus a 5–8 mm isotropic margin depending on tumor location. Organs at risk were delineated depending on the site of the GTV. SBRT was linac-based in 80% of the cases, whereas 7 (20%) patients were treated with Cyberknife. For each patient, daily image-guided radiotherapy was performed.
Statistical analysis
Clinical end-points were time to next-line systemic treatment (NEST), radiological progression-free survival (r-PFS) defined as any radiological progression (in-field and/or out-field) after SBRT to oligoprogressive lesions, and overall survival (OS). Toxicity was registered according to Common Terminology Criteria for Adverse Events (CTCAE) v4.0. The Fisher’s exact test was used to compare the distribution of categorical variables according to patients’ outcomes. The Kaplan–Meier method and log-rank test were used for univariate survival analysis, and the Cox proportional hazards model (with time since start of the radiation treatment as the time variable) for multivariate analysis (MVA). Regarding the latter, all variables were entered in an initial multivariable model, and the final model was then selected via backward elimination. In some cases, the final regression model consisted of a model with a single variable included, whose HR and 95% CI therefore coincided with that of the univariate model (see "Results").
Results
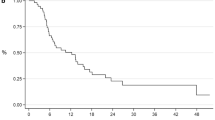
Oligoprogression during first-line ARTT occurred after a median time of 11.7 months (IQR, 7.8–17.5) and was mainly characterized by the presence of ≤ 2 sites of oligoprogression (Table 2). SBRT was delivered to a median total dose of 30 Gy (IQR, 27–36) in 3–5 fractions with a Biological Effective Dose (BED) > 100 Gy in all cases, using an α/β ratio of 3 [7]. At the end of follow-up, 13 patients were still in first-line ARTT, 9 were in second-line therapy, and 12 were dead from disease. The median follow-up after SBRT at oligoprogressive sites was 25.0 months (IQR, 16.0–30.7). One and 2-year NEST-free survival (Fig. 1a) were 58.6% (95% CI 39.4–73.6%) and 32.9% (95% CI 15.8–51.1%), respectively; the median NEST-free survival was 16.97 months (IQR, 6.47—not reached). Regarding r-PFS (Fig. 1b), 1- and 2-year values were 54% (95% CI 34.9–69.7%) and 24.5% (95% CI 10–42.5%); the median r-PFS was 13.47 months (IQR, 5.5–21.93). One and 2-year OS were 86.1% (95% CI 67.2–94.6%) and 74.9% (95% CI 54.3–87.2%), respectively (Fig. 1c); the median OS was 38.3 months (IQR, 17.43–39.43).
At univariate analysis (Table 3), NEST-free survival was significantly affected by the number of metastases (> 3 vs ≤ 3) at mHSPC diagnosis (HR 2.44, 95% CI 0.99–6.01, p = 0.05), the metastatic burden (poly vs oligometastatic) at mHSPC (HR 3.52, 95% CI 1.45–8.55, p = 0.006), and the PSA value (> 7 vs ≤ 7 ng/ml) at mCRPC diagnosis (HR 0.30, 95% CI 0.10–0.85, p = 0.024). The multivariate analysis confirmed the association of the latter two variables with worse NEST-free survival, as well as of PSADT ≤ 3 months at mCRPC diagnosis (Table 3). At univariate analysis, a worse r-PFS was associated with polymetastatic burden at mHSPC, PSA value ≤ 7 ng/ml at mCRPC diagnosis, and time interval between the diagnosis of mHSPC and mCRPC < 12 months. These findings were confirmed at MVA (Table 3). Eventually, PSADT ≤ 3 months at mCRPC diagnosis was negatively associated with r-PFS (HR 2.79, 95% CI 1.06–7.33, p = 0.037).
In our analysis, none of the variables reported in Table 3 affected overall survival except for the high metastatic burden at metastatic hormone-sensitive prostate cancer diagnosis, which was associated with a decreased OS (HR 4.68, 95% CI 1.17–18.79, p = 0.029).
Regarding toxicity, no patient developed acute and/or late grade ≥ 2 toxicity.
Discussion
Metastatic-CRPC treatment consists of ARTT, chemotherapy, radium-223, immunotherapy with Sipuleucel-T [22]. Despite improvements in systemic therapy, mCRPC is still a poor-prognosis disease state. Regarding ARTT, multiple resistance mechanisms have been identified including alterations in AR signaling, and activation of AR-independent pathways [23]. In the subset of oligoprogressive patients during ARTT, MDT could potentially ablate resistant clonogens delaying progression and time to NEST [17,18,19,20].
In our series, SBRT to all oligoprogressive lesions led to a median NEST-free survival of 16.97 months and to a median r-PFS of 13.47 months. These results are concordant with those of Berghen et al. [17], reporting a median NEST-free survival of 16 months (95% CI 10–22) and a median PFS of 10 months (95% CI 6–15). After SBRT to all oligoprogressive lesions in mCRPC patients, Deek et al. [20] reported a median time to next systemic therapy of 15.6 months (95% CI 13.80–21.10) and a median PFS of 10.83 months (95% CI 7.47–13.57). Eventually, the authors reported that the addition of MDT in this setting was associated with improved time to next-line systemic treatment and distant metastasis-free survival when compared with a cohort of patients treated with change in systemic treatment alone. There are two main differences between our series and the one by Deek [20]. In fact, we had no control group of “SBRT-untreated” patients, and our population was more homogeneous because of receiving an anti-androgen receptor agent as first-line treatment at mCRPC diagnosis, whereas in the other study, 26 (38%) patients underwent chemotherapy.
Regarding OS, in our cohort, the median OS was 38.3 months, with a 2 year OS of 74.9% (Fig. 1c). Taking into account that oligoprogression occurred after a median time of 11.7 months from the start date of first-line ARTT, these patients experienced a sustained period of disease control thanks to the ability of SBRT to sterilize resistant clones allowing the maintenance of the current systemic therapy. In this scenario, it is of crucial importance to understand which patients are suitable for SBRT during disease progression. Several studies investigated the role of ablative radiotherapy in the hormone-sensitive setting, demonstrating the positive impact of such treatment modality in terms of ADT-free survival [11] and progression-free survival [12]. In the mCRPC setting, there are some ongoing phase II trials investigating the role of SBRT in combination with standard-of-care therapy, such as FORCE (NCT03556904), ARTO (NCT03449719), and DECREASE trial (NCT04319783). In our series, we sought to find clinical variables that might affect the outcome in mCRPC patients with oligoprogression during ARTT. At multivariate analysis, a high metastatic burden at HSPC diagnosis, a PSA value < 7 ng/ml at diagnosis of mCRPC, a PSADT ≤ 3 months at diagnosis of mCRPC, and a time interval between metastatic hormone-sensitive prostate cancer, and mCRPC < 12 months were all associated with worse outcome (Table 3). Our results, which are hypothesis generating, suggest that these variables reflecting the activity of the tumor are important prognostic factors in the oligoprogressive mCPRC setting. Regarding PSADT, it has been identified as a significant independent risk factor for death [24,25,26]. More specifically, PSADT < 3 months seems to be a related with shorter metastasis-free survival and OS [27]. We found that patients with an absolute PSA value > 7 ng/ml at diagnosis of mCRPC have a better prognosis compared with those expressing values < 7 ng/ml. Although conflicting data have been reported on PSA absolute value as a prognostic factor [28,29,30], our result could be related to the low PSA secretion in a subset of aggressive and de-differentiated mCRPC, but we cannot rule out a selection bias. Anyway, PSADT reflecting the rate of PSA increase as a function of time seems to be a reliable parameter of disease kinetics and should be considered as a prognostic factor in mCRPC [30, 31].
This study has all the limitations of a retrospective analysis with a low number of patients, although imaging modalities and SBRT treatment at mCRPC oligoprogression are quite homogeneous. Regarding imaging, we used choline-PET to stage mCRPC patients, and during the follow-up to evaluate r-PFS after SBRT. Although contrast-enhanced CT, MRI and bone scan are considered as gold standard at this stage [21], they have a poor accuracy for detecting “low-burden” disease and for assessing response to treatment [16]. Compared with standard imaging, prostate cancer-specific PET radiotracers could improve the early detection of disease progression allowing the addition of MDT to the current treatment strategy and/or the switch to a second-line of systemic therapy. In mCRPC, Ceci et al. [32] reported a positive predictive value of 99% and a negative predictive value of 81% of choline-PET in detecting lesions, which do not respond to treatment. Due to the lack of accuracy of PSA in this setting [33, 34], choline-PET might be used as a reliable tool to predict the response to therapy [35,36,37]. For instance, a retrospective analysis [35] showed that choline-PET was able to identify radiological progression in 16/32 (50%) castration-resistant patients with decreasing PSA values during therapy with docetaxel. In patients undergoing therapy with abiraterone or enzalutamide, choline-PET-based radiologic response seems to be more useful to predict clinical outcome compared with PSA decrease [37]. In the scenario of mCRPC, new imaging modalities capable of assessing the tumor burden before and during systemic therapy will help to better define the most appropriate treatment strategy.
In conclusion, from our analysis, we could hypothesize that patients with a more aggressive disease phenotype are not suitable for ablative radiotherapy to go on with first-line ARTT at oligoprogression. In these patients, it would be advisable to start a new systemic treatment maybe in combination with progression-directed therapy, although to date, there is currently no consensus on timing and sequencing of the available treatment options [1]. This work, as the few others to date available in literature [14, 17,18,19,20], arises the question of how progression-directed therapy might best be integrated into the treatment of mCRPC patients. In the next future, beyond currently available clinical parameters advanced imaging techniques and blood-based biomarkers will help better understanding disease behavior allowing to an improvement in the prognostic classification. Tumor progression is a complex phenomenon in which mixed clonal populations show different sensibility to treatment. This observation highlights the potential for systemic and local treatment integration, and prompts to develop clinical trials focused on this treatment strategy in specific settings and to improve selection criteria.
Data availability
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Code availability
The software application for data storage is Excel and the software application for data analysis is SPSS.
References
Nuhn P, De Bono JS, Fizazi K, et al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol. 2019;75:88–99.
Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II randomized study. J Clin Oncol. 2019;37:1558–65.
Wong AC, Watson SP, Pitroda SP, et al. Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT). Cancer. 2016;122:2242–50.
Lancia A, Ingrosso G, Carosi A, et al. Oligometastatic cancer in elderly patients: the “blitzkrieg” radiotherapy approach. Aging Clin Exp Res. 2019;31:109–14.
Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38:2830–8.
Mazzola R, Francolini G, Triggiani L, et al. Metastasis-directed therapy (SBRT) Guided by PET-CT 18F-CHOLINE versus PET-CT 68Ga-PSMA in castration-sensitive oligorecurrent prostate cancer: a comparative analysis of effectiveness. Clin Genitourin Cancer. 2020;S1558–7673(20):30191–9.
Ost P, Jereczek-Fossa BA, As NV, et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multi-institutional analysis. Eur Urol. 2016;69:9–12.
Detti B, Bonomo P, Masi L, et al. Stereotactic radiotherapy for isolated nodal recurrence of prostate cancer. World J Urol. 2015;33:1197–203.
Ingrosso G, Trippa F, Maranzano E, et al. Stereotactic body radiotherapy in oligometastatic prostate cancer patients with isolated lymph nodes involvement: a two-institution experience. World J Urol. 2017;35:45–9.
Triggiani L, Alongi F, Buglione M, et al. Efficacy of stereotactic body radiotherapy in oligorecurrent and in oligoprogressive prostate cancer: new evidence from a multicentric study. Br J Cancer. 2017;116:1520–5.
Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446–53.
Phillips R, Shi WY, Deek M, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020;6:650–9.
Lohaus F, Zöphel K, Löck S, et al. Can local ablative radiotherapy revert castration-resistant prostate cancer to an earlier stage of disease? Eur Urol. 2019;75:548–51.
Triggiani L, Mazzola R, Magrini SM, et al. Metastasis-directed stereotactic radiotherapy for oligoprogressive castration-resistant prostate cancer: a multicenter study. World J Urol. 2019;37:2631–7.
Yoshida S, Takahara T, Arita Y, et al. Progressive Site-Directed Therapy for Castration-Resistant Prostate Cancer: Localization of the Progressive Site as a Prognostic Factor. Int J Radiat Oncol Biol Phys. 2019;105:376–81.
Lancia A, Zilli T, Achard V, et al. Oligometastatic prostate cancer: The game is afoot. Cancer Treat Rev. 2019;73:84–90.
Berghen C, Joniau S, Ost P, et al. Progression-directed therapy for oligoprogression in castration-refractory prostate cancer. Eur Urol Oncol. 2019;S2588–9311(19):30138–45.
Valeriani M, Marinelli L, Macrini S, et al. Radiotherapy in metastatic castration resistant prostate cancer patients with oligo-progression during abiraterone-enzalutamide treatment: a mono-institutional experience. Radiat Oncol. 2019;14:205.
Moyer CL, Phillips R, Deek MP, et al. Stereotactic ablative radiation therapy for oligometastatic prostate cancer delays time-to-next systemic treatment. World J Urol. 2019;37:2623–9.
Deek MP, Taparra K, Phillips R, et al. Metastasis-directed therapy prolongs efficacy of systemic therapy and improves clinical outcomes in oligoprogressive castration-resistant prostate cancer. Eur Urol Oncol. 2020;S2588–9311(20):30058–64.
Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–18.
Ingrosso G, Detti B, Scartoni D, et al. Current therapeutic options in metastatic castration-resistant prostate cancer. Semin Oncol. 2018;45:303–15.
Bonkhoff H, Berges R. From pathogenesis to prevention of castration resistant prostate cancer. Prostate. 2010;70:100–12.
Semeniuk RC, Venner PM, North S. Prostate-specific antigen doubling time is associated with survival in men with hormone-refractory prostate cancer. Urology. 2006;68:565–9.
Armstrong AJ, Garrett-Mayer ES, Yang YC, et al. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res. 2007;13:6396–403.
Daskivich TJ, Regan MM, Oh WK. Distinct prognostic role of prostate-specific antigen doubling time and velocity at emergence of androgen independence in patients treated with chemotherapy. Urology. 2007;70:527–31.
Antonarakis ES, Chen Y, Elsamanoudi SI, et al. Long-term overall survival and metastasis-free survival for men with prostate-specific antigen-recurrent prostate cancer after prostatectomy: analysis of the Center for Prostate Disease Research National Database. BJU Int. 2011;108:378–85.
Huang SP, Bao BY, Wu MT, et al. Impact of prostate-specific antigen (PSA) nadir and time to PSA nadir on disease progression in prostate cancer treated with androgen-deprivation therapy. Prostate. 2011;71:1189–97.
Fleming MT, Morris MJ, Heller G, Scher HI. Post-therapy changes in PSA as an outcome measure in prostate cancer clinical trials. Nat Clin Pract Oncol. 2006;3:658–67.
Miyazawa Y, Sekine Y, Shimizu N, et al. An exploratory retrospective multicenter study of prognostic factors in mCRPC patients undergoing enzalutamide treatment: focus on early PSA decline and kinetics at time of progression. Prostate. 2019;79:1462–70.
Yamamoto Y, Okuda Y, Kanaki T, et al. Clinical indicators for predicting prognosis after radium-223 administration in castration-resistant prostate cancer with bone metastases. Int J Clin Oncol. 2020. https://doi.org/10.1007/s10147-020-01776-w.
Ceci F, Castellucci P, Nanni C, et al. PET/CT imaging for evaluating response to therapy in castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:2103–4.
Loblaw DA, Walker-Dilks C, Winquist E, et al. Systemic therapy in men with metastatic castration-resistant prostate cancer: a systematic review. Clin Oncol (R Coll Radiol). 2013;25:406–30.
Armstrong AJ, Garrett-Mayer E, de Wit R, et al. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2010;16:203–11.
Ceci F, Castellucci P, Graziani T, et al. (11)C-Choline PET/CT in castration-resistant prostate cancer patients treated with docetaxel. Eur J Nucl Med Mol Imaging. 2016;43:84–91.
De Giorgi U, Caroli P, Burgio SL, et al. Early outcome prediction on 18F-fluorocholine PET/CT in metastatic castration-resistant prostate cancer patients treated with abiraterone. Oncotarget. 2014;5:12448–58.
De Giorgi U, Caroli P, Scarpi E, et al. 18F-fluorocholine PET/CT for early response assessment in patients with metastatic castration-resistant prostate cancer treated with enzalutamide. Eur J Nucl Med Mol Imaging. 2015;42:1276–83.
Funding
None.
Author information
Authors and Affiliations
Contributions
Study concept and design: Ingrosso, Detti, Lancia, Valeriani. Acquisition of data: Ingrosso, Detti, Fodor, Borghesi, Triggiani, Trippa, Russo, Bruni, Francolini, Marinelli. Analysis and interpretation of data: Caini, Detti, Ingrosso, Lancia. Drafting of the manuscript: Ingrosso, Detti, Caini, Livi, Aristei, Di Muzio. Critical revision of the manuscript for important intellectual content: Magrini, Triggiani, Maranzano, Musio, Valeriani, Fodor, Livi. Statistical analysis: Caini, Ingrosso, Detti. Supervision: Magrini, Livi, Musio, Maranzano, Di Muzio, Aristei. All authors have made a substantial contribution to research design, or the acquisition, analysis or interpretation of data. All authors have drafted the paper and revised it critically and have approved the submitted final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was waived by the local Ethics Committee of University of Perugia in view of the retrospective nature of the study and all the procedures being performed were part of routine care. The study was performed according to the Declaration of Helsinki and written informed consent was obtained for all patients.
Informed consent
All patients provided informed consent for this retrospective multi-institutional analysis.
Consent for publication
All patients provided informed consent for publication, and no identifying information is included in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ingrosso, G., Detti, B., Fodor, A. et al. Stereotactic ablative radiotherapy in castration-resistant prostate cancer patients with oligoprogression during androgen receptor-targeted therapy. Clin Transl Oncol 23, 1577–1584 (2021). https://doi.org/10.1007/s12094-021-02553-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02553-5