Abstract
Purpose
This study evaluated the efficacy and safety of nivolumab treatment beyond progressive disease (PD) in non-small cell lung cancer (NSCLC).
Patients/methods
Medical records of consecutive patients with advanced NSCLC who received nivolumab between December 2015 and December 2018 were reviewed. Clinical outcomes of three groups of eligible patients who received nivolumab as a second-line treatment after PD were compared based on Response Evaluation Criteria in Solid Tumors v1.1. We conducted subgroup analyses in patients with and without new lesions at first PD.
Results
Twenty-eight patients continued nivolumab treatment beyond PD (TBP). Post PD, 46 patients switched to other anti-cancer treatment (OAT), and 21 received no further anti-cancer treatment (NAT). There were no significant differences in overall survival (OS) or survival post progression (SPP) between TBP and OAT groups (OS: 15.6 vs. 13.4 months, P = .40, SPP: 12.2 vs. 9.3 months, P = .42). Subgroup analyses indicated that among patients without new lesions at first PD, SPP was longer in the TBP than in the OAT groups (12.6 vs. 9.3 months, P = .22, HR: 0.64; 95% CI 0.31‒1.31). The frequency of immune-related adverse events leading to discontinuation during nivolumab beyond PD was equivalent to that for pre-PD (10.7 vs. 12.6%).
Conclusions
No significant benefits were associated with continuation of nivolumab for advanced NSCLC patients. Continuation of nivolumab beyond PD could be a more useful option in patients without new lesions at first PD. Treatment-related toxicities require attention during nivolumab treatment not only before PD but also beyond PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nivolumab, an immune checkpoint inhibitor, is a fully human antibody specific to programmed cell death 1 (PD-1). It blocks the interaction between PD-1 on activated T cells and its ligands, leading to efficacy against multiple tumor types [1,2,3,4,5,6,7]. Checkmate 017/057/078 demonstrated the clinical benefits and favorable tolerability of nivolumab for previously treated advanced non-small cell lung cancer (NSCLC) patients compared with docetaxel [1, 2, 8].
The response to immunotherapy may sometimes differ from those to previous treatments, showing a slower response and pseudo-progression [9]. Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 indicated the possibility that patients may benefit from continuing immunotherapy following progressive disease (PD). Although response evaluation criteria for immunotherapy, such as immune-related response criteria (irRC), immune-related RECIST (irRECIST), and modified immune-related RECIST (iRECIST), may help evaluate the response to immunotherapy more appropriately, these are complicated and not likely to be widely used in clinical practice [10,11,12,13].
In addition, with regard to anti-cancer treatments other than immunotherapy, continuation of EGFR-TKI beyond PD, rather than its discontinuation, may prolong the overall survival (OS) of patients with EGFR activating mutations [14, 15].
Some studies have suggested that continuation of immunotherapy beyond PD may be beneficial [16,17,18,19,20]. Based on these studies, patients with advanced NSCLC may benefit from continuing nivolumab beyond PD. However, only a few studies have examined whether nivolumab beyond PD is effective and safe for patients with advanced NSCLC. Therefore, the objective of the current study was to estimate the efficacy and safety of nivolumab beyond PD in patients with advanced NSCLC, as well as the clinical features of patients who benefited from nivolumab beyond PD.
Patients and methods
Design
This cross-sectional single-center retrospective study was approved by the institutional review board of the National Hospital Organization (NHO) Kinki-Chuo Chest Medical Center (approval number 2019–014). We used an opt-out method to allow patients and families to refuse to participate in the study.
Patients
The medical records of 296 consecutive patients with advanced NSCLC who received nivolumab in our institution between 17 December 2015 and 31 December 2018 were retrospectively reviewed. Of the 296 patients, those who received nivolumab as a second-line treatment were included. Patients who had been lost to follow-up or nivolumab treatment or had not been diagnosed with PD using RECIST v1.1 by 28 February 2019 were excluded. The medical records of patients who were eligible on 28 February 2019 were included in the analysis on 26 July 2019. These patients were classified into three groups based on the following treatment after RECIST PD: continuing nivolumab treatment beyond PD [TBP], switching to other anti-cancer therapy [OAT], and receiving no further anti-cancer therapy [NAT]. RECIST PD was estimated via radiological examinations performed in clinical settings. We defined patients receiving nivolumab beyond PD as those receiving the last dose of nivolumab ≥ 2 weeks following first PD using RECIST v1.1.
Outcomes
We evaluated baseline characteristics [age, sex, ECOG performance status, smoking history, histology, EGFR mutation, presence of brain metastasis, bone metastasis, pleural effusion, overall response rate (ORR), disease control rate (DCR), and progression free survival (PFS)], OS, duration of nivolumab beyond PD, and survival post progression (SPP). Further, we evaluated new lesions at first PD, c-reactive protein (CRP), albumin (Alb), neutrophil-to-lymphocyte ratio (NLR), advanced lung cancer inflammation index (ALI) at baseline, and first progression using RECIST v1.1 during nivolumab treatment.
Safety was assessed in all patients who were eligible. Adverse events were graded for severity using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
In addition, we conducted subgroup analyses of SPP in patients presenting with and without new lesions at first PD.
Statistical analysis
The characteristics of patients in TBP and OAT groups were compared using the Mann–Whitney U test or Fisher’s exact test.
PFS was defined as the period from the date of initiation of nivolumab treatment to the date of first progression. OS was defined as the period from the date of initiation of nivolumab treatment to the date of death due to any cause. Duration of nivolumab beyond PD was defined as the period from the date of first progression to the date of clinical PD. SPP was defined as the period from the date of first progression to the date of death due to any cause. Data for patients not reported as deceased at the time of analysis were censored at the date they were last known to be alive. PFS, OS, duration of nivolumab beyond PD, and SPP were estimated using the Kaplan–Meier method, and the log-rank test was used to assess differences between TBP and OAT groups, as well as differences between TBP and the groups not continuing nivolumab treatment beyond PD (NTBP, i.e. OAT and NAT groups). Univariable Cox proportional hazard regression models were adopted to determine hazard ratios. All statistical analyses were performed using EZR software, version 1.38. Statistical significance was set at P < 0.05.
Results
Patient characteristics at baseline and at the time of PD
The patient selection process is shown in Fig. 1. Of the 296 consecutive patients with advanced NSCLC who received nivolumab in our institution between 17 December 2015 and 31 December 2018, 144 who received nivolumab monotherapy as a second-line treatment were identified. Five patients lost to follow-up were excluded and 44 patients who were found to have not been diagnosed with PD using RECIST v1.1 by 28 February 2019 during nivolumab treatment were also excluded. Ninety-five patients were eligible on 28 February 2019 and the medical records of these patients were included in the analysis on 26 July 2019. Post PD, 28 patients (29%) continued nivolumab, 46 patients (48%) switched to OAT, and 21 patients (22%) received NAT (Fig. 1).
Patient characteristics are shown in Table 1. The median ages of TBP, OAT, and NAT groups were 73 (range: 45‒85), 71 (range: 55‒84), and 68 (range: 51‒84), respectively. ORRs in the TBP, OAT, and NAT groups were 25% [95% confidence interval (CI) 10.7‒44.9], 2.2% (95% CI 0.1‒11.5), and 0.0% (95% CI 0.0‒13.3), respectively. DCRs in the TBP, OAT, and NAT groups were 64.3% (95% CI 44.1‒81.4), 58.7% (95% CI 43.2‒73.0), and 47.6% (95% CI 25.7‒70.2), respectively. PFSs in the TBP, OAT, and NAT groups were 3.4 months (95% CI 1.7‒4.9), 2.2 months (95% CI 1.8‒3.0), and 2.9 months (95% CI 0.9‒4.3), respectively.
Although all baseline characteristics were relatively comparable between TBP and OAT groups, there were significant differences with respect to ORR (P = 0.026), NLR at first PD using RECIST v1.1 (P = 0.011), and ALI at first PD using RECIST v1.1 (P = 0.019).
Efficacy of nivolumab beyond progressive disease
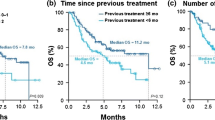
The median duration of nivolumab beyond PD was 3.8 months (95% CI 2.8‒6.6). Following the first PD, no patients in the TBP group experienced a decrease in target lesions (> 30%) compared to that of baseline. At the time of the final analysis, two patients (7%) in the TBP group were still receiving nivolumab, while 26 (93%) had discontinued nivolumab. OS in the TBP, OAT, NAT, and NTBP groups was 15.6 (95% CI 10.8–25.7), 13.4 (95% CI 8.3–20.4), 4.5 (95% CI 1.7–6.2), and 8.3 months (95% CI 6.4–11.0), respectively (Fig. 2a; Online Resource 1). There was no significant difference between the TBP group and the OAT group (P = 0.40), although the difference between the TBP group and the NTBP group was significant (P = 0.026). SPPs in the TBP, OAT, NAT, and NTBP groups were 12.2 (95% CI 5.8–26.6), 9.3 (95% CI 6.4–13.8), 0.7 (95% CI 0.4–1.7), and 5.3 months (95% CI 2.7–6.9), respectively (Fig. 2b; Online Resource 2). There was no significant difference between the TBP group and the OAT group (P = 0.42), although the difference between the TBP group and the NTBP group was significant (P = 0.024).
a Kaplan–Meier curves for overall survival in TBP, OAT, and NAT groups. b Kaplan–Meier curves for survival post progression in TBP, OAT, and NAT groups. TBP continuing nivolumab treatment beyond progressive disease, OAT switching to other anti-cancer treatment, NAT receiving no further anti-cancer treatment
Univariate analyses indicated that, for the TBP and OAT groups, a long OS was significantly associated with no smoking history, CRP at first progression < 1.0, ALI at first progression ≥ 18, and best response PR or SD. Continuation of nivolumab at first PD was not associated with a long OS (HR = 0.78; 95% CI 0.44‒1.39; P = 0.40) (Table 2). A long SPP was significantly associated with no smoking history, non-squamous cell lung cancer, baseline CRP < 1.0, first PD CRP < 1.0, and first PD ALI ≥ 18. Continuation of nivolumab at first PD was not associated with a long SPP (HR = 0.79; 95% CI 0.45‒1.40; P = 0.42) (Table 3).
Subgroup analyses of patients with and without new lesions at first PD were conducted. In the subgroup analysis of patients without new lesions at first PD (n = 57), SPP in the TBP group (n = 19) tended to be longer than that in the OAT group (n = 38) (12.6 vs. 9.3 months, P = 0.22, HR = 0.64; 95% CI 0.31‒1.31) (Fig. 3a). On the other hand, subgroup analyses of patients with new lesions at first PD (n = 17) indicated that median SPPs in the TBP (n = 9) and OAT groups (n = 8) were 10.7 and 9.3 months, respectively (P = 0.78; HR = 1.18; 95% CI 0.38‒3.61) (Fig. 3b).
a Kaplan–Meier curves for survival post progression in patients without new lesions at first progressive disease in TBP and OAT groups. b Kaplan–Meier curves for survival post progression in patients with new lesions at first progressive disease in TBP and OAT groups. TBP continuing nivolumab treatment beyond progressive disease, OAT switching to other anti-cancer treatment, N/A not available
Treatment exposure and response durations of patients in the TBP group are shown in Online Resource 3.
Safety
During nivolumab pre-PD, Grade 2‒4 treatment-related toxicities leading to temporary or permanent discontinuation of nivolumab occurred in 1 (3.6%), 8 (17.4%), and 3 (14.3%) patients in the TBP, OAT, and NAT groups, respectively, and in 12 (12.6%) of all patients. One patient in the TBP group stopped receiving nivolumab due to Grade 3 liver damage. One patient temporarily stopped receiving nivolumab due to tuberculosis, although it remains unclear whether it was related to the treatment or not.
During nivolumab treatment beyond PD, Grade 2‒4 treatment-related toxicities leading to discontinuation of nivolumab occurred in 3 patients (10.7%). One patient died due to acute liver involvement and interstitial lung disease, one stopped receiving nivolumab due to Grade 3 diarrhea, and another stopped receiving nivolumab due to Grade 2 interstitial lung disease.
Of the 27 patients in the TBP group who discontinued nivolumab treatment, 16 (59.3%) switched to OAT, while 11 (40.7%) did not receive any anti-cancer therapy.
Discussion
This retrospective study indicated that there was no significant difference in OS between TBP and OAT groups (OS: 15.6 vs. 13.4 months; P = 0.40), while the difference between TBP and NTBP groups was significant (15.6 vs. 8.3 months; P = 0.026). Furthermore, the difference in SPP between TBP and OAT groups (12.2 vs. 9.3 months; P = 0.42) was not significant, while the difference between TBP and NTBP groups was (12.2 vs. 5.3 months; P = 0.024). Subgroup analyses indicated that, in patients without new lesions at first PD, SPP tended to be longer in the TBP group than in the OAT group (12.6 vs. 9.3 months; P = 0.22; HR: 0.64; 95% CI 0.31‒1.31). The frequency of immune-related adverse events leading to discontinuation during nivolumab beyond PD was equivalent to that of pre-PD (10.7 vs. 12.6%).
The current study indicated the absence of a significant difference in OS and SPP between TBP and OAT groups, although median OS and SPP in the TBP group were a little longer than those in the OAT group. Recent studies have suggested that continuing immunotherapy beyond PD may be beneficial [16,17,18,19,20]. It has been suggested that nivolumab treatment beyond PD may be associated with improved survival in patients with melanoma and renal cell carcinoma [16,17,18]. Following a phase III OAK study, Gandara et al. suggested that atezolizumab, which is an immune checkpoint inhibitor like nivolumab, is effective beyond PD. Furthermore, Ricciuti et al. conducted a retrospective study and reported that OS of patients with NSCLC continuing nivolumab beyond PD was significantly longer than that of patients switching to a different regimen or receiving no further therapy (P < 0.0001) [20]. The less favorable results of nivolumab treatment beyond PD observed in our study, as compared with those of previous studies, may be due to the difference in patient characteristics. While we defined nivolumab beyond PD as nivolumab ≥ 2 weeks after first PD using RECIST v1.1, Ricciuti et al. defined it as nivolumab ≥ 6 weeks after first PD using RECIST v1.1. This may have led to the more favorable results observed in patients receiving nivolumab beyond PD in the previous study.
According to the univariate analyses of TBP and OAT groups, continuation of nivolumab at first PD was not associated with long OS (HR = 0.78; 95% CI 0.44‒1.39; P = 0.40) or SPP (HR = 0.79; 95% CI 0.45‒1.40; P = 0.42), while long OSs and SPPs were significantly associated with CRP at first PD (< 1.0) and ALI at first PD (≥ 18). Thus, the favorable results observed in patients continuing nivolumab beyond PD by previous studies [16,17,18,19,20] may not have been due to continuing nivolumab beyond PD, but to good clinical characteristics. The results of our study challenge the previously reported efficacy of nivolumab beyond PD.
On the other hand, subgroup analyses showed that SPP in patients without new lesions at first PD in the TBP group tended to be longer than that in the OAT group (12.6 vs. 9.3 months; P = 0.22; HR = 0.64; 95% CI 0.31‒1.31). Thus, continuation of nivolumab beyond PD may be a more useful option for patients without new lesions at first PD. Furthermore, recent studies have reported that local consolidative therapy may improve survival in advanced NSCLC patients with oligometastasis [21,22,23,24]. Therefore, nivolumab treatment beyond PD may be more effective in combination with local consolidative therapy.
With regard to the safety of nivolumab beyond PD, the current study indicated that the frequency of Grade 2‒4 treatment-related toxicities leading to discontinuation of nivolumab was not higher for nivolumab beyond PD (10.7%) than for nivolumab pre-PD (12.6%). Large clinical studies have shown that 7‒10% of patients with NSCLC who were treated with nivolumab exhibited Grade 3 or 4 toxicity events [1, 2]. The frequency of Grade 2–4 treatment-related toxicities leading to discontinuation of nivolumab during nivolumab beyond PD observed in this study was not considered to be higher than that observed in previous studies. Furthermore, Ricciuti, et al. reported that there was no significant difference in the rate of Grade 3 or 4 adverse events between patients who continued nivolumab beyond PD and those who did not (11.6% vs. 8.6%) [20]. Therefore, nivolumab treatment beyond PD may be relatively well tolerated by patients with advanced NSCLC.
However, the present study indicated that treatment-related toxicities occurred not only during nivolumab pre-PD but also beyond PD. In addition, one patient died due to immune-related adverse events. The patient had a medical history of usual interstitial pneumonia. He was considered to have PD due to lung metastasis following 10 cycles of nivolumab. Thereafter, he suffered acute liver involvement and ILD exacerbation during the 42nd cycle of nivolumab beyond PD, suggesting that patients with ILD should avoid continuation of nivolumab beyond PD. Attention also needs to be paid to treatment-related toxicities during nivolumab beyond PD.
This study was beset with certain limitations. Firstly, it was conducted at a single center using a relatively small sample size. Secondly, this study was retrospective and involved significantly different patient characteristics at first PD. However, it is difficult to conduct a prospective study wherein patient characteristics at first PD are well balanced. Thirdly, response evaluation criteria for immunotherapy, such as irRC, iRECIST, and irRECIST, were not used. However, these are complicated and not likely to be widely used in clinical practice. Thus, the use of simpler radiological findings to estimate whether continuing nivolumab is beneficial to patients may be warranted.
In conclusion, there were no significant benefits associated with continuation of nivolumab for advanced NSCLC patients. Continuation of nivolumab beyond PD may be especially useful for patients without new lesions at first PD. Attention must be paid to treatment-related toxicities during nivolumab beyond PD.
Data availability
The authors state that they have full control of all primary data and that they agree to allow the journal to review their data if requested.
Change history
22 February 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12094-022-02794-y
References
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2016;373:123–35. https://doi.org/10.1056/NEJMoa1504627.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. https://doi.org/10.1056/NEJMoa1507643.
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. https://doi.org/10.1056/NEJMoa1412082.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. https://doi.org/10.1056/NEJMoa1510665.
Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67. https://doi.org/10.1056/NEJMoa1602252.
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. New Engl J Med. 2015;372:311–9. https://doi.org/10.1056/NEJMoa1411087.
Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, Armand P, Fanale M, Ratanatharathorn V, Kuruvilla J, Cohen JB. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283–94. https://doi.org/10.1016/s1470-2045(16)30167-x.
Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, Zhang L, Tu HY, Wu L, Feng J, Zhang Y. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol. 2019;14:867–75. https://doi.org/10.1016/j.jtho.2019.01.006.
Saiki M, Yoshizawa T, Dotsu Y, Ariyasu R, Koyama J, Sonoda T, Uchibori K, Nishikawa S, Kitazono S, Yanagitani N, Horiike A. Correlation between serum adenosine deaminase activity and efficacy of anti-programmed cell death-1 antibody. Lung Cancer. 2019;133:4–9. https://doi.org/10.1016/j.lungcan.2019.04.022.
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, Hodi FS. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e152152. https://doi.org/10.1016/s1470-2045(17)30074-8.
Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. https://doi.org/10.1158/1078-0432.Ccr-09-1624.
Ribas A, Chmielowski B, Glaspy JA. Do we need a different set of response assessment criteria for tumor immunotherapy? Clin Cancer Res. 2009;15:7116–8. https://doi.org/10.1158/1078-0432.Ccr-09-2376.
Bohnsack O, Hoos A, Ludajic K. Adaptation of the immune related response criteria: irrecist. Ann Oncol. 2014. https://doi.org/10.1093/annonc/mdu342.23(25:iv369-iv369).
Nishie K, Kawaguchi T, Tamiya A, Mimori T, Takeuchi N, Matsuda Y, Omachi N, Asami K, Okishio K, Atagi S, Okuma T. Epidermal growth factor receptor tyrosine kinase inhibitors beyond progressive disease: a retrospective analysis for Japanese patients with activating EGFR mutations. J Thorac Oncol. 2012;7:1722–7. https://doi.org/10.1097/JTO.0b013e31826913f7.
Le X, Puri S, Negrao MV, Nilsson MB, Robichaux J, Boyle T, Hicks JK, Lovinger KL, Roarty E, Rinsurongkawong W, Tang M. Landscape of EGFR-dependent and -independent resistance mechanisms to osimertinib and continuation therapy beyond progression in EGFR-mutant NSCLC. Clin Cancer Res. 2018;24:6195–203. https://doi.org/10.1158/1078-0432.Ccr-18-1542.
Long GV, Weber JS, Larkin J, Atkinson V, Grob JJ, Schadendorf D, Dummer R, Robert C, Márquez-Rodas I, McNeil C, Schmidt H. Nivolumab for patients with advanced melanoma treated beyond progression: Analysis of 2 Phase 3 clinical trials. JAMA Oncol. 2017;3:1511–9. https://doi.org/10.1001/jamaoncol.2017.1588.
Escudier B, Motzer RJ, Sharma P, Wagstaff J, Plimack ER, Hammers HJ, Donskov F, Gurney H, Sosman JA, Zalewski PG, Harmenberg U. Treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in CheckMate 025. Eur Urol. 2017;72:368–76. https://doi.org/10.1016/j.eururo.2017.03.037.
George S, Motzer RJ, Hammers HJ, Redman BG, Kuzel TM, Tykodi SS, Plimack ER, Jiang J, Waxman IM, Rini BI. Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma treated beyond progression: a subgroup analysis of a randomized clinical trial. JAMA Oncol. 2016;2:1179–86. https://doi.org/10.1001/jamaoncol.2016.0775.
Gandara DR, von Pawel J, Mazieres J, Sullivan R, Helland Å, Han JY, Aix SP, Rittmeyer A, Barlesi F, Kubo T, Park K. Atezolizumab treatment beyond progression in advanced NSCLC: results from the randomized, phase III OAK study. J Thorac Oncol. 2018;13:1906–18. https://doi.org/10.1016/j.jtho.2018.08.2027.
Ricciuti B, Genova C, Bassanelli M, De Giglio A, Brambilla M, Metro G, Baglivo S, Dal Bello MG, Ceribelli A, Grossi F, Chiari R. Safety and efficacy of nivolumab in patients with advanced non-small-cell lung cancer treated beyond progression. Clin Lung Cancer. 2019;20(178–85):e172. https://doi.org/10.1016/j.cllc.2019.02.001.
Gomez DR, Tang C, Zhang J, Blumenschein GR Jr, Hernandez M, Lee JJ, Ye R, Palma DA, Louie AV, Camidge DR, Doebele RC. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37:1558–655. https://doi.org/10.1200/jco.19.00201.
Hu F, Li C, Xu J, Guo J, Shen Y, Nie W, Zheng X, Wang L, Zhang H, Han B, Zhang X. Additional local consolidative therapy has survival benefit over EGFR tyrosine kinase inhibitors alone in bone oligometastatic lung adenocarcinoma patients. Lung Cancer. 2019;135:138–44. https://doi.org/10.1016/j.lungcan.2019.07.024.
Xu Q, Zhou F, Liu H, Jiang T, Li X, Xu Y, Zhou C. Consolidative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first-line EGFR-TKIs. J Thorac Oncol. 2018;13:1383–92. https://doi.org/10.1016/j.jtho.2018.05.019.
Petty WJ, Urbanic JJ, Ahmed T, Hughes R, Levine B, Rusthoven K, Papagikos M, Ruiz JR, Lally BE, Chan M, Clark H. Long-term outcomes of a phase 2 trial of chemotherapy with consolidative radiation therapy for oligometastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2018;102:527–35. https://doi.org/10.1016/j.ijrobp.2018.06.400.
Acknowledgements
We thank all patients who were involved in our research.
Funding
None.
Author information
Authors and Affiliations
Contributions
TE and AT contributed to the study conception and design. Data collection was performed by TE, AT, YA, KA, SK, and YT. Analysis was performed by all authors. The first draft of the manuscript was written by TE. AT revised the paper critically and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Akihiro Tamiya reports grants and personal fees from AstraZeneca, Ono Pharmaceutical, and Bristol-Myers Squibb and personal fees from Chugai Pharmaceutical, Eli Lilly, MSD, Taiho Pharmaceutical, Kissei, Boehringer Ingelheim, and Pfizer, all outside the submitted work. Yoshihiko Taniguchi reports grants from Ono Pharmaceutical and Bristol-Myers Squibb, all outside the submitted work. Shinji Atagi reports grants and personal fees from Chugai Pharmaceutical, MSD, Ono Pharmaceutical, AstraZeneca, Taiho Pharmaceutical, Boehringer Ingelheim, Eli Lilly, Pfizer, and BMS and personal fees from Hisamitsu and Kyowa Hakko Kirin and grants from F. Hoffmann La Roche AG, all outside the submitted work. The remaining authors have no conflict of interest.
Ethics approval
This study was approved by the institutional review board of the National Hospital Organization (NHO) Kinki-Chuo Chest Medical Center (approval number 2019-014).
Consent to participate
We used an opt-out method, so that patients and families could refuse to participate in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: to expand the given names of all the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12094_2020_2452_MOESM1_ESM.tif
Supplementary file1 (TIF 144 kb) Online Resource 1 Kaplan-Meier curves for overall survival in TBP and NTBP groups. TBP: continuing nivolumab treatment beyond progressive disease, NTBP: not continuing nivolumab treatment beyond progressive disease
12094_2020_2452_MOESM2_ESM.tif
Supplementary file2 (TIF 147 kb) Online Resource 2 Kaplan-Meier curves for survival post progression in TBP and NTBP groups. TBP: continuing nivolumab treatment beyond progressive disease, NTBP: not continuing nivolumab treatment beyond progressive disease
12094_2020_2452_MOESM3_ESM.tif
Supplementary file3 (TIF 189 kb) Online Resource 3 Treatment exposure and response durations in patients continuing nivolumab beyond progressive disease
Rights and permissions
About this article
Cite this article
Enomoto, T., Tamiya, A., Matsumoto, K. et al. Nivolumab treatment beyond progressive disease in advanced non-small cell lung cancer. Clin Transl Oncol 23, 582–590 (2021). https://doi.org/10.1007/s12094-020-02452-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02452-1