Abstract
Background
The associations between red and processed meat consumption and esophageal cancer risk remain inconclusive. We performed a systematic review and meta-analysis to analyze these associations.
Methods
We searched PubMed and EMBASE to identify studies published between the databases’ dates of inception and May 2019.
Results
We ultimately selected 33 eligible studies for analysis. We found that the summary relative risks for the associations between meat consumption and esophageal cancer risk were positive for the case–control studies (P < 0.05), but negative for the cohort studies included in the analysis (P > 0.05). Subtype analysis indicated that red and processed meat consumption was not associated with the risks of esophageal adenocarcinoma (P > 0.05) and esophageal squamous cell carcinoma (P > 0.05) in the cohort studies.
Conclusions
We found case–control but not cohort studies to associate consumption of red and processed meat with the risk of esophageal cancer. Further large prospective studies are needed to validate these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer (EC) is one of the most fatal malignancies. GLOBOCAN 2018 has reported 572,034 new cases and 508,585 deaths of EC occurring worldwide [1]. Eastern Asia is one of the regions with a high rate of EC related mortality [2]. Given the increasing incidence of EC and the high mortality rate associated with the disease, novel strategies for preventing EC are urgently needed. An increasing number of studies have recently focused on the dietary factors associated with the risk of EC. For example, several studies reported that drinking beverages at high temperatures and low fruit and vegetable are risk factors for EC [3,4,5]. Additionally, fish consumption has been reported to be associated with a decreased the risk of EC [6]. The consumption of red and processed meat also has been shown to be associated with many chronic diseases [7,8,9]. However, the associations between red and processed meat consumption and the risk of EC remain unclear. Some studies have shown that meat consumption is positively associated with EC [10, 11], while other studies have found no evidence of an association between the two phenomena [12, 13].
Thus, given the large burden imposed by EC worldwide and the controversial evidence regarding the risk factors that may be associated with the disease, we conducted a systematic review and meta-analysis with the following objectives: (1) to provide an update regarding the relationship between red and processed meat consumption and the risk of EC using a larger body of evidence and the results of a quantitative analysis of the eligible data pertaining to the relationship that were published up to May 2019; and (2) to evaluate the dose–response associations between red and processed meat consumption and EC risk.
Methods
Selection criteria
The selection criteria were as follows: (1) studies including patients whose diseases were diagnosed by endoscopy with biopsy; (2) studies including patients whose histological features were not consistent with those normally identified by the gold standard diagnostic test, i.e., endoscopy with biopsy; (3) narrative reviews, systematic reviews and meta-analyses; letters; commentaries; case reports; editorials; and studies in which only the abstract was obtained, were excluded from the analysis; (4) studies regarding the consumption of meats that did not specifically cite red or processed meat were excluded from the analysis, as were studies including patients with Barrett’s esophagus, gastrointestinal stromal tumors, precancerous lesions and other digestive tract tumors; (5) we limited the language of the studies included in the analysis to English and included only studies involving humans in the analysis.
Search strategy
We searched PubMed and EMBASE for studies on the relationship between red and processed meat consumption and EC risk published between the databases’ dates of inception and May 2019. The search terms included: “meat/meats”, “beef”, “pork”, “lamb”, “mutton”, “bacon”, “ham”, “sausage”, “hot dogs”, “diet/dietary”, “lifestyle/lifestyles” and “food/foods” in combination with “gastrointestinal/aerodigestive/digestive/alimentary/esophageal/oesophageal/esophagus/oesophagus”. The reference lists of the included studies were also searched manually to identify additional relevant literature. The two sets of keywords were combined individually, and the eligibility of each study was judged independently by two authors (Zhanwei Zhao and Fei Wang).
Study quality
Study quality was assessed using the Newcastle–Ottawa Scale (NOS) [14]. The NOS assesses study quality based on three factors: the ability of case–control or cohort studies to ascertain the exposure or outcomes of interest, respectively; the selection of the study populations and the comparability of the populations. Two researchers (Zhanwei Zhao and Fei Wang) independently assessed the quality of the studies, and disagreements regarding quality were resolved by reaching a consensus with the assistance of a third researcher (Chaojun Zhang). The NOS ranges from 0 to 9 stars, and studies receiving scores of seven or more stars are considered high-quality studies.
Data extraction
A data extraction sheet was generated for each study and included information pertaining to the first author, year of publication, country, study type, study population, study period, method of dietary assessment, dietary exposures measured, dietary exposure categories, adjusted relative risks (RRs) and 95% confidence intervals (CIs) (highest to lowest), adjusted variables and NOS score.
Statistical analysis
The data were collected and analyzed using SPSS 17.0 (Chicago, IL, USA). RevMan5.3 (The Cochrane Collaboration, Oxford, UK) and STATA version 12.1 (STATA Corporation, College Station, TX, USA) software were used for the data synthesis and analysis.
Random-effects models were used to pool the summary relative risks/odds ratios (RRs/ORs) and 95% CIs. The median or mean level of meat intake for each category was assigned to each corresponding RR for each study. When the corresponding data were not reported, the midpoint of the upper and lower boundaries of each category was designated the average intake value. When the highest category was open-ended, we assumed the open-ended interval to be the same as the adjacent interval. If the lowest category was open-ended, we assumed the lowest boundary to be 0.
Heterogeneity between studies was detected using Q (a P < 0.1 represented statistically significant heterogeneity) and I2 statistics (I2 < 50% was indicative of low heterogeneity, and I2 > 50% was indicative of substantial heterogeneity) [15]. Subgroup analyses, in which the studies were assessed according to their geographic areas, sample sizes, publication years, quality scores, questionnaires and adjustments (smoking, alcohol, BMI, energy intake, physical activity and dietary fiber intake), were conducted to identify the sources of the heterogeneity between studies. Meta-regression analyses were conducted to determine if geographic area, sample size, publication year and quality score were significant sources of between-study heterogeneity (P < 0.1 was indicative of a significant source of heterogeneity).
Publication bias was assessed using funnel plots, Begg’s test and Egger’s test (P < 0.1 was indicative of significant publication bias) [16]. Sensitivity analyses were conducted to investigate the influence of a specific study on the pooled risk estimate by removing one study in each turn.
Results
Literature selection, study characteristics and quality scores
Thirty-three studies met the criteria for inclusion in the analysis and provided 56 separate estimates (red meat = 28, processed meat = 24) of the associations between red and processed meat consumption and EC risk (Fig. 1). The analysis included 1,156,150 participants and 11,449 cases. The quality scores ranged from 5 to 9 (Table 1).
Red meat
High vs low consumption
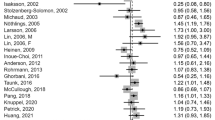
Twenty-two case–control studies with 28 estimates were included in the analysis and the pooled RR for the relationship between red meat consumption and EC was 1.44 (1.20–1.72). Three cohort studies with six estimates were included in the analysis, and the pooled RR for the relationship between red meat consumption and EC was 1.10 (0.80–1.53) (Fig. 2a). Subtype analyses of case–control studies demonstrated that (Fig. 2b) red meat consumption was associated with esophageal squamous cell carcinoma (ESCC) (RR = 1.74, 95% CI = 1.12–2.69), while analysis of cohort studies yielded negative results (RR = 1.43, 95% CI = 0.48–4.23). Subtype analysis of case–control studies yielded positive results (RR = 1.66, 95% CI = 1.22–2.46), while analysis of cohort studies yielded negative results (RR = 0.87, 95% CI = 0.60–1.28) regarding the relationship between red meat consumption and esophageal adenocarcinoma (EAC) (Fig. 2c).
Heterogeneity
We noted high heterogeneity (P < 0.01, I2= 68%) between the case–control studies, but did not observe significant heterogeneity (P = 0.10, I2= 46%) between the cohort studies.
Publication bias
As only three cohort studies were included in the analysis, tests for publication or small study bias were not conducted. Sensitivity analysis of the included cohort studies showed that the changes in the recalculated RRs were not significant, as the RRs ranged from 1.46 (0.57–3.73) when Yu 1993 (12.8%) was excluded from the analysis to 1.28 (0.99–1.65) when Keszei 2012 (3.3%) was excluded from the analysis.
Dose–response analysis
Two cohort studies were included in the analysis, and the pooled RR for a 100 g/day increase in red meat consumption was 1.16 (0.81–1.67) and was without heterogeneity (P = 0.57, I2= 0%). These results demonstrated that red meat consumption was non-significantly positively associated with the risk of EC. Non-linear dose–response analysis was not conducted because of the small number of studies included in the analysis.
Processed meat
High vs low consumption
Nineteen case–control studies were included in the analysis and the pooled RR for the relationship between processed meat consumption and EC was 1.50 (1.22–1.85). Three cohort studies with six estimates were included in the analysis, and the pooled RR for the relationship between processed meat consumption and EC was 1.21 (0.78–1.88) (Fig. 3a). Subtype analysis of case–control studies indicated that processed meat consumption was positively associated with ESCC (RR = 1.54, 95% CI = 1.09–2.16) and that significant between-study heterogeneity was present (P < 0.01, I2= 67%); however, analysis of two large cohort studies indicated that processed meat consumption was negatively associated with ESCC (RR = 1.34, 95% CI = 0.61–2.91) (Fig. 3b). Subtype analyses of the three cohort studies (RR = 1.15, 95% CI = 0.81–1.63) and four case–control studies (RR = 1.138 95% CI = 0.96–1.97) demonstrated that processed meat consumption was not associated with EAC (Fig. 3c).
Heterogeneity
Significant heterogeneity (P < 0.01, I2= 55%) was present between the case–control studies and (P = 0.04, I2= 69%) between the cohort studies.
Publication bias
Due to the inclusion of only three cohort studies in the analysis, tests for publication or small study bias were not conducted. Sensitivity analysis of the included cohort studies showed that the changes in recalculated RRs were not significant, as the RRs ranged from 1.11 (0.89-1.37) when Jakszyn 2013 (6.0%) was excluded from the analysis to 1.54 (0.79-3.01) when Keszei 2012 (7.1%) was excluded from the analysis.
Dose–response analysis
Three cohort studies were included in the analysis, and the pooled RR for a 50 g/day increase in processed meat consumption was 1.41 (1.10-1.82). No between-study heterogeneity (P = 0.42, I2= 0%) was present. However, sensitivity analysis demonstrated significant changes in the recalculated RRs, which ranged from 1.22 (0.86–1.71) when Jakszyn 2013 (45.1%) was excluded from the analysis to 1.47 (1.10–1.96) when Keszei 2012 (13.7%) was excluded from the analysis. Non-linear dose–response analysis was not conducted because of the small number of studies included in the analysis.
Discussion
Three previous systematic reviews evaluated the associations between red and processed meat consumption and esophageal cancer risk, namely, the studies by Salehi et al. [49], Qu et al. [50] and Choi et al. [51]. However, some issues were not adequately addressed by these analyses, which reported different results. First, because case–control studies may provide information regarding exposures that was obtained after patient cancer diagnose, the results of the studies may be affected by inaccurate dietary intake measurements and recall bias. Cohort studies are less prone to bias than case–control studies. Thus, performing separate estimates according to study design is important and necessary for evaluating the associations between meat consumption and esophageal cancer risk. Second, two subtypes of esophageal cancer exist, namely, esophageal squamous cell carcinoma and esophageal adenocarcinoma. Thus, performing separate estimates according to subtype is also important and necessary for evaluating the associations between meat consumption and esophageal cancer risk. As mentioned above, there were some differences between two reports with respect to their results. Salehi et al. [49] found that red meat consumption was associated with a significantly increased risk of esophageal squamous cell carcinoma but not esophageal adenocarcinoma and that processed meat consumption was associated with a significantly increased risk of esophageal adenocarcinoma but not esophageal squamous cell carcinoma. In contrast, Qu et al. [50] found that red and processed meat consumption was associated with a significantly increased risk of esophageal squamous cell carcinoma; however, that study did not obtain data regarding esophageal adenocarcinoma. Choi et al. [51] did not provide the detailed data on subtype of EC. Third, Salehi et al. [49] identified relevant studies published up to 2011, Qu et al. [50] identified relevant studies published up to 2012 and Choi et al. [51] identified relevant studies published up to 2012. Many high-quality studies regarding the relationship between red and processed meat consumption and EC risk have appeared during the recent years and an updated meta-analysis of the literature may clarify the impact of these recent studies on the understanding of the relationship between red and processed meat consumption and EC. Thus, to address the above questions, we conducted an updated systematic review and meta-analysis.
Our analysis yielded detailed evidence indicating that increased consumption of red and processed meat increased the risk of EC in the case–control studies; however, we noted no associations between meat consumption and EC risk in the cohort studies. Similarly, subtype analyses of EC showed that red or processed meat consumption was negatively associated with the risk of EAC and ESCC in the cohort studies. Taken together, our detailed findings have clarified the associations between consumption of red and processed meat and the risk of EC have thus provided us with valuable information with which updated dietary recommendations can be updated.
Several potential mechanisms may underlie the effects of red and processed meat consumption on the risk of EC. First, the positive associations observed in the case–control studies may be biologically plausible. Cooking red meat is one of the major sources of carcinogens such as polycyclic aromatic hydrocarbons, heterocyclic amines, nitrate and N-nitroso compounds, which are believed to play an important role in the development of EC [52]. Second, the high iron intake associated with red and processed meat consumption may also play a role in the development of EC by causing oxidative damage and facilitating the endogenous formation of carcinogenic N-nitroso compounds [13, 53]. Finally, bacteriological and virological studies have identified mechanisms that explain the associations between red and processed meat consumption and the risk of EC to a degree. Helicobacter pylori (H. pylori) may be a protective factor of EC [54] and human papillomavirus (HPV) infection may be associated with an increased risk of EC [55]. However, the results of many cohort studies and meta-analyses do not support these hypotheses. For example, a European prospective investigation regarding cancer and nutrition suggested that no association exists between increased unprocessed red meat consumption and the risk of EAC [48]. Although some prospective studies showed that red meat consumption is positively associated with gastrointestinal cancer, their definitions of red meat also included processed red meat, which may have influenced to the above associations [46, 56, 57]. Additionally, Barrett’s esophagus is considered to be the strongest risk factor and only known precursor for EAC [58]. However, the results of our previous study did not support the idea that positive associations exist between high red and processed meat consumption and the risk of Barrett’s esophagus [59]. Thus, additional studies are needed to verify the existence these associations.
Study strengths and limitations
Our study had several strengths. For example, we performed separate analyses according to study design and EC subtype, which provided us with more detailed data and increased the power of the meta-analysis, thereby strengthening its conclusions. Our analysis was based on a significantly large sample and a quantitative analysis of eligible data, which provided us with sufficient reliable, robust and current evidence regarding the relationship between red and processed meat consumption and the risk of EC and increased the statistical power of the analysis. We broadly and systematically searched multiple databases for all investigations of the relationship between red and processed meat consumption and the risk of EC that were published from the databases’ dates of inception to May 2019 and identified all the major published studies regarding this phenomenon. Study selection and data extraction were performed independently and in duplicate by two investigators, which increased the validity of the results. Furthermore, we conducted dose–response analyses to assess these associations rather than merely performing categorical comparisons.
However, several limitations of the present meta-analysis must be taken into consideration.
First, the studies included in the analysis were observational, and residual confounding and unmeasured factors could not be excluded from the study. In particular, most of the studies included in the analysis lacked information regarding H. pylori infection and gastroesophageal reflux. Only two studies [12, 37] examined the role of H. pylori infection in EC development. Thus, the results of the analysis should be interpreted with caution due to the presence of possible confounders, and future analyses should consider studies regarding H. pylori infection and gastroesophageal reflux.
Second, our analyses showed that significant heterogeneity was present among the studies and that this heterogeneity may have been related to the publication year, cases numbers, geographic region, exposure measurement methods, study quality score, meat consumption classifications, and other confounders. Heterogeneity was observed mainly in the analysis comparing the highest and the lowest levels of meat consumption and may be at least partially attributable to differences in the categories of meat consumption among the included studies. We used random-effects models to account for between-study heterogeneity. The ranges from the lowest to the highest categories varied, and the levels of red and processed meat consumption between the lowest and highest categories differed among the included studies, which may have resulted in bias and influenced the accuracy of the results.
Conclusions
The results of the meta-analysis indicate that the case–control studies but not the cohort studies associated the consumption of red and processed meat with the risk of EC. Additional large prospective studies are needed to validate these findings.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. https://doi.org/10.3322/caac.21262.
Islami F, Pourshams A, Nasrollahzadeh D, Kamangar F, Fahimi S, Shakeri R, et al. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study. BMJ. 2009;338:b929.
Liu J, Wang J, Leng Y, Lv C. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer. 2013;133(2):473–85. https://doi.org/10.1002/ijc.28024.
Li B, Jiang G, Zhang G, Xue Q, Zhang H, Wang C, et al. Intake of vegetables and fruit and risk of esophageal adenocarcinoma: a meta-analysis of observational studies. Eur J Nutr. 2014;53(7):1511–21. https://doi.org/10.1007/s00394-014-0656-5.
Jiang G, Li B, Liao X, Zhong C. Poultry and fish intake and risk of esophageal cancer: a meta-analysis of observational studies. Asia Pac J Clin Oncol. 2016;12(1):e82–91. https://doi.org/10.1111/ajco.12114.
Kaluza J, Harris H, Linden A, Wolk A. Long-term unprocessed and processed red meat consumption and risk of chronic obstructive pulmonary disease: a prospective cohort study of women. Eur J Nutr. 2018. https://doi.org/10.1007/s00394-018-1658-5.
Horikawa C, Kamada C, Tanaka S, Tanaka S, Araki A, Ito H, et al. Meat intake and incidence of cardiovascular disease in Japanese patients with type 2 diabetes: analysis of the Japan Diabetes Complications Study (JDCS). Eur J Nutr. 2017. https://doi.org/10.1007/s00394-017-1592-y.
de Batlle J, Gracia-Lavedan E, Romaguera D, Mendez M, Castano-Vinyals G, Martin V, et al. Meat intake, cooking methods and doneness and risk of colorectal tumours in the Spanish multicase-control study (MCC-Spain). Eur J Nutr. 2018;57(2):643–53. https://doi.org/10.1007/s00394-016-1350-6.
Lin S, Wang X, Huang C, Liu X, Zhao J, Yu IT, et al. Consumption of salted meat and its interactions with alcohol drinking and tobacco smoking on esophageal squamous-cell carcinoma. Int J Cancer. 2015;137(3):582–9. https://doi.org/10.1002/ijc.29406.
Zhu HC, Yang X, Xu LP, Zhao LJ, Tao GZ, Zhang C, et al. Meat consumption is associated with esophageal cancer risk in a meat- and cancer-histological-type dependent manner. Dig Dis Sci. 2014;59(3):664–73. https://doi.org/10.1007/s10620-013-2928-y.
O’Doherty MG, Cantwell MM, Murray LJ, Anderson LA, Abnet CC. Dietary fat and meat intakes and risk of reflux esophagitis, Barrett’s esophagus and esophageal adenocarcinoma. Int J Cancer. 2011;129(6):1493–502. https://doi.org/10.1002/ijc.26108.
Ward MH, Cross AJ, Abnet CC, Sinha R, Markin RS, Weisenburger DD. Heme iron from meat and risk of adenocarcinoma of the esophagus and stomach. Eur J Cancer Prev. 2012;21(2):134–8. https://doi.org/10.1097/CEJ.0b013e32834c9b6c.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. https://doi.org/10.1007/s10654-010-9491-z.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557.
Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Yu MC, Garabrant DH, Peters JM, Mack TM. Tobacco, alcohol, diet, occupation, and carcinoma of the esophagus. Cancer Res. 1988;48(13):3843–8.
Rogers MA, Thomas DB, Davis S, Vaughan TL, Nevissi AE. A case-control study of element levels and cancer of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 1993;2(4):305–12.
Tavani A, Negri E, Franceschi S, La Vecchia C. Risk factors for esophageal cancer in lifelong nonsmokers. Cancer Epidemiol Biomarkers Prev. 1994;3(5):387–92.
Castelletto R, Castellsague X, Munoz N, Iscovich J, Chopita N, Jmelnitsky A. Alcohol, tobacco, diet, mate drinking, and esophageal cancer in Argentina. Cancer Epidemiol Biomarkers Prev. 1994;3(7):557–64.
Rolon PA, Castellsague X, Benz M, Munoz N. Hot and cold mate drinking and esophageal cancer in Paraguay. Cancer Epidemiol Biomarkers Prev. 1995;4(6):595–605.
Bosetti C, La Vecchia C, Talamini R, Simonato L, Zambon P, Negri E, et al. Food groups and risk of squamous cell esophageal cancer in northern Italy. Int J Cancer. 2000;87(2):289–94.
Takezaki T, Shinoda M, Hatooka S, Hasegawa Y, Nakamura S, Hirose K, et al. Subsite-specific risk factors for hypopharyngeal and esophageal cancer (Japan). Cancer Causes Control. 2000;11(7):597–608.
Takezaki T, Gao CM, Wu JZ, Ding JH, Liu YT, Zhang Y, et al. Dietary protective and risk factors for esophageal and stomach cancers in a low-epidemic area for stomach cancer in Jiangsu Province, China: comparison with those in a high-epidemic area. Jpn J Cancer Res. 2001;92(11):1157–65.
Chen H, Ward MH, Graubard BI, Heineman EF, Markin RM, Potischman NA, et al. Dietary patterns and adenocarcinoma of the esophagus and distal stomach. Am J Clin Nutr. 2002;75(1):137–44.
Li K, Yu P. Food groups and risk of esophageal cancer in Chaoshan region of China: a high-risk area of esophageal cancer. Cancer Invest. 2003;21(2):237–40.
Levi F, Pasche C, Lucchini F, Bosetti C, La Vecchia C. Processed meat and the risk of selected digestive tract and laryngeal neoplasms in Switzerland. Ann Oncol. 2004;15(2):346–9.
Hung HC, Huang MC, Lee JM, Wu DC, Hsu HK, Wu MT. Association between diet and esophageal cancer in Taiwan. J Gastroenterol Hepatol. 2004;19(6):632–7. https://doi.org/10.1111/j.1440-1746.2004.03346.x.
Yang CX, Wang HY, Wang ZM, Du HZ, Tao DM, Mu XY, et al. Risk factors for esophageal cancer: a case-control study in South-western China. Asian Pac J Cancer Prev. 2005;6(1):48–53.
Wu AH, Tseng CC, Hankin J, Bernstein L. Fiber intake and risk of adenocarcinomas of the esophagus and stomach. Cancer Causes Control. 2007;18(7):713–22. https://doi.org/10.1007/s10552-007-9014-8.
Wang JM, Xu B, Rao JY, Shen HB, Xue HC, Jiang QW. Diet habits, alcohol drinking, tobacco smoking, green tea drinking, and the risk of esophageal squamous cell carcinoma in the Chinese population. Eur J Gastroenterol Hepatol. 2007;19(2):171–6. https://doi.org/10.1097/MEG.0b013e32800ff77a.
Sapkota A, Hsu CC, Zaridze D, Shangina O, Szeszenia-Dabrowska N, Mates D, et al. Dietary risk factors for squamous cell carcinoma of the upper aerodigestive tract in central and eastern Europe. Cancer Causes Control. 2008;19(10):1161–70. https://doi.org/10.1007/s10552-008-9183-0.
Chen YK, Lee CH, Wu IC, Liu JS, Wu DC, Lee JM, et al. Food intake and the occurrence of squamous cell carcinoma in different sections of the esophagus in Taiwanese men. Nutrition. 2009;25(7–8):753–61. https://doi.org/10.1016/j.nut.2009.02.002.
Aune D, De Stefani E, Ronco A, Boffetta P, Deneo-Pellegrini H, Acosta G, et al. Meat consumption and cancer risk: a case-control study in Uruguay. Asian Pac J Cancer Prev. 2009;10(3):429–36.
Wu M, Zhang ZF, Kampman E, Zhou JY, Han RQ, Yang J, et al. Does family history of cancer modify the effects of lifestyle risk factors on esophageal cancer? A population-based case-control study in China. Int J Cancer. 2011;128(9):2147–57. https://doi.org/10.1002/ijc.25532.
Gao Y, Hu N, Han XY, Ding T, Giffen C, Goldstein AM, et al. Risk factors for esophageal and gastric cancers in Shanxi Province, China: a case-control study. Cancer Epidemiol. 2011;35(6):e91–9. https://doi.org/10.1016/j.canep.2011.06.006.
Hajizadeh B, Jessri M, Moasheri SM, Rad AH, Rashidkhani B. Fruits and vegetables consumption and esophageal squamous cell carcinoma: a case-control study. Nutr Cancer. 2011;63(5):707–13. https://doi.org/10.1080/01635581.2011.563028.
O’Doherty MG, Cantwell MM, Murray LJ, Anderson LA, Abnet CC. Dietary fat and meat intakes and risk of reflux esophagitis, Barrett’s esophagus and esophageal adenocarcinoma. Int J Cancer. 2011;129(6):1493–502. https://doi.org/10.1002/ijc.26108.
De Stefani E, Deneo-Pellegrini H, Ronco AL, Boffetta P, Correa P, Aune D, et al. Meat consumption, cooking methods, mutagens, and risk of squamous cell carcinoma of the esophagus: a case-control study in Uruguay. Nutr Cancer. 2012;64(2):294–9. https://doi.org/10.1080/01635581.2012.648299.
Song Q, Wang X, Yu IT, Huang C, Zhou X, Li J, et al. Processed food consumption and risk of esophageal squamous cell carcinoma: a case-control study in a high risk area. Cancer Sci. 2012;103(11):2007–11. https://doi.org/10.1111/j.1349-7006.2012.02387.x.
Di Maso M, Talamini R, Bosetti C, Montella M, Zucchetto A, Libra M, et al. Red meat and cancer risk in a network of case-control studies focusing on cooking practices. Ann Oncol. 2013;24(12):3107–12. https://doi.org/10.1093/annonc/mdt392.
De Stefani E, Boffetta P, Ronco AL, Deneo-Pellegrini H, Correa P, Acosta G, et al. Processed meat consumption and squamous cell carcinoma of the oesophagus in a large case-control study in Uruguay. Asian Pac J Cancer Prev. 2014;15(14):5829–33.
Golozar A, Etemadi A, Kamangar F, Fazeltabar MA, Islami F, Nasrollahzadeh D, et al. Food preparation methods, drinking water source, and esophageal squamous cell carcinoma in the high-risk area of Golestan, Northeast Iran. Eur J Cancer Prev. 2016;25(2):123–9. https://doi.org/10.1097/CEJ.0000000000000156.
Rosato V, Kawakita D, Negri E, Serraino D, Garavello W, Montella M, et al. Processed meat and risk of selected digestive tract and laryngeal cancers. Eur J Clin Nutr. 2019;73(1):141–9. https://doi.org/10.1038/s41430-018-0153-7.
Yu Y, Taylor PR, Li JY, Dawsey SM, Wang GQ, Guo WD, et al. Retrospective cohort study of risk-factors for esophageal cancer in Linxian, People’s Republic of China. Cancer Causes Control. 1993;4(3):195–202.
Cross AJ, Freedman ND, Ren J, Ward MH, Hollenbeck AR, Schatzkin A, et al. Meat consumption and risk of esophageal and gastric cancer in a large prospective study. Am J Gastroenterol. 2011;106(3):432–42. https://doi.org/10.1038/ajg.2010.415.
Keszei AP, Schouten LJ, Goldbohm RA, van den Brandt PA. Red and processed meat consumption and the risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Ann Oncol. 2012;23(9):2319–26. https://doi.org/10.1093/annonc/mdr615.
Jakszyn P, Lujan-Barroso L, Agudo A, Bueno-de-Mesquita HB, Molina E, Sanchez MJ, et al. Meat and heme iron intake and esophageal adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition study. Int J Cancer. 2013;133(11):2744–50. https://doi.org/10.1002/ijc.28291.
Salehi M, Moradi-Lakeh M, Salehi MH, Nojomi M, Kolahdooz F. Meat, fish, and esophageal cancer risk: a systematic review and dose-response meta-analysis. Nutr Rev. 2013;71(5):257–67. https://doi.org/10.1111/nure.12028.
Qu X, Ben Q, Jiang Y. Consumption of red and processed meat and risk for esophageal squamous cell carcinoma based on a meta-analysis. Ann Epidemiol. 2013;23(12):762–70. https://doi.org/10.1016/j.annepidem.2013.09.003.
Choi Y, Song S, Song Y, Lee JE. Consumption of red and processed meat and esophageal cancer risk: meta-analysis. World J Gastroenterol. 2013;19(7):1020–9. https://doi.org/10.3748/wjg.v19.i7.1020.
Samraj AN, Pearce OM, Laubli H, Crittenden AN, Bergfeld AK, Banda K, et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci USA. 2015;112(2):542–7. https://doi.org/10.1073/pnas.1417508112.
Bastide NM, Chenni F, Audebert M, Santarelli RL, Tache S, Naud N, et al. A central role for heme iron in colon carcinogenesis associated with red meat intake. Cancer Res. 2015;75(5):870–9. https://doi.org/10.1158/0008-5472.CAN-14-2554.
Xie FJ, Zhang YP, Zheng QQ, Jin HC, Wang FL, Chen M, et al. Helicobacter pylori infection and esophageal cancer risk: an updated meta-analysis. World J Gastroenterol. 2013;19(36):6098–107. https://doi.org/10.3748/wjg.v19.i36.6098.
Petrick JL, Wyss AB, Butler AM, Cummings C, Sun X, Poole C, et al. Prevalence of human papillomavirus among oesophageal squamous cell carcinoma cases: systematic review and meta-analysis. Br J Cancer. 2014;110(9):2369–77. https://doi.org/10.1038/bjc.2014.96.
Flood A, Velie EM, Sinha R, Chaterjee N, Lacey JJ, Schairer C, et al. Meat, fat, and their subtypes as risk factors for colorectal cancer in a prospective cohort of women. Am J Epidemiol. 2003;158(1):59–68.
Li WQ, Park Y, Wu JW, Ren JS, Goldstein AM, Taylor PR, et al. Index-based dietary patterns and risk of esophageal and gastric cancer in a large cohort study. Clin Gastroenterol Hepatol. 2013;11(9):1130–6. https://doi.org/10.1016/j.cgh.2013.03.023.
Jung KW, Talley NJ, Romero Y, Katzka DA, Schleck CD, Zinsmeister AR, et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett’s esophagus: a population-based study. Am J Gastroenterol. 2011;106(8):1447–55. https://doi.org/10.1038/ajg.2011.130.
Zhao Z, Pu Z, Yin Z, Yu P, Hao Y, Wang Q, et al. Dietary fruit, vegetable, fat, and red and processed meat intakes and Barrett’s esophagus risk: a systematic review and meta-analysis. Sci Rep. 2016;6:27334. https://doi.org/10.1038/srep27334.
Funding
This work was supported by the funds of the Innovation Funds of Navy General Hospital (CXPY201801) and the Bethune Charitable Foundation HZB-20181119-71.
Author information
Authors and Affiliations
Contributions
Zhanwei Zhao, and Fei Wang wrote the main manuscript text, participated in the design of the work. Di Chen participated in the analysis of the data and prepared figures. Chaojun Zhang carried out the study design, the analysis and interpretation of the data and drafted the manuscript. All authors have reviewed the manuscript. Zhanwei Zhao, Fei Wang and Di Chen contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest for each author.
Ethical statement
This work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed. Our meta-analysis did not involve human participants and animals.
Informed consent
All participants provided informed consent prior to their participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, Z., Wang, F., Chen, D. et al. Red and processed meat consumption and esophageal cancer risk: a systematic review and meta-analysis. Clin Transl Oncol 22, 532–545 (2020). https://doi.org/10.1007/s12094-019-02157-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02157-0