Abstract
Our aim was to evaluate factors associated with persistently elevated prostate-specific antigen (PSA) and biochemical recurrence following robotic-assisted radical prostatectomy (RARP). The study population (N = 5300) consisted of consecutive patients who underwent RARP for localized prostate cancer by a single surgeon (VP) from January 2008 through July 2013. A query of our Institutional Review Board-approved registry identified 162 men with persistently elevated PSA (group A), defined as PSA level ≥0.1 ng/ml at 6 weeks after surgery, who were compared with rest of the cohort group having undetectable PSA, group B (<0.1 ng/ml). A univariate and multivariate logistic regression analysis was used to evaluate the significant association between various variables and the following: (1) persistently elevated PSA, (2) BCR (PSA value ≥0.2 ng/ml) on follow-up in the persistent PSA group. On multivariate analysis, only the following parameters were significantly associated with persistent PSA after RARP—preoperative [PSA >10 ng/ml (p = 0.01), Gleason Score ≥8 (p = 0.001) and clinical stage(p = 0.001)]; postoperative [pathologic stage (p = 0.001), extraprostatic extension (EPE, p = 0.01), lymph node positivity (p = 0.001), positive surgical margin (PSM, p = 0.02), Gleason score (p = 0.01) and tumor volume percent (p < 0.001)]. The mean follow-up was 38.1 months. The BCR was significantly higher in group A as compared to group B(52.47 vs 7.9 %) respectively; p = 0.01). The mean time to BCR was significantly lesser in group A as compared to group B(8.9 vs 21.1 months respectively; p = 0.01). The BCR-free survival rates at 1 year and 3 years were significantly lower statistically in the persistent PSA group in comparison to other groups (69.7 vs 97.3 % and 48.5 vs 92.1 %, respectively; p = 0.01). On multivariate logistic regression analysis in patients with persistent PSA on follow-up, preoperative PSA >10 ng/ml, postoperative Gleason score ≥8, postoperative stage ≥pT3, positive pelvic lymph nodes, PSM >3 mm and post-RARP PSA doubling time (DT) <10 months (p < 0.001) were significantly associated with BCR. In patients after RARP, factors associated with aggressive disease (high preoperative PSA, Gleason score ≥8, stage ≥T3, PSM, high tumor volume percent and EPE) predict PSA persistence. Although these patients with persistent PSA after RARP are more likely to have BCR and that too earlier than those patients with undetectable PSA after RARP, a significant proportion of these patients (47.53 %) remain free of BCR. This subset of patients is associated with these favorable parameters (preoperative PSA <10 ng/ml, post-RARP PSA DT ≥10 months, postoperative Gleason score <8, pathologic stage <pT3, PSM <3 mm and no lymph node involvement), thus potentially not requiring any adjuvant treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the use of prostate-specific antigen (PSA) screening and increased public awareness of prostate cancer, the number of cases being diagnosed yearly has been increasing in the United States of America (USA) [1–3]. According to the American Cancer Society in 2014, approximately 233,000 cases of prostate cancer will be diagnosed [4]. Every year, younger and healthier men are being diagnosed with localized prostate cancer, with an annual percentage increase of approximately 9.5 %, as evidenced by data from the Surveillance, Epidemiology and End Results (SEER) registry [4]. Approximately, 90 % of patients are being diagnosed with localized prostate cancer [4]. Radical prostatectomy (RP) is a standard of care for localized prostate cancer, with goals of providing good oncologic and functional outcomes. However, despite various advances in surgical technology, biochemical recurrence (BCR) has been reported in approximately 25–35 % patients after RP within 10 years of surgery and even more so in intermediate–high risk prostate cancer patients (38–51 %) [5–8].
There has been an evolution from open RP through laparoscopic to robot-assisted radical prostatectomy (RARP) [9, 10]. With advances in robotic techniques and increasing experience using the robotic platform, RARP has yielded improved functional outcomes and at least comparable oncological outcomes as compared to open and laparoscopic radical prostatectomy [11, 12]. Long-term oncologic outcomes are still not available for RARP, and thus intermediate-term BCR is often taken as a surrogate marker of oncologic efficacy of RARP [13–17]. PSA has been used as an effective tool to detect the earliest evidence of disease recurrence after RARP. Postoperative PSA values of ≥0.2 ng/ml within 3 months with a second confirmatory value >0.2 ng/ml define BCR [18]. Biochemical recurrence-free survival after RARP has been reported in around 88–98 % at 1–2 years in most of the series [13–17], with only very few studies reporting 86.4 % at 5 years [17] and 81–84.5 % at 7 years [15, 16]. The mean time from BCR until progression to metastases has been reported as 5–8 years in previously reported series; therefore detection of postoperative PSA (≥0.2 ng/ml) gives a lead time, in which adjuvant treatments like radiotherapy and hormonal therapy can be started, although these regimens may alter the progression of disease [19–21].
Following radical excision of the prostate, the PSA should become undetectable within 4 weeks of surgery, as the half-life of PSA is approximately 3 days. It follows that measurement of any detectable PSA at 6 weeks after surgery has been taken as an adverse oncologic surrogate marker, in various published series [22–26]. There are many definitions of persistent PSA in these series; however, PSA ≥0.1 ng/ml has been used in numerous series [21, 23, 27]. The possible causes of persistent PSA have been reported as either undetected vascular systemic micrometastases at the time of surgery, or the presence of residual cancer (in the region of positive surgical margins, PSM), or benign prostate glandular tissue left in situ (apical region due to difficult anatomy and inadequate dissection; posterolateral region due to overzealous preservation of neurovascular bundles resulting in capsular incision at the base region, or in bladder neck preservation surgery) [22–26, 28–30]. Thus, persistent PSA has been reported as a surrogate marker of future BCR (as >50 % but not all of these patients will develop BCR) in these series on RP [21–26]. The various predictors of persistent PSA have been reported after RP ranging from preoperative PSA, PSM, pathologic stage, nodal status, seminal vesicle invasion to postoperative Gleason score [22–26]. However, still quite a significant proportion of these patients do not develop BCR. There are no published factors which can predict BCR in these subset of patients, to avoid unnecessary adjuvant treatment and their side effects in those who are unlikely to develop BCR There are no published series so far, to the best of our knowledge, addressing the predictors and oncologic outcome of persistent PSA after RARP. Therefore, we performed this study to evaluate the predictors and oncologic outcome of persistently elevated PSA after RARP. Our study includes one of the largest cohorts of persistently elevated PSA patients, performed by a single surgeon with experience of more than 8000 cases of RARP, from a high volume tertiary care referral center.
Patients and methods
Study population
From February 2008 through July 2013, 5300 consecutive patients underwent RARP for localized prostate cancer by a single surgeon at our institution, a tertiary referral center. Patients receiving prior radiation, focal therapy for prostate cancer, androgen deprivation therapy or undergoing salvage therapy for failed other localized treatment were excluded from the study. A query of our Institutional Review Board-approved registry identified 162 men with persistently elevated PSA (group A), defined as PSA level ≥0.1 ng/ml at 6 weeks after surgery, who were compared with the rest of the cohort group (N = 5138) having undetectable PSA (<0.1 ng/ml) at 6 weeks after surgery. Our patients had preoperative staging bone scans as well as CT/MRI of abdomen and pelvis whenever required.
Surgical technique
All procedures were performed using a transperitoneal, six-port technique previously described [31]. The periurethral suspension stitch, modified transverse plication for bladder neck reconstruction and modified posterior reconstruction of the rhabdosphincter were performed in all patients [32–34]. Nerve sparing was performed, if appropriate, by athermal early retrograde release of neurovascular bundles using the landmark prostate artery with minimized traction [32]. A standard pelvic lymphadenectomy was performed removing the obturator and external iliac lymph nodes. The urethral catheter was removed 4–5 days after the procedure.
Data collection and management
Perioperative data were collected retrospectively and entered into the IRB-approved Integrated Robotic Assisted Urological Surgery Outcomes Database. Data collected from the patient’s clinical record included patient clinical history, positive biopsy results, PSA level, clinical staging, Gleason score and digital rectal examination. Follow-up information was obtained including final histopathology and entered into the follow-up component of the database.
Since our institute is a tertiary referral center in which most of the patients are referred from outside, the biopsy technique and number of cores were not standardized. However, a dedicated uropathologist from our institute reviewed all the slides. Parameters including primary and secondary Gleason grade and the total number and percentage of positive cores in biopsy were recorded. Postoperative specimen pathologic analyses were performed at our institution, and specimens were processed according to the recommendations of the American Society of Clinical Pathologists. The apex and bladder neck cones were amputated and sectioned in the sagittal plane. The remaining specimen was sectioned transversely at intervals of 4 mm. Positive surgical margins (PSM) were defined as the presence of tumor tissue on the inked surface of the specimen. The PSM were recorded as apex, base, posterolateral and multifocal. Pathologic staging was performed according to the 2002 TNM system.
Follow-up data (including prostate-specific antigen) were collected at 6 weeks, 3 months, 6 months, 9 months, 12 months, 18 months and 1 year intervals to assess oncologic outcomes. Intraoperative complications were classified using the Dindo modification of the Clavien Grading System. Biochemical recurrence was defined as a prostate-specific antigen (PSA) level ≥0.2 ng/ml postoperatively, with a second confirmatory value of PSA >0.2 ng/ml. The PSA doubling time (PSADT) was calculated for each patient using the natural log of 2 (ln2 = 0.693) divided by the gradient of the plot of the log of PSA (y plot) at each sequential PSA measurement (x plot).
Statistical analysis
Demographic and clinical data are presented as frequency distribution and simple percentages. Values of continuous variables are expressed as mean ± the standard deviation and median. Univariate analysis of selected perioperative discrete variables was accomplished by the Chi-square test with the appropriate degrees of freedom or the Fisher’s exact test to assess the equality of proportions. Two-sample t tests were used to test for the equality of means in continuous variables.
Univariate and multivariate logistic regression analyses were used to identify the association between various following parameters and persistently elevated PSA, thus finding possible predictive factors for (1) persistently elevated PSA and (2) BCR on follow-up, in the persistent PSA group. For the multivariate exploration, our preoperative variables included: age, body mass index (BMI), prostate-specific antigen (PSA), clinical stage, American Urological Association (AUA) score, prostate weight, biopsy Gleason grade, and number and percentage of positive cores. Intraoperative variables included: nerve sparing (unilateral, bilateral, none), median lobe and intraoperative complications. Finally, postoperative variables included: stage, Gleason score, tumor volume %, perineural invasion (PNI), extraprostatic extension (EPE), lymphovascular invasion (LVI), seminal vesicle involvement (SVI) and PSADT (only for BCR). The BCR-free survival was calculated according to the method of Kaplan–Meier using time 0 as the date of operation and the date of biochemical recurrence of disease (BCR) as the end point. The equality of event-free distribution was tested with the log-rank algorithm in the two groups.
All p values reported are two sided and were not adjusted for multiple testing. A value of p ≤ 0.050 was considered to indicate significant differences between measurements. Quantitative and qualitative analyses were performed with Number Cruncher Statistical Systems software (version 9, NCSS, Kaysville, UT).
Results
The demographics of the overall cohort are summarized (Table 1). The majority of patients had clinical stage ≤T2 (81.6 %), a preoperative Gleason score ≤7 (82.2 %) and were of D’Amico low–intermediate risk classification (76.2 %).
The univariate logistic regression analysis for persistent PSA is provided (Table 2a, b). Regarding preoperative characteristics, the persistent PSA group had statistically significant higher recordings for BMI (p = 0.02), preoperative PSA (p = 0.01), clinical stage ≥T3 (p = 0.01) and Gleason score ≥8 (p = 0.01). The intraoperative variables including extent of nerve sparing and median lobe were comparable in the two groups (p = 0.51 and 0.67, respectively). In postoperative variables, the persistent PSA group had statistically significant higher EPE (p = 0.01), PSM (p = 0.04), LN involvement (p = 0.01), LVI (p = 0.04), tumor volume percent (p < 0.001), pathologic stage ≥T3 (p > 0.001) and Gleason score ≥8 (p < 0.001) in comparison to the undetectable PSA group. However, PSM location did not affect the persistent PSA status. Multifocal PSMs were most commonly recorded. If unifocal, the most common location was the apex, followed by the posterolateral area and then the base .
In our multivariate logistic regression analysis, the persistent PSA was significantly associated with preoperative PSA (p < 0.001), clinical stage (p < 0.001), preoperative Gleason score (p < 0.001), PSM (p < 0.001), EPE (p = 0.001), LN positivity (p < 0.001), postoperative Gleason score (p < 0.001) and tumor volume percent (p < 0.001), which are all surrogate markers of high volume aggressive disease (Table 3).
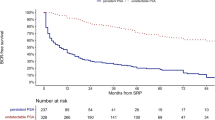
The follow-up results are summarized (Table 4). At the mean follow-up of 38.1 months, BCR was significantly higher and mean time to BCR was significantly shorter in the persistent PSA group in comparison to other groups (52.47 vs 7.9 %, p = 0.01 and 8.9 vs 21.1 months, p = 0.01, respectively). In the remaining 77 patients of the persistent PSA group, who did not have BCR on follow-up, 41 (53.25 %) patients showed stable PSA, 25 showed decrease in PSA (32.47 %) and the remaining 11 (14.28 %) showed a slowly increasing PSA, which did not reach the 0.2 threshold value we place on BCR. The BCR-free survival rates at 1 and 3 years were significantly lower statistically in the persistent PSA group versus the comparator group (69.7 vs 97.3 % and 48.5 vs 92.1 %, respectively; p = 0.01).The Kaplan–Meier curve for BCR-free survival also highlighted statistically favorable outcomes in the undetectable PSA group in comparison to the persistent PSA group (log rank p = 0.01) (Fig. 1). Adjuvant radiotherapy and hormonal therapy were also required in a significantly higher number of patients in the persistent PSA group (p = 0.02 and 0.03, respectively).
On multivariate logistic regression analysis of variables affecting BCR in the persistent PSA group, the following parameters were found to have statistically significant association with BCR: preoperative PSA >10 ng/ml (HR: 2.35; 95 % CI: 1.93–5.31; p < 0.001), PSM >3 mm (HR: 1.76, 95 % CI: 1.54–2.67; p < 0.001), PSADT <10 months (HR: 3.17, 95 % CI: 2.89–4.75; p < 0.001), lymph node involvement (HR: 2.73, 95 % CI: 1.41–4.67; p < 0.001) and postoperative Gleason score ≥8 (HR: 3.93, 95 % CI: 2.21–6.65; p < 0.001) (Table 5).
Discussion
Prostate cancer is the most common noncutaneous malignancies in men in the USA [1–3]. With advances in robotic techniques and the increase in the experience using the robotic platform, RARP has shown improved functional outcomes and at least comparable oncological outcomes as compared to open and laparoscopic radical prostatectomy [9–12]. Biochemical recurrence is an important surrogate marker of oncologic outcome of RARP. However, despite an era of prostate screening and advancements in robotic techniques, BCR has been reported in 15–20 % patients at 5–7 years RARP [13, 16, 17]. Due to the association of BCR with disease progression, adjuvant treatment, metastatic potential and cancer-specific mortality, various predictors have been published including preoperative PSA, PSM, pathologic stage, pathologic Gleason score, lower surgical volume and persistently elevated PSA at 6 weeks after surgery. These factors have been used for preoperative patients counseling and appropriate selection timing of adjuvant treatments [19–21].
After surgical resection of the prostate, the PSA should become undetectable within 4 weeks. Therefore, detectable PSA at 6 weeks after surgery has been considered as an adverse oncologic outcome [19–21]. The possible explanation of this persistent PSA is as follows. First, there could be systemic micrometastases in blood circulation, undetected at the time of surgery. These tumor cells are postulated to gain activity and are thus a potential source of PSA [22–25]. Second, there could be residual prostate tumor in the region of positive surgical margins, as a result of iatrogenic capsular incision in localized disease and tumor burden in locally advanced disease [26, 27, 35]. Third, the benign prostate glands left in situ during surgery at various locations could be a potential source of PSA [28–30]. In the apical region, inadequate dissection due to difficult anatomy and efforts in leaving a longer intrapelvic urethral stump could be possible causes. In the bladder neck area, bladder neck preservation and dissection too close to the prostate lead to capsular incision, leaving benign prostate tissue in situ. In the posterolateral region, in patients with low tumor burden with attempts of complete bilateral nerve sparing, iatrogenic capsular incision can result in the same outcome. The presence of benign prostate tissue in situ also might suggest that principles of radical surgery have been compromised, leading to more chances of disease recurrence. Paul et al. reported residual benign prostate glands in 73.6 % patients, mostly in the apical region [30]. However, it failed to affect oncologic outcome in those patients. Kohl et al. reported benign prostate glands at the surgical margins in 54 % patients, mainly in the apical region [28]. However, the presence of such tissue did not affect the BCR outcomes of these patients.
Many definitions for clinically significant persistently elevated PSA have been reported (ranging from ≥0.03 to ≥0.1 ng/ml) [19–27]. These series have reported significantly higher BCR, disease progression, need for adjuvant radiotherapy/hormonal therapy, metastases and cancer-specific mortality after RP in this subset of patients [19–27]. Audenet et al. reported persistently elevated PSA (≥0.1 ng/ml) in 34.58 % patients, in a cohort of 240 patients after RP [23]. Biochemical recurrence was found in 51.8 % patients at a median follow-up of 44 months. In a study by Rogers et al., 8.36 % patients developed persistently elevated PSA (≥0.1 ng/ml) after RP. At a mean follow-up of 5.3 years, 47 % of these patients developed distant metastases with cancer-specific mortality in 21 % patients. However, 38 % of these patients remained free of distant metastases up to a follow-up of 7 years [21]. In another study reported by Moreira et al., persistently elevated PSA (≥0.03 ng/ml) was found in 26 % of patients after RP. Following a multivariate analysis, independent predictors of persistent PSA were higher preoperative PSA, body mass index and high biopsy Gleason score. These patients were four times more likely to have BCR in comparison to the other group [22]. Similarly, Eisenberg et al. reported detectable ultrasensitive PSA (>0.05) in 13 % of patients after RP. The BCR at 5 years was significantly lower in this subset in comparison to the other group (67 vs 86 %, p < 0.01) [26]. In another study reported by Naselli et al., persistent PSA (≥0.1 ng/ml) was found in 10.3 % patients after RP. Biochemical recurrence was found in 72.7 % of these patients at a median follow-up of 6 months, needing a second treatment. On multivariate analysis, preoperative PSA, pathologic stage and nodal status were significant independent predictors of persistent PSA [25]. Importantly, to the best of our knowledge, there are no previously published series investigating the predictors of persistently elevated PSA following RARP or the oncological sequelae of this scenario.
In our study of 5300 patients of RARP, 3.07 % patients (162) had persistently elevated PSA (≥0.1 ng/ml). On univariate and multivariate logistic regression analysis, we found the following variables as predictors of the persistent PSA—preoperative PSA (p < 0.001), clinical stage (p < 0.001), preoperative Gleason score (p < 0.001), PSM (p < 0.001), EPE (p = 0.001), LN positivity (p < 0.001), postoperative Gleason score (p < 0.001) and tumor volume percent (p < 0.001). Notably, these characteristics are all surrogate markers of high volume aggressive disease, similar to previously reported series of RP. The location of PSM (s) did not affect the presence of persistent PSA status.
At a mean follow-up of 38.1 months, the BCR was significantly higher and mean time to BCR was significantly shorter in the persistent PSA group in comparison to the other group (52.47 vs 7.9 %, p = 0.01 and 8.9 vs 21.1 months, p = 0.01, respectively).The BCR-free survival rates at 1 year and 3 years were significantly lower statistically in the persistent PSA group in comparison to the other group (69.7 vs 97.3 % and 48.5 vs 92.1 %, respectively; p = 0.01). The Kaplan–Meier curve for BCR-free survival also highlighted statistically significant favorable outcomes in the undetectable PSA group in comparison to the persistent PSA group (log rank p = 0.01) (Fig. 1). Adjuvant radiotherapy and hormonal therapy were also required in a significantly higher number of patients in the persistent PSA group (p = 0.02 and 0.03, respectively). Notwithstanding, 47.53 % (73/162) of patients with persistent PSA remained free of BCR at a mean follow-up of 38.1 months. On further analysis of this subset of patients, 41 (53.25 %) showed stable PSA, while 25 showed a decrease in PSA (32.47 %) and the remaining 11 (14.28 %) showed a slow increase in PSA which did not reach our BCR threshold. We performed a multivariate logistic regression analysis of variables affecting BCR in the persistent PSA group and found that the following parameters were found to have statistically significant association with BCR—preoperative PSA >10 ng/ml (p < 0.001), PSM >3 mm (p < 0.001), PSADT <10 months (p < 0.001), lymph node involvement (p < 0.001), ≥pT3 (p < 0.001) and postoperative Gleason score ≥8 (p < 0.001). Thus, patients in the persistent PSA group with markers of less aggressive disease (preoperative PSA <10 ng/ml, PSM <3 mm, postoperative Gleason score <8, pathologic stage <T3, no pelvic lymph node involvement and PSADT ≥10 months) are less likely to develop BCR and need adjuvant treatment. However, this could also be attributed to benign prostate glands present in situ producing a slow stable PSA. Furthermore, the use of cautery at the prostate margins during RARP induces tissue ischemia, promotes the formation of postoperative granulation tissue and thus may decrease the capacity of tissue to produce PSA.
This preoperative ‘intelligence’ might be used for comprehensive patients counseling first preoperatively, highlighting which patients are more likely to develop persistent PSA after RARP. Subsequently, postoperatively also, this information can be utilized for counseling of these patients explaining what subset of patients are likely to remain free of BCR and need for adjuvant treatment (27, 36). It is hoped that this may go some way in meeting patients’ expectations and guide clinicians in judiciously utilizing secondary treatment in these patients.
Our study has some limitations. First, this is a retrospective analysis and is prone to bias associated with retrospective analyses including recall bias. However, this study is strengthened by the large patient numbers and is therefore statistically powered. Secondly, our preoperative biopsy technique was not standardized and most of our patients were referred to our tertiary care referral center. However, all pathological specimen slides were reviewed by our dedicated uropathologist. Third, in our series, all the cases were performed by a single surgeon. Therefore, these results may not be applicable to series with low volume RARP cases; however, this series offers data with homogeneity in surgical technique and practice. Fourth, our follow-up was limited to 38.1 months; therefore, long-term oncologic outcomes including cancer-specific mortality could not be evaluated. However, this is the first series of RARP addressing the issue of predictors and oncologic outcome of persistently elevated PSA. Moreover, these results need external validation by large multicenter prospective randomized controlled trials.
Conclusions
In patients after RARP, factors associated with aggressive disease (high preoperative PSA, Gleason score ≥8, stage ≥T3, PSM, high tumor volume percent and EPE) predict PSA persistence. Patients with persistent PSA after RARP are more likely to have BCR and that too earlier than those patients with undetectable PSA after RARP. However, there are a significant proportion of these patients (47.53 %) who remain free of BCR. This subset of patients is associated with favorable parameters (preoperative PSA <10 ng/ml, post RARP PSA DT ≥10 months, postoperative Gleason Score <8, pathologic stage <pT3, PSM <3 mm and no lymph node involvement). This subset might potentially avoid adjuvant treatment.
References
Jani AB, Johnstone PA, Liauw SL, Master VA, Brawley OW (2008) Age and grade trends in prostate cancer (1974–2003): a surveillance, epidemiology, and end results registry analysis. Am J Clin Oncol 31:375–378
Crawford ED (2003) Epidemiology of prostate cancer. Urology 62:3–12
Minino AM, Smith BL (2001) Deaths: preliminary data for 2000. Natl Vital Stat Rep 49:1–40
Edwards BK, Noone AM, Mariotto AB et al (2014) Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 120(9):1290–1314
Dorin RP, Daneshmand S, Lassoff MA et al (2012) Long term outcomes of open radical retropubic prostatectomy for clinically localized prostate cancer in the prostate specific antigen era. Urology 79:626–631
D’Amico AV, Whittington R (1998) Malkowicz SBet al.Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280:969–974
Han M, Partin AW, Pound CR et al (2001) Long term biochemical disease free and cancer specific survival following anatomic radical retropubic prostatectomy. The 15 years Johns Hopkins experience. Urol Clin North Am 28:555–565
Heidenreich A, Bastian PJ, Bellmunt J et al (2014) EAU guidelines on prostate cancer.Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 65:124–137
Ficarra V, Cavalleri S, Novara G, Aragona M, Artibani W (2007) Evidence from robot-assisted laparoscopic radical prostatectomy: a systematic review. Eur Urol 51:45–56
Ficarra V, Borghesi M, Suardi N et al (2013) Long-term evaluation of survival, continence and potency (SCP) outcomes after robot-assisted radical prostatectomy (RARP). BJU Int 112(3):338–345
Ficarra V, Novara G, Artibani W, Cestari A, Galfano A, Graefen M, Guazzoni G, Guillonneau B, Menon M, Montorsi F, Patel V, Rassweiler J, Van Poppel H (2009) Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol 55:1037–1063
Coelho RF, Rocco B, Patel MB (2010) Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a critical review of outcomes reported by high-volume centers. J Endourol 24(12):2003–2015
Sooriakumaran P, Haendler L, Nyberg T et al (2012) Biochemical recurrence after robot assisted radical prostatectomy in a European single centre cohort with a minimum follow up time of 5 years. Eur Urol 62:768–774
Patel VR, Sivaraman A, Coelho RF et al (2011) Pentafecta: a new concept for reporting outcomes of robot-assisted laparoscopic radical prostatectomy. Eur Urol 59(5):702–707
Patel VR, Coelho RF, Chauhan S et al (2010) Continence, potency and oncological outcomes after robotic-assisted radical prostatectomy: early trifecta results of a high-volume surgeon. BJU Int 106(5):696–702
Suardi N, Ficarra V, Willemesen P et al (2011) Long term biochemical recurrence rates after robot assisted radical prostatectomy: analysis of a single center series of patients with a minimum follow up of 5 years. Urology 79:133–138
Menon M, Bhandari M, Gupta N et al (2010) Biochemical recurrence following robot assisted radical prostatectomy: analysis of 1384 patients with a minimum 5 year follow up. Eur Urol 58:838–846
Cookson MS, Gunnar Aus, Burnett AL et al (2007) Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association prostate guidelines for localized prostate cancer update report and recommendations for a standard in the reporting of surgical outcomes. J Urol 177:540–545
Moreira DM, Presti JC, Aronson WJ et al (2009) Natural history of persistently elevated prostate specific antigen after radical prostatectomy: results from the SEARCH database. J Urol 182:2250–2256
Pound CR, Partin AW, Eisenberger MA et al (1999) Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281(17):1591–1597
Rogers CG, Khan MA, Miller MC et al (2004) Natural history of disease progression in patients who fail to achieve an undetectable prostate specific antigen level after undergoing radical prostatectomy. Cancer 101(11):2549–2556
Moreira DM, Presti JC, Aronson WJ et al (2009) Definition and preoperative predictors of persistently elevated prosate specific antigen after radical prostatectomy: results from the SEARCH database. BJUI 105:1541–1547
Audenet F, Seringe E, Drouin SJ et al (2012) Persistently elevated prostate specific antigen at six weeks after radical prostatectomy helps in early identification of patients who are likely to recur. World J Urol 30:239–244
D’Amico AV, Chen MH, Roehl KA et al (2005) Identifying patients at risk for significant versus clinically insignificant postoperative prostate specific antigen failure. J Clin Oncol 23:4975–4979
Naselli A, Introini C, Andreatta R et al (2009) Prognostic factors of persistently detectable PSA after radical prostatectomy.International. J Urol 16:82–86
Eisenberg ML, Davies BJ, Cooperberg MR et al (2010) Prognostic implications of an undetectable ultrasensitive prostate specific antigen level after radical prostatectomy. Eur Urol 57:622–630
Song DY, Thompson TL, Ramakrishnan V et al (2002) Salvage radiotherapy for rising or persistent PSA after radical prostatectomy. Urology 62(2):281–287
Kohl SK, Balaji KC, Smith LM et al (2007) Clinical significance of benign glands at surgical margins in robotic radical prostatectomy specimens. Urology 69(6):1113–1116
Shah R, Bassily N, Wei J et al (2000) Benign prostatic glands at surgical margins of radical prosatatectomy specimens: frequency and associated risk factors. Urology 56(5):721–725
Paul R, Hoppmann M, Randenborgh HV et al (2004) Residual benign prostatic glands at the urethrovesical anastomosis after radical retropubic prostatectomy: prediction and impact on disease outcome. Eur Urol 46:321–326
Patel VR, Tully AS, Holmes R et al (2005) Robotic radical prostatectomy in the community setting—the learning curve and beyond: initial 200 cases. J Urol 174:269–272
Ko YH, Coelho RF, Sivaraman A et al (2013) Retrograde versus antegrade nerve sparing during robot-assisted radical prostatectomy: which is better for achieving early functional recovery? Eur Urol 63(1):169–177
Patel VR, Coelho RF, Palmer KJ et al (2009) Periurethral suspension stitch during robot assisted laparoscopic radical prostatectomy: description of the technique and continence outcomes. Eur Urol 56:472–478
Coelho RF, Chauhan S, Orvieto MA et al (2011) Influence of modified posterior reconstruction of the rhabdosphincter on early recovery of continence and anastomotic leakage rates after robot assisted radical prostatectomy. Eur Urol 59:72–80
Yossepowitch O, Briganti A, Eastham JA et al (2014) Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol 65:303–313
Matsumoto K, Mizuno R, Tanaka N et al (2014) Optimal timing of hormonal therapy for prostate specific antigen recurrence after radical prostatectomy. Med Oncol 31:45
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest arising from this work.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 [5]. Informed consent was obtained from all patients for being included in the study.
Rights and permissions
About this article
Cite this article
Kumar, A., Samavedi, S., Mouraviev, V. et al. Predictive factors and oncological outcomes of persistently elevated prostate-specific antigen in patients following robot-assisted radical prostatectomy. J Robotic Surg 11, 37–45 (2017). https://doi.org/10.1007/s11701-016-0606-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-016-0606-8