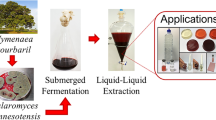
Abstract
Natural pigments are becoming increasingly popular owing of their reliability. Microbial pigments provide an alternative to natural colours. A total of 24 fungal cultures were collected from leaf bits of Senna auriculata, with one strain (FNG1) producing an extracellular red orange pigment. Nigrospora oryzae was confirmed by using physical criteria and molecular phylogenetic study by using ITS and β- tubulin analysis. In EtOAc, the crude red pigment was the most soluble. The TLC analysis was used to partly purify the natural pigment. The partially purified fungal pigment was used in successive bioprospecting studies. The antimicrobial activity of the partially purified sample was assessed against eight human pathogens, with Leucobacter AA7 showing the largest zone of inhibition (200–500 µg/mL). The compound's DPPH scavenging activity enhanced from 38.2 to 67.9%, with an IC50 value of 34.195 ± 2.33 µg/mL. Cancer cells were suppressed by partly pure fungal pigment, but non-cancerous HEK 293 cells were unaffected. The GC–MS analysis was used to characterize the molecule present in the partly purified pigment. In addition, the cotton textiles have the greatest staining capability for crude mycobial pigment, which dyes quickly and has a negative cytotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural pigments are colours that are sourced from nature and are primarily generated by plants, animals, and microorganisms [1]. The pervasive use of colour in many facets of human life, such as clothes, food, and furniture, has made life more "colourful." The use of pigments as colouring agents may be traced back to prehistoric periods, as evidenced by a large quantity of archaeological data [2]. The ability to see colours serves a multitude of functions in living creatures, including camouflaging defence systems, aposematic warning signals that promote sexual attraction, and our food choice tendencies. Science, art, and philosophy are just few of the fields that study colour because of its significance, attraction, and profound effect on human instincts and inclinations [3, 4]. Natural colours, generally termed pigments, are the end products of metabolic processes in living organisms [5] stated that carotenoids, anthocyanins, and a lot more are types of such metabolites. Identical colours to those found in nature, such as beta carotene and canthaxanthin, are synthetically produced. On the other hand, inorganic colours are also produced and include titanium dioxide, gold, silver, and other similar elements. Natural pigments have been accumulating for a long time, and as people became even more aware of the adverse effects of synthetic colourants, they were increasingly concerned about their continuing growth. Natural pigments such as carotenoids, chlorophylls, quinones, flavonoids (anthocyanins), and phycobiliproteins are used to produce a broad spectrum of colours. Microorganisms are the most promising of these because of the relative simplicity of culture and extraction, as well as the genetic variety they contain [6].
In contrast to most other microbes, fungi have the unique ability to produce powerful colours that may be utilised as a dye or to colour food [7]. Since they produce chemicals with excellent light and chemical durability, a diverse colour range, a high yield, and a reliable supply, fungi have attracted the curiosity of researchers as a potential natural source of pigments [8, 9]. Fungi are responsible for the synthesis of a multitude of colors, many of which have biological properties and some of which are cytotoxic. Fungi are responsible for the production of a diverse array of pigments, many of which exhibit many biological features, some of which are cytotoxic [10]. Even in the most hostile settings, the existence of fungi makes it feasible for those fungi to synthesise new secondary metabolites since fungi are such a varied and prolific group of eukaryotic creatures on the planet. Fungal life is pervasive and may be found in both terrestrial and aquatic environments [11]. Aspergillus, Penicillium, Fusarium, and Trichoderma are just a few of the fungal species which may create a spectrum of colors as intermediary metabolites [12]. Carotenoids, polyketides, polyketide-derivates/azaphilones and melanins are all examples of fungal pigments [13]. It is currently obvious that a single species of fungus is capable of producing a variety of distinct pigments, each of which has a unique set of biological characteristics. Fungi's ability to produce these colours serves a vital function in the organism [5]. To withstand extreme conditions, fungus produce melanin, which also protects them from UV rays [14]. Monascus is responsible for the creation of six unique pigments, each of which is produced from polyketides and serves to distinguish the colours yellow, orange, and red yellow pigments include monascin and ankaflavin. The two typical orange Monascus pigments, monascorubrin and rubropunctatin, are essential precursors in the synthesis of food colourants [15]. Fungi are next to plants in the number of different pigments that they contain, with over one hundred having been identified thus far. One of the most essential qualities that can be used to identify different kinds of fungus is the variety of colours that they could take on. Its own pigments protect it from harmful ultraviolet (UV) radiation and may also provide resilience to infection from microorganisms. The pigments found in fungus are quite distinct from those found in higher plants, which do not include chlorophyll or the anthocyanins that are responsible for the myriad of colours seen in flowers [5].
When it comes to stimulating economic growth and providing new career opportunities, the textile sector is unrivalled. It is of tremendous importance to the textile industry because fungal pigments have high colourfastness and staining qualities; they merit manufacture under controlled circumstances, have no seasonal changes, and are biodegradable [16]. The dyeing industries have the responsibility of minimising the negative impact they have on the surrounding ecosystem. The textile industry has high hopes for fungal dyes because of their many potential benefits; yet, they have fallen short owing to their inconsistent fixation. Moreover, the commercial dyeing of fungal pigments is not standardised since there are no recognised methods. Hence, various innovative fungal pigments and a reliable method for standardising the industrial dyeing of fungal dyes are required for use in standardised industrial applications. This should be done as soon as possible. It has been suggested that stable and consistent fungal pigments might replace synthetic dyes in the textile industry. This is due to the pigments' potential usage as replacements in the textile industry. Certain pigments have the potential to absorb ultraviolet light, and when used as textile dyes, they may protect human skin from the harmful effects of UV radiation [17]. The objective of the research focuses on the exploration of the pigment that is produced by the fungal strain that has been utilised for textile dyeing, along with the characterization of the pigment after it has been purified and assessments for its cytotoxicity.
Materials & Methods
Isolation of Pigment Producing Endophytic Fungi
The mature, disease-free leaves of Senna auriculata were gathered from the Nagamalai hills in the Madurai region of TamilNadu, where they were analysed for endophytic fungi. The area of investigation lies in 9°54'N latitude and 78° 00' E longitude and at an elevation of 150 m. The hill is 1500 ft at its tallest point and stretches for over four kilometres. The leaves were cleaned for 5 min under running tap water before being sliced into small leaf fragments (5 mm2). Surface sterilisation was carried out using 70% ethanol for 30 s, 90 s in 4% sodium hypochlorite, followed by three washes in sterile distilled water. Fifty leaf segments were plated on agar containing potato dextrose (PDA) supplemented with 50 μgmL-1 streptomycin. The plates were incubated at 25 °C (± 2˚ C) with a 12:12 light and dark cycle to monitor fungal growth [18]. Pure cultures were raised from the emerging hyphal tips and used for further studies. Suitable control plates were maintained throughout the study to validate the surface sterilization process.
Identification of Pigment-Producing Endophytic Fungi Using Morphological and Molecular Identification
A light microscope was used to analyse the morphology of an isolated pigment-producing endophytic fungus strain grown on PDA medium. Extraction of genomic DNA was performed for molecular identification according to the procedure outlined by Cenis [19]. The fungal strain was identified by amplifying the internal spacer region spanning the ITS1-5.8S-ITS2 segment with universal ITS primers (ITS1-forward primer (5' TCCGTAGGTGAACCTGCGG 3') and ITS4-reverse primer (5' TCCTCCGCTTATTGATATGC 3'). Polymerase chain reaction (PCR) was carried out in a 25 L reaction including 1 μL of DNA template, 1.25 μL of Forward and Reverse Primers, and 12.5 μL of 2 × DNA master mix (Amplicon). PCR amplification was carried out in a Himedia’s Prima-96™ Thermocycler instrument with the following reaction conditions: Denaturation at 94º Celsius for 4 min, 3 °C ycles for thirty seconds at 94º Celsius for denaturation, annealing at 58.2º Celsius for 1 min, extension at 72º Celsius for 2 min, and extension at 72º Celsius for 7 min [20]. The amplicons were confirmed on 1% agarose gels, purified and sanger sequencing was carried out by Bio kart India Pvt. Ltd., Bangalore, India. The amplified sequence was analysed using NCBI and BLAST, and a phylogenetic tree was constructed using MEGA X.
ITS2 Secondary Structure Prediction of Endophytic Fungi and Phylogenetic Analysis
The ITS2 region of the fungal isolate was extracted from the ITS2 Database (http://its2.bioapps.biozentrum.uni-wuerzburg.de/) [21]. The ITS2 sequence and the secondary structure for all the sequences were exported to 4SALE V1.7.1 for generating synchronous alignment of sequence and secondary structures. Compensatory Base Change (CBC) were analysed and the genera specific consensus secondary structure were modelled. The phylogenetic tree was reconstructed using Phangorn package implemented in the R framework as previously reported. The phylogenetic tree was rebuilt by using the Phangorn tool that was functioning within the R environment [22]. The tree was built using the maximum Likelihood (ML) technique using a General Time Reversible (GTR + I + G) substitution model. The 1000 repetitions were used to calculate the branch support values in the phylogenetic trees [18].
Extraction of Fungal Pigment
Endophytic fungal isolates were cultured in 200 ml of a broth made from potato dextrose (PDB) for pigment synthesis. About, 5 mm diameter Agar plugs containing mycelia (7 days old) was used as the inoculums and the flasks were inoculated and incubated for 21 days at 25 °C (± 2 ˚C) with 12:12 h light and dark cycle. After incubating for 21 days, the fungal culture containing the mycelia was filtered using filter paper. From the filtrate of the fungal culture, the extracellular components were extracted using ethyl acetate (1:2 V/V) [23]. After collecting the organic phase, the solvent was removed from the mixture by passing it through a rotary vacuum evaporator at low. For further bioprospecting research, the remaining dry material was used.
Characterization of Crude Mycobial Pigment
Solubility Test
Tests for solubility was carried out through a modified form of the procedure outlined by Ahmad et al. [24]. Crude fungal pigment was dissolved in the concentration of 1 mg/ml with following solvents Acetone, Benzene, Chloroform, Ethanol, Ethyl acetate and Methanol. The mixer was vortex for 5 min at the room temperature. The λmax for each solution was measured from (300–600 nm) by UV—8453 Agilent spectrophotometer where the λmax of the red orange pigment was recorded at (450–550 nm).
Purification of Mycobial Pigment
TLC Separation and Sample Preparation
The fungal crude extract was combined with ethyl acetate before being loaded onto thin-layer silica gel plates (Silica gel F254, Merck, Germany) for solvent-based separation (chloroform: methanol—9:1 ratio). The bioactive components were subsequently extracted by drying the running lane. Both 254 nm and 366 nm UV light systems were used to observe the chromatogram in the TLC chamber. Based on the spot resolution, the optimal mobile phase was selected. The silica residue was centrifuged to remove the remaining particles, and the resulting supernatant was collected. Biological activity was assessed in the silica-free supernatant. Rf was determined by dividing solute distance by solvent distance.
Assessment of Antibacterial Assay
The anti—bacterial activity was evaluated using the agar well diffusion technique, according to Perez [25]. The fungal crude extract and partially purified fungal pigment was determined against eight distinct human pathogen bacterial strains: Escherichia coli MTCC 1687, Bacillus cereus ATCC14579, Klebsiella aerogenes MTCC 4031, Salmonella typhi A MTCC 733, and Staphylococcus aureus MTCC 7443, Enterobacter cloacae GS1, Leucobacter AA7. The bacterial cultures (8- hour old) were swabbed (sterile cotton swabs) on the Muller Hinton Agar (MHA) plates. The plates were perforated six times with a sterile corkborer of six-millimeter diameter. Each well had 50 µl of partly purified fungal extract (200–500 µg/mL) applied to it and left to diffuse at ambient temperature. The antibiotic streptomycin used as a standard for bacterial species while water was used as control. The plates had been incubated for twenty-four hours at 37 °C. The triplicate plate was kept.
Assessment of Antioxidant Activity
The antioxidant capacity of the partially purified extracts of pigment-producing fungus was evaluated using the DPPH and reducing power assays. Each study was carried out three times, with the findings averaged.
DPPH Assay
The 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) approach was used to evaluate the scavenging of free radical activity of the partly purified fungal extract, as described by Nuerxiati et al. [26]. A 100 μL fungal extract 10–100 μg/mL was diluted with methanol and mixed with a 100 μL DPPH solution (0.5 mmol/L in 95% methanol) on a 96-well microtiter plate for 30 min at room temperature. Fungal extract at different concentration (10μL-100μL) was diluted with methanol and was added with 100μL DPPH solution in 96-well microtiter plate. The plate was incubated in the dark for 30 min at room. After 30 min, the absorbance at 515 nm was assessed. The following formula was used to calculate the percentage of free radicals that were neutralized:
where A0 = absorbance of the control, and A1 = absorbance of the test extracts. The positive control, ascorbic acid in methanol, was employed to assess the radical scavenging capacity of the fungal extracts.
Reducing Power Assay
To evaluate the extract's reductive potential, it was examined in accordance with Oyaizu [27]. Phosphate buffer of pH 6.6 (0.2 mol/L) and Potassium Ferricyanide (2.5 mL, 1% w/v) were added to 1 mL of distilled water containing a fungal extract of varying concentrations (10–100 g/mL), the mixture was centrifuged for 10 min at 3000 rpm. The absorbance was assessed in spectrophotometer at 700 nm after mixing the surface layer (2.5 mL) with equal parts of filtered water and Ferric chloride (0.5 mL, 0.1% w/v). High absorption value, implying a higher reductive potential.
Anticancer Activity
Cell Line and Culturing
The HeLa cervical cancer cell, A549 Lung Cancer cell line and HEK 293 Human Embryonic Kidney cell line were acquired from the NCL cell collection in Pune. HeLa, HEK 293, and A549 cells were used for the experiments. These cells were cultured in (DMEM, Himedia), to which were administered 10% heat-inactivated FBS (Himedia) and 1% antibiotic cocktail (penicillin (10,000 units/mL), fungizone (10,000 μg/mL), and streptomycin (25 μg/mL)). Cell monolayers were cultured in tissue culture flasks in a humidified 37 °C, 5% CO2 gas incubator.
Cytotoxicity Evaluation by MTT Assay
The MTT analysis was made to examine the sample's potential cytotoxicity. A confluent HeLa, HEK 293, and A549 cell monolayer was distributed at a density of 1 × 104 cells/well in 96-well plates. The cells were cultured for 24 and 48 h at 37 °C in a 5% CO2 incubator with crude and partly purified extract at various doses (25—250 μg/mL). A medium control, which was a blank medium, and a cell control, which were cells that had not been treated with an extract, were also included in the same plates. The MTT colorimetric test was utilized to assess the vitality of the cells, as the protocol outlined by Mosmann [28], with few modifications. Each well contained 10 µL solution of MTT (10 mg/mL in phosphate buffered saline (PBS)) for 4 h incubation at 37 °C in an incubator with 5% CO2. To dissolve the MTT formazan, approximately 200 μL of dimethyl sulfoxide were added for every well. The absorbance at 595 nm was read using a microplate reader (BioTek- Agilent, USA). Using the formula below, the optical density value was converted to a percentage of viability:
The average results of the triplicates were used to figure out the IC50 concentration and the percentage of living cells.
Dual Acridine Orange and Ethidium Bromide (AO/EB) Fluorescent staining
Acridine orange and ethidium bromide double stain was used as described by Gohel et al. [29]. AO/EB labelling distinguishes between living and dead cells by monitoring cellular and nuclear changes. A total of 2 × 104 HEK 293, HeLa, and A549 cells were introduced into each well of a 6-well culture plate and incubated for 24 h. Following a 24-h incubation at 37 °C with 5% CO2, the cells were treated with the fungal extract at the IC50 concentration. The cells were rinsed with PBS before being stained with AO (100 µg/ml) and EB (100 µg/ml). The untreated cells serve as a baseline for comparison. The living and apoptotic cell morphology was studied using a fluorescent microscope (Ti Eclipse) at a magnification of 400.
GC–MS Analysis
Metabolite profiles were determined using an Agilent GC 7890A / MS5975C equipped with an Agilent DB5MS column (30 m × 0.25 mm × 0.5 m) and 99.99% pure helium gas (carrier gas) flowing at 1 ml/min using the gas chromatography mass spectrometry (GC–MS) method. The temperature of the column was adjusted from its initial setting of 50 ºC / min to 300 ºC at a rate of 12ºC/ min and maintained there for 2 min. An injection volume of 1 µL was employed (split ratio of 5:1), and a constant temperature of 250˚C was maintained at the injection port's base. The mass spectral scan range was set between 50 and 550 (m/z) and the ionization voltage was 70 eV. Compounds were identified by searching for mass spectra in the National Institute of Standards and Technology's USA library database, and then classified into appropriate groups using a web-based natural product classifier. (https://npclassifier.ucsd.edu/#) [30].
Textile Dyeing
The lab scale dyeing studies were carried out on cotton fabric employing the fungal pigment extract with mordanting. A mordant is a chemical or material that is used to increase the affinity of cloth and extracted dyes. In this study, numerous mordants were used, including Aluminium potassium sulphate (alum), ferrous sulphate, lemon juice (lemon), and myrobalan. Experiments with mordanting used a pre-mordanting procedure that included simultaneously treating the mordant and the liquid extract. The extract in liquid form without the mordant served as a control. Mordant was utilized at a concentration of 5%. Experiments with mordants and dyes were carried out in a water bath heated to (60° C) for 1 h. The material to liquor ratio (MLR) is 1:50 (fabric weight: extract in w/v). Once the procedure was done, the cotton was removed and air dried. The cloth was washed under running water and air-dried. Once the procedure was done, the cotton was removed and air dried. The cloth was washed under running water and air-dried.
Wash Fastness and Rubbing Tests
Test for wash fastness and rubbing was carried out in AZO Textile Dyeing Laboratory, Tirupur, Tamilnadu, India. At 30 °C, the color wash fastness after washing and rubbing was tested using the ISO 105 C06 (MODIFIED-A2S): 2010 and ISO 105X12: 2016 methods. Colorfastness after being washed. The resistance to washing and rubbing was rated on a scale of 1 to 5. On this scale, 1 denotes very poor, 2 denotes poor, 3 denotes moderate, 4 denotes good, and 5 is the best. Dyed cotton textiles were then clipped (1.5 cm) following the fastness and rubbing tests in order to make the shade card [31].
Results
Isolation and Identification of Mycobial Pigment
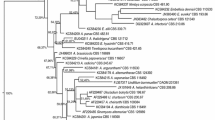
The plant specimen for the study was collected from Nagamalai hills in Madurai district, where the area of investigation lies in 9°54'N latitude and 78° 00' E longitude and at an elevation of 150 m. A total of twenty-four isolates were isolated from Senna auriculata leaf tissues, including one unique strain (FNG1) that generated an extracellular red orange colour. Macroscopic and microscopic examination of the FNG1 isolate revealed that its colonies were flat, whole bordered, floccose in the centre, and comprised of grey aerial mycelia that aged to black and produced a soluble red orange pigment. According to these characteristics described above, strain FNG1 was identified as one certain species of the genus Nigrospora. The FNG1 isolate's identification was confirmed using molecular techniques. A neighbour- joining tree was constructed from the ITS and β- tubulin sequencing and their closet matching refences sequencing was matched with NCBI database and the phylogram of combined sequences showed in (Fig. 1) using MEGA X. Using the ITS2 database, ITS2 secondary structure prediction was employed. Nigrospora sequences had a length of 159 base pairs (bp) in their ITS2 region, and four helices were present in their secondary structures. The homology modelling was found to be similar with the Nigrospora oryzae. No CBCs could be discerned among the species pairs of Nigrospora (Table S1), nevertheless they formed well supported clades in the phylogenetic tree (Fig. S1), which confirms the species as Nigrospora oryzae.
Solubility Test and Fungal Pigment Purification
The fungal crude pigment was tested in a variety of solvents to check their solubility. The coloured crude extract was soluble in a wide variety of solvents and exhibited a vivid red colour in Acetone, Benzene, Chloroform, Ethanol, Ethyl acetate, and Methanol, with a maximum absorbance range of 400–550 nm. The highest solubility was found in Ethyl acetate (Fig. 2A). The crude extract was purified, characterised, and preliminary purified using TLC (Fig. 2B) A red orange colour was detected in UV radiation between the wavelengths of 254 and 366 nm. The red orange spot was verified by a single spot. As a result, the partly purified fungal pigment was employed for additional bioprospecting investigation.
Antimicrobial and Antioxidant Potential of Partially Purified Fungal Pigment
Positive results were found when a partially purified fungal pigment was evaluated against eight human pathogens (Fig. 3) The greatest zone of inhibition observed was in Leucobacter AA7, which was tolerant to all concentrations (200–500 µg/mL), whereas B. cereus ATCC14579 produced the zone of inhibition from 300–500 µg/mL. Salmonella typhi A MTCC 733, Staphylococcus aureus MTCC 7443 formed the zone of inhibition at the range of 400–500 µg/mL. There was no obvious zone of inhibition found against Escherichia coli MTCC 1687, Pseudomonas aeruginosa MTCC 1688, Enterobacter cloacae GS1, or Klebsiella aerogenes MTCC 4031, as shown in the Table 1.
The partly purified fungal pigment's DPPH radical scavenging ability was denoted in the (Fig. 4A) DPPH radical absorbance reductions induced by the antioxidant were a result of radical scavenging via hydrogen donation. There was a noticeable shift in colour from purple to yellow as a result of the reaction. The extract showed scavenging activity from 38.2% to 67.9%. The IC50 values were calculated to be 34.195 ± 2.33 µg/mL, which was equivalent to the standard, as shown in the Table 2.
In the assay for reducing power, the ability to reduce was assessed by the rate at which Fe3 + was converted to Fe2 + . The reaction mixture's greater absorbance was a measure of the samples' enhanced reduced power capability. During the reducing power assay, the yellow test solution changes to various hues of green and blue in response to the reducing power of the antioxidant samples. (Fig. 4A) shows the reducing power of the fungal extract. The extract had strong reduced power, which increased with concentration. The IC50 values were determined to be 46.515 ± 3.57 µg/mL, which was comparable to that of standard, showed in the Table 2.
Cell Cytotoxicity/Viability Assay
The cytotoxicity of the partly purified fungal pigment was evaluated using the HeLa, A549, and HEK 293 cell lines. The IC50 value was plotted in the graph (Fig. 5A) The IC50 value of HeLa cell line for the partially purified compound (24H-386 ± 3.45 µg/mL, 48H-339 ± 4.23 µg/mL), A549 cell (24H-414 ± 2.4 µg/mL, 48H-397 ± 2.89 µg/mL), for HEK 293 cell line (24H-698 ± 4.67 µg/mL, 48H-609 ± 6.7 µg/mL). Cancer cells were significantly inhibited by the extracts. At the same time, meagre cytotoxicity was observed on the non-cancerous HEK 293 cells, which suggest that it is safe to use in the food and pharmaceutical formulations. As a result, the isolate has the potential to be used as an anticancer therapeutic agent.
Morphological analysis was made, and it was observed that the IC50 treated cells displayed cellular morphological changes indicative of unhealthy cells whereas the control cells seemed normal (Fig. 5B). Similarly, Regular, spherical, and polygonal cell aggregates made up the control cells. Compared to control, cells treated with IC50 concentration, the cancer cells appeared to shrink, took on a spherical form, and their patterns of cell spreading were constrained with nuclear fragmentation and chromatin condensation (Fig. 6).
The viability of cells was determined using AO/EB dual staining at the IC50 concentration for 24 h in HeLa, A549 and HEK 293 cell line. Using the fluorescence imaging the cellular variation was observed. The control cells continually exhibited a green florescence light, indicating their viability. Late-stage apoptosis was identified by orange-stained cells, whereas necrotic cells were marked by red staining. When comparing with the control cells, The AO/EB-stained apoptotic cells also showed nuclear breakage, chromatin condensation, cytoskeletal disintegration and plasma membrane blebbing (Fig. 6).
GC–MS Analysis
The GC–MS peaks' spectral properties were compared to those of compounds in the NIST library, and then the NP classifier method was used to categorise the compounds according to their similarities to natural products. Metabolites were classified into five major classes based on their pathways such as: alkaloids (43.8%), fatty acids (18.8%), shikimates and phenylpropanoids (18.8%), polyketides (6.3%), and terpenoids (12.5%) showed in the (Fig. 4B). The metabolites recognized in the GCMS are Hexanoic acid, 2,7-dimethyloct-7-en-5-yn-4-yl ester, Benzenemethanol,.alpha.-(phosphinomethyl)-, 2-Methyl-2-vinyloxirane, 2-Butenenitrile, 2-chloro-3-(4-methoxyphenyl)-, o-Methoxy-.alpha.,.alpha.-dimethylbenzyl alcohol, Phenol, 2,4-bis(1,1-dimethylethyl), Benzoic acid, 4-ethoxy-, ethyl ester, 2,4-Dimethoxyphenyl isocyanate, Acetamide, 2-(3,4-dimethoxyphenyl)-N-(9-oxo-9H-fluoren-2-yl)-, 7,9-Di-tert-butyl-1-oxaspiro (4,5) deca-6,9-diene-2,8-dione, 1H-Indole, 1-(trimethylsilyl)-5-[(trimethylsilyl)oxy]-, Phthalic acid, 2,7-dimethyloct-7-en-5-yn-4-yl isobutyl ester, Octadecanoic acid, 4,8,12-trimethy l-, methyl ester, 1H-Indole, 5-methyl-2-phenyl-, 5-Methyl-2-phenylindolizine, Trimethyl[4-(1,1,3,3,-tetramethylbutyl)phenoxy]silane, Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester, Tetrasiloxane, decamethyl-, 1,1,1,3,5,5,5-Heptamethyltrisiloxane, 2,4,6-Cycloheptatrien-1-one, 3,5-bis-trimethylsilyl-. Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester has the highest area percentage Table 3.
Textile Dyeing—Color Fastness to Laundering and Rubbing
Table 4 shows the results of a study comparing the wash fastness to laundering and rubbing of cotton fabric coloured using various mordanting methods and temperatures. In Wash fastness to laundering the Sample 1 & 2 shows the grey scale rating of 1, where else Sample 3 & 5 shows 1–2 scale rating and sample 4 shows 2 scale rating, which emphasize that among the sample 4 only has little high scale rating when compared to other samples. In Fastness to rubbing (Wet and Dry) sample 1 shows the grey scale rating of (-4-, 4–5), Sample 2 (-3-, 4–5), sample 3 (2–3, 3–4), sample 4 (-4-, 4–5) and sample 5 (3–4, -4-), here sample 4 has shown the highest rating and the least rating was observed in the sample 3. Standard testing techniques revealed that cotton fabric coloured with Fungal extract after being pre-treated with lemon had excellent fastness against rubbing (Wet and Dry), as shown by a grey scale grading of (4, 4–5). But all the dyed fabrics showed poor fastness to laundering. The dyed cotton fabrics are shown in the (Fig. 7). Due to the difficulty associated with the washing and rubbing resistance of naturally coloured samples, the findings for wash fastness and stains on textiles were adequate.
Discussion
A growing desire for products derived from natural sources has resulted in an emerging market for natural colourants. Endophytic fungus generates a wide range of chemically distinct colours, they are investigated as a potential source because of their easy accessibility [32]. Senna auriculata (L.) Roxb., a Fabaceae family member, is a traditional therapeutic plant used in India's Ayurveda and Siddha medical systems to cure a number of ailments. Almost every part of the plant has been studied for its potential therapeutic value, including the flowers, leaves, seeds, barks, and roots. It was once used to treat metabolic disorders, skin disorders, diabetes, asthma, rheumatism, dysentery, and skin ailments. [33]. An endophytic fungus Nigrospora sp., was discovered to generate a deep red orange colour extracellularly isolated from S. auriculata in the present study. Early literature had revealed that pure cultures of fungi such as Blakeslea, Eurotium, Fusarium, Isaria, Monascus, Nigrospora, Paecilomyces, Penicillium, and Talaromyces, where the colored pigment that has been released in solid and liquid mediums. [34,35,36,37,38,39].
In this study, microscopic examinations, together with phenotypic and genotypic features, allow the fungus to be classified as Nigrospora sp. when cultured on PDA media at 28℃, the mycelium of this species expands fast, forming cottony white colonies. Colonies mature in 3–4 days without sporulation, and during this time red orange metabolites are secreted extracellularly. When left to incubate longer, the red extracellular pigments are bleached and the colour changes to a brownish tan. The use of ITS markers as DNA barcodes in the accurate identification of a wide variety of fungus, with a barcode gap that is most clearly defined between interspecific and intraspecific changes, has been described in the past [40]. The fungal isolate's ITS and beta-tubulin sequences were compared to those in the NCBI database to construct a phylogenetic tree. In a multitude of investigations, ITS2 ribotyping has been shown to be superior to other methods for determining phylogenies [18]. In addition to the clades created in the phylogenetic tree, ITS2 secondary structure prediction and CBCs provided extra support for the clades formed in the phylogenetic tree.
The crude red orange pigment was solubilized in a variety of solvents, with Ethyl acetate showing the highest rate of solubility. This conclusion is consistent with previous research that revealed that varying solvents altered the solubilization potential of crude microbial pigments. Pigments produced by Epicoccum nigrum, Lecanicillium aphanocladii, and Penecillium flavigenum were found to be soluble in ethyl acetate by [41]. According to a study by [35], Nigrospora sp. is capable of producing an orange-red pigment with a high solubility in ethyl acetate and similar observations were also recorded in the Aspergillus sp. by [42].
Microbial pigments exhibit distinct bioprospecting properties, including antioxidant, antibacterial, anticancer, anti-inflammatory, antiangiogenic, and immunosuppressive activities. In light of these pharmacological advantages, the scope of this research was expanded to include an examination of pigment's cytotoxic and antioxidant capacities [43]. [44] reported that Ethyl acetate extract of the endophytic fungus Nigrospora sp. suppressed bacterial growth. In the present study, the bactericidal activity of partly purified red orange pigments was assessed using the well diffusion method. Sensitivities to red orange pigments have been seen in both gram-negative and gram-positive bacteria, although the sensitivity varies depending on the organism. Leucobacter AA7 was susceptible for all the concentration and showed maximum zone of inhibition. The red orange pigment was most effective to the gram-positive pathogens. The red orange pigments rendered several gram-negative and gram-positive bacteria inactive.
In addition to antibacterial action, the partly purified fungal extract displayed radical scavenging activity and power reduction capacity. When examined by the DPPH free radical scavenging experiment, the partly purified fungal extract had an IC50 value of 34.195 ± 2.33 µg/mL, with the greatest scavenging activity at 67.9%. [44] showed in their investigation that the radical-scavenging DPPH activity of Nigropsora sp. extract exhibited an IC50 value of 9.28 μg/mL. In the reducing power test, the ability of a compound to reduce is contingent not only on the electron donor but also on the molecule's capacity to quench free radicals [45]. The partially purified fungal extract also showed great reducing power potential with a IC50 value of 46.515 ± 3.57 μg/mL. According to [46], their results showed that 29 of the 13 endophytic fungi's crude extracts had a reducing power activity, which ranging from 0.14 -12.13 mg/mL of fungal extract. The antioxidant compounds aid in the prevention of cancer, along with ageing and the mechanisms that lead to neurodegeneration [47].
According to [48] research, one method for determining a potential for a new treatment is to first extract naturally occurring bioactive molecules and then test those compounds to determine if they have any pharmacological value. Several different endophytes have been linked to the development of new compounds that have shown promise in anticancer studies by [49]. The MTT test was used to evaluate the cytotoxicity of a partially purified fungal extract against two human carcinomas (A549, HeLa cell line, and HEK 293 as a control). Fungal pigment extract showed the strongest cytotoxic effect in a dose-dependent activity. Upon treatment the fungal pigment extract was more effective in A549 & HeLa cells and was less cytotoxicity to the control cells. The study was similar with [50], where A549 and HeLa cells showed antitumor activity of 82.4% by endophytic fungal cultures obtain from Actinidia macrosperma. For HeLa cells, the IC50 values for T. purpureogenus and N. sphaerica extracts were below 200 µg/ml, whereas the IC50 values for Aspergillus sp. and T. stipitatus extract for HeLa cells were 30.1 ± 1 µg/ml and 117.2 µg/ml, respectively [51]. When evaluating the safety of a cytotoxic treatment is appropriate to use, the assessment of the drug's impact on non-cancer cell lines is one of the most essential factors. Efficient cytotoxic therapies induce distinct pathways in malignant and non-cancerous tissues, making tumor cells more exposed to the drug's effects [52]. When the anticancer activities of melanin pigment were examined on HEK 293 cells after 24 h, the percentage of cell viability was 17.70.1 when 100 μg/mL of melanin was utilised. The highest IC50 value (μg/mL) was recorded as 64.11 ± 0.00 µg/mL [53]. The partially purified fungal pigment showed robust anticancer activity against A549 and HeLa cells while being relatively noncytotoxic to HEK 293 cells, making it a promising candidate for application as an antitumor drug at the same time for other industrial and pharmaceutical applications.
The presence of a wide range of phytochemical groups was confirmed by Gas chromatography of the partly purified fungal compounds, many of which have been shown to have activity against malignancy, microbes, and possess antioxidant and other activity. Majorly the metabolites are classified into five classes such as alkaloids, fatty acids, shikimates and phenylpropanoids, polyketides and terpenoids. Hexanoic acid, 2,7-dimethyloct-7-en-5-yn-4-yl ester possess antibacterial activity, but their mechanisms are yet to known [54]. Cognitive functions, neural plasticity, and Cholinergic transmission are just a few of the many neurological processes that benzenemethanol, alpha. -(phosphinomethyl)- aids to [55]. It has been discovered that the naturally occurring substance phenol 2, 4-bis (1, 1-dimethylethyl) serves numerous purposes in the fields of agriculture, food processing, and medicine. In the field of medicine, it is used for its antioxidant, anticancer, antifungal, and antibacterial characteristics, as well as its ability to protect against the cognitive dysfunction caused by trimethyltin (TMT) [56]. Benzoic acid-4-ethoxy-ethyl ester is a potential natural additive for preventing food deterioration microorganisms [57]. Antimicrobial and anticancer activity was reported in 7,9-di-tert-butyl-1-oxaspiro [4.5] deca-6,9-diene-2,8-dione and Phthalic acid, 2,7-dimethyloct-7-en-5-yn-4-yl isobutyl ester- [58, 59]. Octadecanoic acid, 4,8,12-trimethy l-, methyl ester- naturally occur as Stearic acid ester and major possess antioxidant, anti-inflammatory and anticancer activity [60]. Hemolytic, pesticide, flavour, antioxidant properties are reported in Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester [61]. Antifungal, antimicrobial acitivity, anti-inflammatory, antioxidant and anticancer properties are reported in methyl-2-phenyl-1H-indole- [62, 63]. The compound Trimethyl [4-(1,1,3,3, -Tetramethylbutyl) Phenoxy] Silane acts as vitamin D derivatives to cure rickets and antioxidant potential [64]. According to [65] the 2,4,6-Cycloheptatrien-1-one,3,5-bis-trimethylsilyl- possess Antibacterial, nematocidal and used as binder, 1,1,1,3,5,5,5-Heptamethyltrisiloxane- showed Anti-inflammatory, anti-microbial and used as an additive.
According to studies, Nigrospora sp. Red orange pigment might be employed as a textile dye. The red pigment imposed the most strain on cotton fibres, as measured by how lengthy the fabric remained the colour after washing. Additionally, studies have revealed that the fungal pigment might be utilised to create natural textile colours [38]. When the fungal extract is combined with water, it is easy to bond and display its colour. When mordant is present, the net positive charge of cotton yarn rises, facilitating the rapid drawing of pigment down the fibre orientation. It was observed that fungal extract dye combined with pre-mordanting reveals that lemon juice produces the finest colour fastness. It can also be demonstrated that pre-mordanting with myrobalan produces better results than other synthetic mordants, such as alum and ferrous sulphate. It is possible to notice that. On the other hand, both ferrous sulphate and alum, which are synthetic mordants, exhibit poor colour fastness on the cotton fabric, but alum performed much better than ferrous sulphate. Myrobalan and alum, on the other hand, performed much better than ferrous sulphate. Similarly, [66] reported that the use of lemon juice in a pre-mordanting process improved the fabric's colourfastness. Concurrently, red pigments which were produced by Nigrospora aurantiaca, Penicillium purpurogenum, Alterneria alternata, Scytalidium cuboideum, Aspergillus niger, Talaromyces verruculosus, and Trichoderma virens demonstrated the maximum degree of staining and exceptional fastness to washing in cotton textiles [39, 67,68,69,70,71]. In addition, these fungal pigments have no negative effects on the cloth and cause no irritation to the human skin. As a result, there is potential for the variety of applications by using fungal pigments to expand into the clothing and textile sectors.
Conclusion
There is a significant need for more environmentally friendly alternatives to synthetic colourants because of the irreparable harm that they do to ecosystems and human health. Fungal pigment, one of the microbial pigments, has recently been investigated for its use in textile dyeing. It is obvious that, A fungal endophyte, Nigrospora sp., cultured from S. auriculata was identified to synthesize an extracellular reddish orange pigment. The results obtained from the studies highlights that the pigment possess antioxidant, antimicrobial, and anticancer properties confirms the promising applications in the pharmaceutical and food industries. The bioactive metabolites found in the partly purified pigment and their medicinal potential were discovered using GC–MS analysis. Fungal pigments are effective substitutes for synthetic colours in cotton garments. The research shows that fungal pigments may be utilised as non-toxic cotton textile dyes. These dyes offer exceptional colour fastness to washing and rubbing and may be used on cotton. As a result, the findings of our research indicate that this pigment has prospective applications in the textile and pharmaceutical sectors. Pigment-producing microbes play an important part in the advancement of biotechnology and may potentially be employed in the textile, cosmetic, food, feed, and pharmaceutical industries.
Data availability
The data supporting the findings are provided in the supplementary file.
6 References
Samanta AK, Agarwal P (2009) Application of natural dyes on textiles 34:384–399
Narsing Rao MP, Xiao M, Li WJ (2007) Fungal and bacterial pigments: secondary metabolites with wide applications. Front Microbiol 8:1113
Garber LL Jr, Hyatt EM, Nafees L (2016) The effects of food color on perceived flavor: a factorial investigation in India. J Food Prod Mark 22(8):930–948
Elliot AJ, Maier MA (2014) Color psychology: effects of perceiving color on psychological functioning in humans. Annu Rev Psychol 65:95–120
Mukherjee G, Mishra T, Deshmukh SK (2017) Fungal pigments: an overview. Dev Fungal Biol Appl Mycol 525–541
Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM (2006) Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr 46(2):185–196
Babitha S, Soccol CR, Pandey A (2007) Effect of stress on growth, pigment production and morphology of Monascus sp. in solid cultures. J Basic Microbiol 47(2):118–126
Fouillaud M, Venkatachalam M, Girard-Valenciennes E, Caro Y, Dufossé L (2016) Marine-derived fungi producing red anthraquinones: new resources for natural colors?. In: 8th international conference of pigments in food, “coloured foods for health benefits”.
Chen W, Chen R, Liu Q, He Y, He K, Ding X, Kang L, Guo X, Xie N, Zhou Y, Lu Y (2017) Orange, red, yellow: biosynthesis of azaphilone pigments in Monascus fungi. Chem Sci 8(7):4917–4925
Durán N, Teixeira MF, De Conti R, Esposito E (2002) Ecological-friendly pigments from fungi. Crit Rev Food Sci Nutr 42(1):53–66
Ramesh C, Vinithkumar NV, Kirubagaran R, Venil C, Dufossé L (2019) Multifaceted applications of microbial pigments: current knowledge, challenges and future directions for public health implications. Microorganisms 7(7):186
Atalla MM, Elkhrisy EAM, Youssef YA, Mohamed AA (2011) Production of textile reddish brown dyes by fungi. Malay J Microbiol 7(1):33–40
Meruvu H, Dos Santos JC (2021) Colors of life: a review on fungal pigments. Crit Rev Biotechnol 41(8):1153–1177
Kunwar A, Adhikary B, Jayakumar S, Barik A, Chattopadhyay S, Raghukumar S, Priyadarsini KI (2021) Melanin, a promising radioprotector: Mechanisms of actions in a mice model. Toxicol Appl Pharmacol 264(2):202–211
Feng Y, Shao Y, Zhou Y, Chen W, Chen F (2016) Monascus pigments. Ind Biotechnol Vit Biopigm Antioxidants 497–535
Mapari SA, Thrane U, Meyer AS (2010) Fungal polyketide azaphilone pigments as future natural food colorants? Trends Biotechnol 28(6):300–307
Venil CK, Velmurugan P, Dufossé L, Renuka Devi P, Veera Ravi A (2020) Fungal pigments: potential coloring compounds for wide ranging applications in textile dyeing. J Fungi 6(2):68
Sundaresan N, Jagan EG, Kathamuthu G, Pandi M (2019) Internal transcribed spacer 2 (ITS2) molecular morphometric analysis based species delimitation of foliar endophytic fungi from Aglaia elaeagnoidea, Flacourtia inermis and Premna serratifolia. PLoS ONE 14(4):e0215024
Cenis JL (1992) Rapid extraction of fungal DNA for PCR amplification. Nucleic Acids Res 20(9):2380
White TJ, Bruns T, Lee SJWT, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols Guide Methods Appl 18(1):315–322
Ankenbrand MJ, Keller A, Wolf M, Schultz J, Förster F (2015) ITS2 database V: Twice as much. Mol Biol Evol 32(11):3030–3032
Schliep PKP (2011) phylogenetics analysis in R. Bioinformatics 27(4):592–593
Raj KG, Manikandan R, Arulvasu C, Pandi M (2015) Anti-proliferative effect of fungal taxol extracted from Cladosporium oxysporum against human pathogenic bacteria and human colon cancer cell line HCT 15. Spectrochim Acta Part A Mol Biomol Spectrosc 138:667–674
Ahmad WA, Yusof NZ, Nordin N, Zakaria ZA, Rezali MF (2012) Production and characterization of violacein by locally isolated Chromobacterium violaceum grown in agricultural wastes. Appl Biochem Biotechnol 167:1220–1234
Perez C (1990) Antibiotic assay by agar-well diffusion method. Acta Biol Med Exp 15:113–115
Nuerxiati R, Abuduwaili A, Mutailifu P, Wubulikasimu A, Rustamova N, Jingxue C, Aisa HA, Yili A (2019) Optimization of ultrasonic-assisted extraction, characterization and biological activities of polysaccharides from Orchis chusua D. Don (Salep). Int J Biol Macromol 141:431–443
Oyaizu M (1986) Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutrit Dietet 44(6):307–315
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Gohel A, McCarthy MB, Gronowicz G (1999) Estrogen prevents glucocorticoid-induced apoptosis in osteoblasts in vivo and in vitro. Endocrinology 140(11):5339–5347
Kim HW, Wang M, Leber CA, Nothias LF, Reher R, Kang KB, Cottrell GW (2021) NPClassifier: A deep neural network-based structural classification tool for natural products. J Natl Prod 84(11):2795–2807
Kannathasan K, Kokila P (2021) Dyeing of cotton fabric by Caesalpinia sappan aqueous extract at different temperatures and mordants. Curr Bot 12:188–191
Westphal KR, Wollenberg RD, Herbst FA, Sørensen JL, Sondergaard TE, Wimmer R (2018) Enhancing the production of the fungal pigment aurofusarin in Fusarium graminearum. Toxins 10(11):485
Nille GC, Mishra SK, Chaudhary AK, Reddy KRC (2021) Ethnopharmacological, phytochemical, pharmacological, and toxicological review on Senna auriculata (L) Roxb: a special insight to antidiabetic property. Front Pharmacol 2180
Velmurugan P, Kamala-Kannan S, Balachandar V, Lakshmanaperumalsamy P, Chae JC, Oh BT (2010) Natural pigment extraction from five filamentous fungi for industrial applications and dyeing of leather. Carbohyd Polym 79(2):262–268
Arumugam GK, Srinivasan SK, Joshi G, Gopal D, Ramalingam K (2015) Production and characterization of bioactive metabolites from piezotolerant deep sea fungus Nigrospora sp. in submerged fermentation. J Appl Microbiol 118(1):99–111
Torres FAE, Zaccarim BR, de Lencastre Novaes LC, Jozala AF, Santos CAD, Teixeira MFS, Santos-Ebinuma VC (2016) Natural colorants from filamentous fungi. Appl Microbiol Biotechnol 100:2511–2521
Lebeau J, Venkatachalam M, Fouillaud M, Petit T, Vinale F, Dufossé L, Caro Y (2017) Production and new extraction method of polyketide red pigments produced by ascomycetous fungi from terrestrial and marine habitats. J Fungi 3(3):34
Suwannarach N, Kumla J, Nishizaki Y, Sugimoto N, Meerak J, Matsui K, Lumyong S (2019) Optimization and characterization of red pigment production from an endophytic fungus, Nigrospora aurantiaca CMU-ZY2045, and its potential source of natural dye for use in textile dyeing. Appl Microbiol Biotechnol 103:6973–6987
Mishra R, Kalra R, Dilawari R, Deshmukh SK, Barrow CJ, Goel M (2021) Characterization of an endophytic strain Talaromyces assiutensis, CPEF04 with evaluation of production medium for extracellular red pigments having antimicrobial and anticancer properties. Front Microbiol 12:665702
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Fungal Barcoding Consortium, Fungal Barcoding Consortium Author List, Bolchacova E, Voigt K (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci 109(16):6241–6246
da Costa N, Souza P, Luiza Bim Grigoletto T, Beraldo A, de Moraes L, Abreu LM, Henrique Souza Guimarães L, Santos C, Ribeiro Galvão L, Gomes Cardoso P (2016) Production and chemical characterization of pigments in filamentous fungi. Microbiology 162(1):12–22
Narendrababu BN, Shishupala S (2017) Spectrophotometric detection of pigments from aspergillus and penicillium isolates. J Appl Biol Biotechnol 5(1):053–058
El-Sayed ESR, Gach J, Olejniczak T, Boratyński F (2022) A new endophyte Monascus ruber SRZ112 as an efficient production platform of natural pigments using agro-industrial wastes. Sci Rep 12(1):12611
Pawle G, Singh SK (2014) Antimicrobial, antioxidant activity and phytochemical analysis of an endophytic species of Nigrospora isolated from living fossil Ginkgo biloba. Curr Res Environ Appl Mycol 4(1):1–9
Singh N, Rajini PS (2004) Free radical scavenging activity of an aqueous extract of potato peel. Food Chem 85(4):611–616
Khalil D, El-Zayat SA, El-Sayed MA (2020) Phytochemical screening and antioxidant potential of endophytic fungi isolated from Hibiscus sabdariffa. J Appl Biotechnol Rep 7(2):116–124
Xing R, Yu H, Liu S, Zhang W, Zhang Q, Li Z, Li P (2005) Antioxidant activity of differently regioselective chitosan sulfates in vitro. Bioorg Med Chem 13(4):1387–1392
Salvador-Reyes LA, Engene N, Paul VJ, Luesch H (2015) Targeted natural products discovery from marine cyanobacteria using combined phylogenetic and mass spectrometric evaluation. J Nat Prod 78(3):486–492
Elkhouly HI, Hamed AA, El Hosainy AM, Ghareeb MA, Sidkey NM (2021) Bioactive secondary metabolite from endophytic Aspergillus tubenginses ASH4 isolated from Hyoscyamus muticus: antimicrobial, antibiofilm, antioxidant and anticancer activity. Pharmacognosy J 13(2)
Lu Y, Chen C, Chen H, Zhang J, Chen W (2012) Isolation and identification of endophytic fungi from Actinidia macrosperma and investigation of their bioactivities. Evidence-based complementary and alternative medicine
Taritla S, Kumari M, Kamat S, Bhat SG, Jayabaskaran C (2021) Optimization of physicochemical parameters for production of cytotoxic secondary metabolites and apoptosis induction activities in the culture extract of a marine algal-derived endophytic fungus Aspergillus sp. Front Pharmacol 12:542891
Navya PN, Kaphle A, Srinivas SP, Bhargava SK, Rotello VM, Daima HK (2019) Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Convergence 6:1–30
Polapally R, Mansani M, Rajkumar K, Burgula S, Hameeda B, Alhazmi A, Bantun F, Almalki AH, Haque S, El Enshasy HA, Sayyed RZ (2022) Melanin pigment of Streptomyces puniceus RHPR9 exhibits antibacterial, antioxidant and anticancer activities. PLoS ONE 17(4):e0266676
Aji N, Kumala S, Mumpuni E, Rahmat D (2022) Antibacterial activity and active fraction of Zingiber officinale Roscoe, Zingiber montanum (J. Koenig) link ex A., and Zingiber zerumbet (L.) roscoe ex sm against propionibacterium acnes. Pharmacognosy J 14(1)
Olasehinde TA, Odjadjare EC, Mabinya LV, Olaniran AO, Okoh AI (2019) Chlorella sorokiniana and Chlorella minutissima exhibit antioxidant potentials, inhibit cholinesterases and modulate disaggregation of β-amyloid fibrils. Electron J Biotechnol 40:1–9
Ren J, Wang J, Karthikeyan S, Liu H, Cai J (2019) Natural anti-phytopathogenic fungi compound phenol, 2, 4-bis (1, 1-dimethylethyl) from Pseudomonas fluorescens TL-1. Indian J Biochem Biophys 56(2):162–168
Arokiyaraj S, Bharanidharan R, Agastian P, Shin H (2018) Chemical composition, antioxidant activity and antibacterial mechanism of action from Marsilea minuta leaf hexane: methanol extract. Chem Cent J 12(1):1–11
El-Fayoumy EA, Shanab SM, Gaballa HS, Tantawy MA, Shalaby EA (2021) Evaluation of antioxidant and anticancer activity of crude extract and different fractions of Chlorella vulgaris axenic culture grown under various concentrations of copper ions. BMC Complem Med Therapies 21(1):1–16
Eid K, El-Sayed AN, Shoala T (2018) Gas chromatography-mass spectrometry (GC-MS) analysis of sugar beet leaf extracts in response to exogenous application of resistance inducers to manage sugar beet powdery mildew. Egypt J Phytopathol 46(1):257–277
Ganesh M, Mohankumar M (2017) Extraction and identification of bioactive components in Sida cordata (Burm. f.) using gas chromatography–mass spectrometry. J Food Sci Technol 54:3082–3091
Tyagi T, Agarwal M (2017) Research article antioxidant properties and phenolic compounds in methanolic extracts of Eichhornia crassipes. Res J Phytochem 11:85–89
Salim SA (2018) In vitro induction of callus from different explants ofterminalia arjuna (roxb) Wight and arn. And detection of its active secondary metabolites using gc-ms analysis. Plant Arch 18(2):2519–2527
Mannaa M, Kim KD (2018) Biocontrol activity of volatile-producing Bacillus megaterium and Pseudomonas protegens against Aspergillus and Penicillium spp. predominant in stored rice grains: study II. Mycobiology 46(1):52–63
Abed RM, Salman MD (2022) Chemical composition ethanol and methanol extract of lycoperdon pyriforme. Int J Health Sci 6(S1):13749–13760
Sami SA (2022) New insights into the identification of bioactive compounds from Willughbeia edulis Roxb. through GC–MS analysis. Beni-Suef Univ J Basic Appl Sci 11(1):89
Zubairu A, Mshelia YM (2015) Effects of selected mordants on the application of natural dye from onion skin (Allium cepa). Sci Technol 5(2):26–32
Sastrawidana IDK, Maryam SY, Sukarta IN (2016) Natural dyeing of silk and cotton fabric with red pigment from Penicillium purpurogenus which is isolated from goat milk contaminated soil. J Natl Sci Res 6:32–37
Hinsch EM, Weber G, Chen HL, Robinson SC (2015) Colorfastness of extracted wood-staining fungal pigments on fabrics: a new potential for textile dyes. J Textile Apparel Technol Manag 9(3)
Aishwarya AD (2014) Extraction of natural dyes from fungus—an alternate for textile dyeing. J Natl Sci Res 4(7):1–7
Devi S, Karuppan P (2015) Reddish brown pigments from Alternaria alternata for textile dyeing and printing. Indian J Fibre Text Res 40(3):315–319
Chadni Z, Rahaman MH, Jerin I, Hoque KMF, Reza MA (2017) Extraction and optimization of red pigment production as secondary metabolites from Talaromyces verruculosus and potential use in textile industries. Mycology 8:48–57
Acknowledgements
The authors are grateful to Mr. S. Suresh Kumar, Fungal and Cancer Biology Lab, School of Biotechnology, Madurai Kamaraj University, for his support for the work. The authors acknowledge “DST-PURSE and RUSA” Madurai Kamaraj University, Madurai, India for the instrument facility. Also, the authors acknowledge King Saud University, Riyadh, Saudi Arabia, for funding this research through Researchers Supporting Project No: RSPR2023/11.
Funding
This work was supported by grants from the RUSA” Madurai Kamaraj University, Madurai, India.
Author information
Authors and Affiliations
Contributions
IM: Investigation, methodology, conceptualization, writing—original draft & editing. MT: investigation, methodology, conceptualization, writing—review & editing. ASB: investigation and methodology. JXS, RR, AA, NP: Writing—review & editing. MP: Supervision, conceptualization, Writing—review & editing.
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mani, I., Thangavel, M., Surendrababu, A. et al. Unveiling the Bioprospecting Efficacy and Textile Dyeing of a Novel Endophytic Mycobial Red Pigment. Indian J Microbiol 64, 618–634 (2024). https://doi.org/10.1007/s12088-024-01211-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-024-01211-y