Abstract
Viili has been traditionally regarded as healthy food; viili exopolysaccharides (VEPS) function as antioxidants, but the molecular and cellular mechanisms, especially its immune functions, remain largely unclear. To assess VEPS’s immunological roles, VEPS were separated by Sevage’s method and purified by anion exchange chromatography. Cell proliferation, phagocytosis, releases of nitric oxide (NO), interleukin (IL)-1β, and IL-6, the inducible nitric oxide synthase (iNOS) gene expression by reverse transcription polymerase chain reaction (RT-PCR) and iNOS protein by Western blotting, and morphology by scanning electron microscopy in lipopolysaccharides (LPS)/VEPS-stimulated and non-stimulated RAW264.7 macrophages were analyzed. VEPS increased cell proliferation at 50–200 μg/mL. The uptake of neutral red for the indication of phagocytosis and releases of NO, IL-6, and IL-1β were enhanced after exposure to LPS and VEPS. Gene expressions of iNOS, IL-6, and IL-1β and protein expressions of iNOS were increased with VEPS. The RAW264.7 cell treated with VEPS became flattened, a strong indication of the activation of macrophages. We concluded that VEPS promoted the activation of macrophages in which NO, IL-6, and IL-1β were involved; the release of NO and other cytokines may eventually activate lymphocytes, increasing nonspecific (innate) and specific immunity in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Viili, a semisolid yogurt that originated in Finland, has a ropey, gelatinous consistency and a sour taste that resulted from microbial action of lactic acid bacteria (LAB) and a surface-growing fungus, Geotrichum candidum, which forms a velvet-like surface. In addition, viili also contains yeast: Kluveromyces marxianus and Pichia fermentans. Among the mesophilic LAB strains, the slime-forming LAB cremoris produces a phosphate-containing exopolysaccharide (EPS). The basic structure of viili EPS is mainly composed of d-glucose, d-galactose, l-rhamnose, and phosphate, with an average molecular weight of about 2,000 KDa and repeating unit of “→4-β-Glcp-(1 → 4)-β-d-Galp (1 → 4)-β-d-Glcp-(1→” as well as groups of -l-Rhap and -d-Galp-1-p attached to each side of Galp [1, 2]. It has been claimed to have various functional benefits, including its antioxidation, anti-inflammation, anticancer, anti-aging, and enhancement of natural immunity [3–6].
Macrophages not only constitute a principal component of the innate immune system, but also perform pivotal roles in acute inflammatory responses and atherosclerosis [7]. Lipopolysaccharide (LPS)-stimulated macrophages can generate a variety of inflammatory mediators, such as nitric oxide (NO), prostaglandin E2, interleukin (IL)-1β and tumor necrosis factor-a, and matrix metalloprotease-9 [8], but NO is the most crucially active gas molecule, important for inter- and intracellular signal transmission; in the meantime, it also mediates immune and inflammatory processes. NO plays a critical role in communicating a variety of physiological and pathological signalings [9, 10]. Therefore, NO production might be reflective of the inflammation process and may provide a measure to assess the effects of drugs or functional foods on the inflammatory process; however, excessive NO is toxic, which is possible to form free radical groups that lead to a toxic ONOO− molecule [11, 12].
The aim of this study was to examine the effects of viili exopolysaccharides (VEPS) in RAW264.7 macrophage cells and to observe phagocytosis, the release of NO, IL-6, and IL-1β, the gene expression of IL-6 and IL-1β, the gene and protein expression of inducible nitric oxide synthase (iNOS), as well as the morphology change, so as to investigate the potential possible roles of VEPS in human immunity.
MATERIALS AND METHODS
Chemicals
3-(4,5-Dimethyl thiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT), LPS and dimethyl sulfoxide (DMSO), penicillin, and streptomycin were purchased from Sigma-Aldrich Chemicals (St Louis, MO, USA). RPMI1640 medium with l-glutamine 10 % fetal bovine serum (FBS) was purchased from GIBCO (Invitrogen, CA, USA); NO assay kit was purchased from Beyotime (Shanghai, China). RNA mini kit was purchased from Qiagen (Hilden, Germany); RevertAidTM First Strand cDNA Synthesis Kit was from Fermentas (Hanover, MD, USA).
Extraction and Purification of VEPS
The growth medium used for the production of VEPS was reconstituted skim milk autoclaved at 121 °C for 15 min. Fermentation was carried out at 28 °C for18 h with 5 % inoculum. The protein of viili was removed using Sevage and isoelectric point method, ethanol-precipitated VEPS were further purified by DEAE–cellulose (OH―) ionic exchange chromatography [13]. An elution with a filled height of 45 cm in 2.5-cm × 50-cm column was performed at a flow rate of 25 mL/h with distilled water and 0.05, 0.10, 0.15, and 0.2 M NaCl subsequently. The peak of VEPS-V-1 polysaccharide was determined with phenol–sulfuric acid method, following a dialysis in distilled water for 3 days and freeze-drying.
Cell Proliferation of VEPS and LPS-Stimulated RAW264.7 Macrophages
RAW264.7 cell line was a gift from Prof. Yang Xiao, Beijing Institute of Biotechnology, Chinese Academy of Military Medical & Sciences. The cells were maintained in RPMI1640 medium supplemented with l-glutamine (1 mM), 10 % (v/v) heat-inactivated FBS, penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37 °C in 5 % (v/v) CO2 incubator. In general, all experiments were carried out when cells were grown to 80–90 % confluence. About 5,000 cells were seeded in each well of a 96-well plate (100 μL/well) for MTT assay. After 24 h, the 80-μL medium containing different concentrations of VEPS (final concentrations at 50, 100, 200 μg/mL) or a final concentration of 1 μg/mL of LPS (always the same for following assays, otherwise addressed) was separately added. The different concentrations of VEPS and LPS were dissolved in DMSO (not more than 0.01 % (v/v)) or control only with medium and incubated for another 24 h. Subsequently, the medium was removed and replaced with fresh medium containing 0.5 mg/mL of MTT. The wells were then incubated for 4 h at 37 °C before the supernatant was removed. The absorbance was measured at 490 nm after formazan crystals were solubilized with 150 μL of DMSO. Six duplicates for each concentration were performed, and the cell proliferation rate was calculated using the optical density (OD) value.
Neutral Red Assay of Phagocytosis of RAW264.7
Phagocytosis of RAW264.7 was evaluated through neutral red method according to previous reports [14]. The 8 × 104/well of RAW264.7 cells was set in six duplicates, treated with different concentrations of VEPS, LPS (as positive control), or blank, and incubated for 24 h. The cells were then washed with phosphate buffered saline (PBS) three times and incubated with 100 μL of 0.075 % neutral red in PBS for 30 min, followed by floating with 150 μL of lysis solution (glacial acetic acid/ethanol = 1:1) after three times of prewarmed PBS washes. Subsequently, the plates were gently oscillated at 4 °C overnight, and the plate’s absorbance was read at 560 nm to measure the neutral red that was taken by the macrophages.
Measurement of Nitric Oxide
The nitrite concentration in the culture medium was measured as an indicator of NO production using the Griess reaction. RAW264.7 cells (1.6 × 105 cells/well) were treated with 50, 100, 200 μg/mL of VEPS, LPS (positive control), and only culture media as blank control, respectively. The 50 μL of cultural supernatant and an equivalent amount of Griess reagent (1 % sulfanilamide, 0.1 % N-1-naphthalenediamine dihydrochloride, and 2.5 % H3PO4) were transferred to a new 96-well plate; NO levels were determined by measuring nitrite levels through the absorbance at 540 nm. Nitrite levels in the samples were calculated from a standard curve with known concentrations of sodium nitrite [15].
Measurement of Interleukin IL-6 and IL-1β
The secretion of IL-6 and IL-1β by the RAW264.7 cells was assayed using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, MN, USA) according to the manufacturer’s instructions. The cells (3 × 105 cells/well) in 24-well plates were treated with different concentrations of VEPS, LPS, or blank and incubated for 24 h. The cytokine levels in the supernatant were analyzed in the sensitive range through a standard curve.
Gene Expression of iNOS, IL-6, IL-1β by RT-PCR
Total RNA was extracted from approximately 2 × 106 of cells for each test following the manual (Qiagen, CA, USA). The full integrity of total RNA was determined by 1 % agarose gel. The reverse transcription was carried out as follows: 1 μL of RibolockTM RNase inhibitor, 1 μL of Oligo(dT)18 primer, 2 μL of 10 mM dNTP mix, 4 μL of 5 × reaction buffer, 2 μL of template RNA (100 ng/μL), 1 μL of RevertAidTM reverse transcriptase, and nuclease-free water was added to 20 μL of the final volume, mixed, and incubated at 42 °C for 1 h, then at 70 °C for 5 min for the termination of reaction. The cDNA was kept at −20 °C. The iNOS PCR products with the fragment size of 222 bp were sense 5′-GCATGGACCAGTATAAGGCAAGCA-3′ and antisense 5′-GCTTCTGGTCGATGTCATGAGCAA-3′ based on the literature [16]. The IL-6 with the size of 383 bp were sense 5′-CTT CTTGGGACTGATGCT GGTG-3′ and antisense 5′-CGC TGG CTT TGT CTT TCT TGT TA-3′ [17]. The IL-1β with the size of 229 bp were sense 5′-GCCCATCCTCTGTGACTCAT-3′and antisense 5′-AGGCCACAGGTATTTTGTCG-3′ based on the literature [18], and the β-actin with the size of 202 bp were sense 5′-TGGAGAAGAGCTATGAGCTGCCTG-3′ and antisense 5′-GTGCCACCAGACAGCACTGTGTTG-3′ [19], synthesized by Invitrogen (Shanghai, China). Reverse transcription polymerase chain reaction (RT-PCR) was performed with the following: 5 μL of 10 × Taq reaction buffer, 2 μL of template cDNA, 1.5 μL of primers each, 1 μL of dNTP mix (10 mM), 1 μL of Taq DNA polymerase and water up to 50 μL. Reaction started with 94 °C for 30 s, then 94 °C for 30 s, 58 °C for 45 s, and 72 °C for 45 s for 35 cycles, and finally, an extension of 10 min at 72 °C, and it was kept at 4 °C. The correct fragment of PCR was confirmed by a commercial sequencing service company (BGI, Beijing, China).
Protein Expression of iNOS by Western Blotting Analysis
For Western blotting analysis, the cells were washed with ice-cold PBS and lysed for 10 min in lysis buffer. Equal amounts (60 μg) of protein were separated using 10 % SDS-polyacrylamide gels and then transferred to polyvinylidene fluoride membrane. After blotting in 5 % nonfat milk in TBST buffer, the membrane was soaked in blocking buffer and then incubated overnight with primary antibodies against iNOS, followed by horseradish peroxidase-conjugated secondary antibodies. Proteins were detected by enhanced chemiluminescence (Amersham, Piscataway, NJ, USA).
Morphology of RAW264.7 Cells by Scanning Electron Microscopy
The sample for scanning electron microscopy (SEM) was carried out by mounting 20 μL of 106/mL of cell suspension onto coverslip in six-well plate, and the cells were cultured in a CO2 incubator for 15 min; subsequently, fresh medium was added and incubated for 24 h to allow for attachment, then incubated for another 24 h after the desired amount of VEPS or LPS was added. The cells were washed with PBS three times and then fixed for 2 h at 4 °C with 2.5 % glutaraldehyde in 0.2 M (cacodylate) buffer, pH 7.4. The dehydration was carried out with a gradient of 30, 50, 70, 80, 90, and 100 % ethanol at a 3-min interval of each concentration. The cell surface of macrophage RAW264.7 cells were coated with gold spray and examined by SEM (Hitachi, Su1510, Tokyo, Japan); images were collected in TIFF (PC-SeM, Hitachi, Tokyo, Japan) and edited with photoshop without any addition of artifacts.
Statistical Methods
Experimental results were presented as mean ± standard deviation (SD). The level of statistical significance employed in all cases was p < 0.05; t test was used, and statistical calculations were performed using the statistical software SAS 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
VEPS Increased Cell Proliferation of RAW264.7
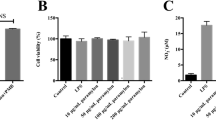
The cytotoxic effects of VEPS on RAW264.7 cells were investigated to establish the appropriate concentration range of VEPS treatment for the analysis of cell proliferation, NO release, phagocytosis, iNOS gene expression, and morphology of cell activation. By MTT assay, non-cytotoxicity was observed with an appropriate concentration of 8 × 104 cell/mL within VEPS’s concentration from 50 to 200 μg/mL. VEPS increased the RAW264.7 cell proliferation significantly, and LPS stimulated RAW264.7 macrophage cell proliferation up to threefold (Fig. 1); synergy was also seen together (data not shown). MTT results were consistent with the cell counts under microscope.
VEPS Increased Phagocytosis of RAW264.7 Macrophage Cells
The phagocytosis activity of RAW264.7 was measured by neutral fuchsin (red) uptake method. With the final concentration of VEPS increasing, from 50 to 200 μg/mL, the OD value increased, which indicated that VEPS increased the phagocytosis of RAW264.7 macrophages. The results are presented by mean ± SD (n = 4) (Fig. 2).
VEPS-Induced NO Production in RAW264.7 Cells
The effects of VEPS on NO production in RAW264.7 cells in comparison with the control were assessed by measuring the amount of nitrite released into the culture medium. To investigate whether NO production was modulated by VEPS, NO production was quantified by Griess assay for the supernatant. NO was very low in the non-stimulated RAW264.7 cells but increased moderately with VEPS (Fig. 3) and greatly with LPS.
VEPS Increased Gene and Protein Expression of iNOS
Both LPS and VEPS clearly induced iNOS, as demonstrated by RT-PCR with the subtraction of β-actin, a housekeeping gene. The gene expression of iNOS was increased by VEPS (Fig. 4). VEPS also increased the protein expression of iNOS shown by Western blotting (Fig. 5). The expression of iNOS protein by Western blotting was consistent with the results of gene expression by RT-PCR analysis.
Gene expression of iNOS in macrophage RAW264.7 cell with LPS and VEPS by RT-PCR. a M MW marker; lanes 1–5 for control, LPS, and VEPS with different concentrations indicated, respectively. b The relative optical density (ROD) was plotted after contracting the housekeeping gene β-actin. **p < 0.05 compared with the control group.
Protein expression of iNOS in macrophage RAW264.7 cell with different concentrations of VEPS by Western blotting. a Western blot: lanes 1–5 for control, LPS, and VEPS at different concentrations indicated, respectively. b The expression values plotted by ROD after contracting the housekeeping β-actin protein. **p < 0.01 and *p < 0.05 compared with the control group.
VEPS Increased Secretion and Gene Expression of IL-6 and IL-1β
The secreted interleukins, IL-6 and IL-1β that were detected by ELISA method, show that VEPS increased IL-6 and IL-1β, even though weaker than LPS (Fig. 6). The gene expression of IL-6 (Fig. 7a) and IL-1β (Fig. 7b) was significantly increased compared with that of the control (Fig. 7c).
Interleukin genes expression of macrophage RAW264.7 influenced with VEPS and LPS by RT-PCR. a IL-6, b IL-1β, and c the expression value plotted by ROD after contracting the housekeeping gene β-actin. M molecular weight, lanes 1–5 for control, LPS, and VEPS at different concentrations indicated, respectively. **p < 0.01, *p < 0.05 for significance compared with the control group.
Morphological Changes of RAW264.7 by VEPS
The macrophages became typically flattened after stimulation with LPS and VEPS compared with the control under SEM observation (Fig. 8). This is due to the fact that cytoskeletons were affected during faster proliferation. Macrophages adopted a flattened appearance with cytoplasmic protrusions spreading across the surface with LPS, and VEPS synergistically presented the typically activated states; with higher concentration, the macrophages appeared longer and flatter (Fig. 8b–h) compared with the control (Fig. 8a). Combined with morphology change, it is clear that VEPS have a very similar function as LPS but in a milder way.
DISCUSSION
VEPS from Lactococcus lactis subsp. cremoris were able to strongly induce NF-κB and various cytokines via two swine intestinal receptors: poRP105 and poMD-1 [20, 21], which inspired our investigation in mouse macrophage RAW264.7 cells, a model much closer to humans. Macrophages are normally accumulated in the small intestine and play crucial roles in innate and adapted immunity as host defense against infection, which is triggered by a large range of different compounds; quite often, polysaccharides or LPS are involved. In our assay, VEPS have shown the ability to increase cell proliferation of RAW264.7 at concentrations of 50, 100, and 200 μg/mL and also with striking increases in phagocytesis, NO secretion, iNOS gene and protein expressions, secretion and gene expression of IL-6 and IL-1β, as well as flatting morphology. In the positive control, LPS has always shown that it is much more potent compared to the VEPS groups; synergy was also observed when VEPS were used in the presence of LPS (data not shown).
NO is recognized as a mediator and regulator of inflammatory responses. There are three isoforms of nitrite oxide synthase, but iNOS is the most important key enzyme in NO synthesis, directly affecting many physiological and pathological functions, for example, relaxing the cardiovascular vessel, increasing the blood flow, as well as promoting cytokine emission at the events of inflammation, at the moment of antigen recognition by conjugates between T cell and antigen-presenting cell, or in the presence of specific hormones in vivo [22–25]. The enhancement of NO production and gene expression by VEPS was in a dose-dependent manner, which strongly suggests that VEPS are an immune mediator/modulator. The secretion of NO increased substantially when both VEPS and LPS were applied, which indicates both of them having a possible similar mechanism because there was also a parallel increase of iNOS by semi-quantitative RT-PCR, and VEPS also increased the iNOS expression of protein by Western blotting analysis. NO is a nonspecific inflammatory mediator, which is normally involved in triggering the proliferation of macrophages, and B and T lymphocytes in specific and nonspecific immunity, and also stimulates the central nervous system and other immune regulations in vivo.
It is common for intestinal macrophages to process the engulfing and ingestion of particles to form a phagosome (or food vacuole), which in turn fuses with a lysosome to form a phagolysosome, where the engulfed materials are eventually digested or degraded and either released extracellularly via exocytosis, or released intracellularly to undergo further processing. Activation of intestinal macrophages by diets is a common duty for some innate and adaptive immunity [26]. After treatment with VEPS, the significant increase of phagocytosis demonstrated by neutral red indicates the activation of cell functions, probably through reversible protein aggregation besides motor molecular mechanisms [27]. The activation of macrophages was also supported by increasing gene expression by RT-PCR and secretion of IL-6 and IL-1β in ELISA analysis.
It is documented that cell morphology of macrophages can be modified from a round to a flat or spread shape when activated by LPS [28]; however, it is the first time to our knowledge that a similar morphology of RAW264.7 became flattened by VEPS. The degree of flattening and spreading shape is consistent with the proliferation, phagocytosis, and NO release and iNOS expression. The process of morphological alteration is involved in a complex, dynamic reorganization of the cytoskeleton actins [29]. However, we believe that these activations by VEPS are reversible physiological processes, which favorably increase the cell proliferation of macrophages and promote immunity, even though it needs to be further studied.
References
Nakajima, H., T. Hirota, T. Toba, T. Itoh, and S. Adachi. 1992. Structure of the extracellular polysaccharide from slime-forming Lactococcus lactis subsp. cremoris SBT 0495. Carbohydrate Research 224: 245–253.
Higashimura, M., B.W. Mulder-Bosman, R. Reich, T. Iwasaki, and G.W. Robijn. 2000. Solution properties of viilian, the exopolysaccharide from Lactococcus lactis subsp. cremoris SBT 0495. Biopolymers 54: 143–158.
Kitazawa, H., T. Yamaguchi, M. Miura, T. Saito, and T. Itoh. 1993. B cell mitogen produced by slime-forming, encapsulated Lactococcus lactis ssp. cremoris isolated from ropy sour milk, viili. Journal of Dairy Science 76: 1514–1519.
Kitazawa, H., M. Tohno, T. Shimosato, and T. Saito. 2007. Development of molecular immunoassay system for probiotics via toll-like receptors based on food immunology. Animal Science Journal 79: 11–21.
Kekkonen, R.A., E. Kajasto, M. Miettinen, V. Veckman, R. Korpela, and I. Julkunen. 2008. Probiotic Leuconostoc mesenteroides ssp. cremoris and Streptococcus thermophilus induce IL-12 and IFN-gamma production. World Journal of Gastroenterology 14: 1192–1203.
Liu, L., J.H. Wu, J.L. Zhang, Z.J. Li, C.L. Wang, M.H. Chen, Y.R. Wang, Y.X. Sun, L.K. Wang, and C. Luo. 2011. A compatibility assay of ursolic acid and foodborne microbial exopolysaccharides by antioxidant power and anti-proliferative properties in hepatocarcinoma cells. Journal of Food, Agriculture & Environment 10: 111–1114.
Lee, M.Y., J.A. Lee, C.S. Seo, H. Ha, H. Lee, J.K. Son, and H.K. Shin. 2011. Anti-inflammatory activity of Angelica dahurica ethanolic extract on RAW264.7 cells via upregulation of heme oxygenase-1. Food and Chemical Toxicology 49: 1047–1055.
Shin, H.Y., C.H. Shin, T.Y. Shin, E.J. Lee, and H.M. Kim. 2003. Effect of bojungikki-tang on lipopolysaccharide-induced cytokine production from peripheral blood mononuclear cells of chronic fatigue syndrome patients. Immunopharmacology and Immunotoxicology 25: 491–501.
Kröncke, K.D., K. Fehsel, and V. Kolb-Bachofen. 1998. Inducible nitric oxide synthase in human diseases. Clinical Experimental Immunology 113: 147–156.
Ohshima, H., and H. Bartsch. 1994. Chronic infections and inflammatory processes as cancer risk factors: Possible role of nitric oxide in carcinogenesis. Mutation Research 305: 253–264.
Korhonen, R., A. Lahti, H. Kankaanranta, and E. Moilanen. 2005. Nitric oxide production and signaling in inflammation. Current Drug Targets Inflammation Allergy 4: 471–479.
Valko, M., D. Leibfritz, J. Moncol, M.T. Cronin, M. Mazur, and J. Telser. 2007. Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology 39: 44–84.
Yang, Z., E. Huttunen, M. Staaf, G. Widmalm, and H. Tenhu. 1999. Separation, purification and characterization of extracellular polysaccharides produced by slime-forming¸ Lactococcus lactis ssp. cremoris strains. International Dairy Journal 9: 631–638.
Papis, E., S.J. Davies, and A.N. Jha. 2011. Relative sensitivity of fish and mammalian cells to the antibiotic, trimethoprim: Cytotoxic and genotoxic responses as determined by neutral red retention, Comet and micronucleus assays. Ecotoxicology 20: 208–217.
Babich, H., M.R. Palace, and A. Stern. 1993. Oxidative stress in fish cells: In vitro studies. Archives Environmental Contamination Toxicology 24: 173–178.
Markewitz, B.A., J.R. Michael, and D.E. Kohan. 1993. Cytokine-induced expression of a nitric oxide synthase in rat renal tubule cells. Journal of Clinical Investigation 91: 2138–2143.
Maa, P., H.T. Liua, W. Peng, Q.S. Xua, X.F. Bai, Y.G. Du, and C. Yu. 2011. Chitosan oligosaccharides inhibit LPS-induced over-expression of IL-6 and TNF-α in RAW264.7 macrophage cells through blockade of mitogen-activated protein kinase (MAPK) and PI3K/Akt signaling pathways. Carbohydrate Polymers 84: 1391–1398.
Liang, Q., Q. Wu, J. Jiang, J. Duan, C. Wang, M.D. Smith, H. Lu, Q. Wang, P. Nagarkatti, and D. Fan. 2011. Characterization of sparstolonin B, a Chinese herb-derived compound, as a selective Toll-like receptor antagonist with potent anti-inflammatory properties. Journal of Biological Chemistry 286: 26470–26479.
Kousteni, S., T. Bellido, L.I. Plotkin, C.A. O’Brien, D.L. Bodenner, L. Han, K. Han, G.B. DiGregorio, J.A. Katzenellenbogen, B.S. Katzenellenbogen, P.K. Roberson, R.S. Weinstein, R.L. Jilka, and S.C. Manolagas. 2001. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: Dissociation from transcriptional activity. Cell 104: 719–730.
Tohno, M., H. Kitazawa, T. Shimosato, M. Matsumoto, S. Katoh, Y. Kawai, and T. Saito. 2005. A swine toll-like receptor 2-expressing transfectant as a potential primary screening system for immunobiotic microorganisms. FEMS Immunology Medical Microbiology 44: 283–288.
Tohno, M., T. Shimazu, W. Ueda, D. Anzawa, H. Aso, J. Nishimura, Y. Kawai, Y. Saito, T. Saito, and H. Kitazawa. 2007. Molecular cloning of porcine RP105/MD-1 involved in recognition of extracellular phosphopolysaccharides from Lactococcus lactis ssp. cremoris. Molecular Immunology 44: 2566–2577.
Lyons, C.R., G.J. Orloff, and J.M. Cunningham. 1992. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. The Journal of Biological Chemistry 267: 6370–6374.
Jin, M., S.J. Suh, J.H. Yang, Y. Lu, S.J. Kim, S. Kwon, T.H. Jo, J.W. Kim, Y.I. Park, G.W. Ahn, C.K. Lee, C.H. Kim, J.K. Son, K.H. Son, and H.W. Chang. 2010. Anti-inflammatory activity of bark of Dioscorea batatas DECNE through the inhibition of iNOS and COX-2 expressions in RAW264.7 cells via NF-κB and ERK1/2 inactivation. Food and Chemical Toxicology 48: 3073–3079.
Manucha, W., L. Oliveros, L. Carrizo, A. Seltzer, and P. Vallés. 2004. Losartan modulation on NOS isoforms and COX-2 expression in early renal fibrogenesis in unilateral obstruction. Kidney International 65: 2091–2107.
Luo, C., T. Vooder, E. Urgard, and A. Metspalu. 2011. The role of COX-2 and Nrf2/ARE in anti-inflammation and antioxidative stress: Aging and anti-aging. Med Hypotheses 77: 174–178.
Luo, C., M. Kallajoki, R. Gross, M. Mulari, T. Teros, L. Ylinen, M. Mäkinen, J. Laine, and O. Simell. 2002. Cellular distribution and contribution of cyclooxygenase COX-2 to diabetogenesis in NOD mouse. Cell Tissue Research 310: 169–175.
Mogensen, T.H. 2009. Pathogen recognition and inflammatory signaling in innate immune defenses. Clinical Microbiology Reviews 22: 240–273.
Schmidt, A., E. Caron, and A. Hall. 2001. Lipopolysaccharide-induced activation of beta2-integrin function in macrophages requires Irak kinase activity, p38 mitogen-activated protein kinase, and the Rap1 GTPase. Molecular and Cellular Biology 21: 438–448.
Begum, R., M.S. Nur-E-Kamal, and M.A. Zaman. 2004. The role of Rho GTPases in the regulation of the rearrangement of actin cytoskeleton and cell movement. Experimental & Molecular Medicine 36: 358–366.
Acknowledgments
This project was supported by an initial fund from Tianjin city government for “1000 talents plan” program for CL. Authors are thankful to Dr. Vladimir Matveev and the Institute of Cytology, Russian Academy of Sciences, Russia for their critical comments for the preparation of the manuscripts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, J., Li, M., Liu, L. et al. Nitric Oxide and Interleukins are Involved in Cell Proliferation of RAW264.7 Macrophages Activated by Viili Exopolysaccharides. Inflammation 36, 954–961 (2013). https://doi.org/10.1007/s10753-013-9626-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-013-9626-y