Abstract
Bone morphogenetic proteins (BMPs) regulate cell fate during development and mediate cancer progression. In this study, we investigated the role of BMP4 in proliferation, anoikis resistance, metastatic migration, and drug resistance of breast cancer cells. We utilized breast cancer cell lines and clinical samples representing different subtypes to understand the functional effect of BMP4 on breast cancer. The BMP pathway was inhibited with the small molecule inhibitor LDN193189 hydrochloride (LDN). BMP4 signaling enhanced the expression of stem cell genes CD44, ALDH1A3, anti-apoptotic gene BCL2 and promoted anoikis resistance in MDA-MB-231 breast cancer cells. BMP4 enhanced self-renewal and chemoresistance in MDA-MB-231 by upregulating Notch signaling while LDN treatment abrogated anoikis resistance and proliferation of anoikis resistant breast cancer cells in the osteogenic microenvironment. Conversely, BMP4 downregulated proliferation, colony-forming ability, and suppressed anoikis resistance in MCF7 and SkBR3 cells, while LDN treatment promoted tumor spheroid formation and growth. These findings indicate that BMP4 has a context-dependent role in breast cancer. Further, our data with MDA-MB-231 cells representing triple-negative breast cancer suggest that BMP inhibition might impair its metastatic spread and colonization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
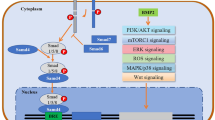
Metastatic breast cancer is the second important cause of cancer-related death in women (Sung et al. 2021), and fewer therapeutic options are available for metastatic cancers. Several signaling mechanisms (Feng et al. 2018; Yousefnia et al. 2020) control metastasis in breast cancer, and bone morphogenetic proteins (BMPs) play an important role in the progression and metastasis of breast cancer (Jiramongkolchai et al. 2016; Zabkiewicz et al. 2017). BMPs, members of the transforming growth factor β (TGF β) superfamily, regulate embryogenesis, homeostasis (Wang et al. 2014), and DNA damage response (Chau et al. 2012). BMPs function through canonical SMAD signaling or non-canonical pathway involving ERK, p38MAPKs, JNK mitogen-activated protein kinases (MAPKs) (Katagiri and Watabe 2016). BMPs were found to have a dual role in cancer (Alarmo and Kallioniemi 2010; Bach et al. 2018b; Ouahoud et al. 2020), promoting tumor progression (Fukuda et al. 2020) or can act as tumor suppressor (Kodach et al. 2009) in a context-dependent manner (Gomez-Puerto et al. 2019).
Cancer stem cells are chemoresistant, contribute towards the development of metastasis (Gomez-Miragaya and González-Suárez 2017; Gomez-Miragaya et al. 2017; Neophytou et al. 2018), and responsible for tumor recurrence at local and distant sites (Ye et al. 2017). Targeting the cancer stem cells or the stem cell properties of cancer cells is necessary to eliminate the chemoresistant cells and prevent the metastatic spread of the cancer cells. Several studies have explored the role of BMPs in breast cancer and provided contradictory reports (Zhang et al. 2016), where knockdown of BMPR1A (Bone morphogenetic protein receptor 1A) reduced the tumor growth and osteolysis (Liu et al. 2018), and BMP4 either inhibited or promoted metastasis of breast cancer cells (Ampuja et al. 2013; Cao et al. 2014; Eckhardt et al. 2020; Guo et al. 2012). BMP2 primarily promoted breast cancer metastasis (Katsuno et al. 2008) by increasing the CD44+CD24− breast cancer stem cell population (Zhang et al. 2018).
Given the contradictory functions attributed to BMP signaling in breast cancer (Zabkiewicz et al. 2017), in the current study, we systematically analyzed the role of BMP4 in regulating the proliferation, anoikis resistance, and chemoresistance of breast cancer cells. For this, we utilized both representative breast cancer cell lines and clinical samples. We found a context-dependent effect of BMP4; it inhibited the proliferation of MCF7 and SkBR3 breast cancer cells while promoting the proliferation of MDA-MB-231 cells that represent aggressive triple-negative breast cancer. Conversely, LDN193189 (LDN), which inhibits BMP receptors, decreased the proliferation and self-renewal of MDA-MB-231 cells while enhancing the self-renewal of MCF7 and SkBR3 cells. Although BMP4 majorly activated canonical SMAD-dependent signaling in all the cell lines tested, it produced diverse functional effects on each cell type. BMP4 significantly enhanced anoikis resistance, an important hallmark of metastatic cells (Kim et al. 2012) in MDA-MB-231 cells, while LDN inhibited anoikis resistance of both MCF7 and MDA-MB-231 cells. Thus, BMP4 has dual, context-dependent, and a possible tumor stage-dependent effect on breast cancer cells. However, BMP inhibition might have therapeutic benefits in preventing invasion, metastasis and induce chemosensitivity in aggressive triple-negative breast cancer.
Methods
Reagents and cell lines
Primary antibodies anti-GAPDH, anti-E-cadherin, anti-N-cadherin, anti-phospho ERK1/2, anti-phospho SMAD1/5, anti-beta catenin, anti-BCL2, anti-BMP4, anti-BMPRIA, anti-BMPRII were purchased from Invitrogen (ThermoFisher Scientific, India), and anti-RHOA antibody was purchased from Cytoskeleton (USA). HRP conjugated secondary antibodies goat anti-rabbit, goat anti-mouse was purchased from Invitrogen (ThermoFisher Scientific, India). Fluorescent conjugated antibodies against phospho SMAD1/8, phospho SMAD2/3, CD44, CD49F, KI67 were purchased from BD biosciences (India), and fluorescent conjugated anti-CD24, anti-EPCAM were purchased from Invitrogen. The authenticated breast cancer cell lines MCF7, SkBR3 and MDA-MB-231, were obtained from National Centre for Cell Sciences (Pune, India). BMP inhibitor LDN193189 was purchased from Sigma-Aldrich (Bangalore, India), and recombinant human BMP4 was from Invitrogen.
Cell cycle analysis
Cell cycle analysis was performed as described earlier (Dattachoudhury et al. 2020). Fixation and permeabilization of the cells was done with ice-cold ethanol at 4 °C. For detecting the G0 population, the cells were stained with fluorescent conjugated anti-KI67 antibody for one hour at room temperature (RT). The cells were stained with propidium iodide (PI) to stain the DNA and analyzed by flow cytometry. The proliferation index of the cells was calculated using the formula: Proliferation index (%) = (S + G2/M)/(G0/G1 + S + G2/M)X100%.
Flow cytometry
The flow cytometric identification of phospho-proteins was performed as described previously (Sharma et al. 2019). Briefly, the cells were fixed with paraformaldehyde at 37 °C and treated with ice-cold methanol to permeabilize the cells. The cells were further stained with anti-phospho protein specific antibodies conjugated with fluorescent dye for one hour at RT and analyzed using flow cytometer.
The cells were stained with fluorescence conjugated antibodies against the cell surface proteins at 4 °C for 30 min to detect cell surface proteins. The cells were stained with PI and analyzed by flow cytometry.
Gene expression analysis
Total RNA from the cells was extracted with RNA extraction kit (ThermoFisher Scientific) according to the manufacturer’s instructions. Tissue samples stored at RNA later were cut and minced with liquid nitrogen and collected with Trizol reagent. Total RNA from the tissue samples was extracted with TRIzol Plus RNA purification kit (ThermoFisher Scientific). Two micrograms of RNA were subjected to reverse transcription using OligodT primers and high-capacity cDNA reverse transcription kit (ThermoFisher Scientific). Gene expression was analyzed using SYBR Green Master Mix (ThermoFisher Scientific) in a CFX96 real-time PCR machine (Bio-Rad Laboratories). Primers used for the study are from previous reports (Akrap et al. 2016). The fold change in expression level was calculated with the ∆∆Ct method.
Actin cytoskeleton staining
F-actin in the breast cancer cells was stained with phalloidin-TRITC (Sigma Aldrich) as described previously (Somaiah et al. 2015). Cells were cultured in cover-slip bottom dishes (Ibidi GmbH, Germany) coated with fibronectin or poly-l-lysine. Fixation of the cells was done using paraformaldehyde (4%) and permeabilization with Triton X-100 (0.1%). F-actin was stained with phalloidin-TRITC overnight at 4 °C, the nucleus was stained with DAPI and imaged with an inverted fluorescent microscope (Zeiss Axio Observer, Zeiss).
Cell migration
The wound healing migration assay was performed by seeding the cells at a density of 20,000 cells/cm2. The cells were allowed to attach for 24–36 h and serum-starved overnight prior to starting the migration assay. The inducers and inhibitors were added to the cells, a scratch was made in the cell monolayer, and the migration of the cells was monitored and documented by imaging. Migration speed was determined based on the distance migrated by the cells at any given time point.
3D spheroid invasion assay was performed as described previously (Dattachoudhury et al. 2020). Firstly, the spheroids were formed with the breast cancer cells by seeding the cells in an agar-coated U-bottom 96-well plate at a density of 0.5–2 × 104 cells/mL. After four days, the spheroids were transferred to 96-well plates coated with collagen (50 µg/mL), and BMP4, or LDN were added and allowed to migrate. Cell invasion from the spheroid was monitored at regular intervals and imaged microscopically.
Anoikis assay
Breast cancer cells were seeded in agar coated 12-well plates and treated with either BMP4 or LDN or its combination for 48 h. Agar coating inhibits the adhesion of the cells to the culture surface; therefore, the cells are maintained in suspension, suitable to study the anoikis resistance of cancer cells. The cells were analyzed for gene expression by flow cytometry, immunoblotting, or tested for self-renewal through colony formation assay. During colony formation, BMP4/LDN were either removed from the pre-treated cells or added again throughout the colony formation period.
Colony assay
For colony formation assay, one hundred cells were seeded in a 6-well plate and allowed to attach for 16–24 h. The cells were treated with BMP4 or LDN for initial 48 h or for the entire duration of the colony formation assay for 14 days. In case of treatment for 48 h, fresh media without BMP4/LDN was added, and colonies were stained with crystal violet and counted microscopically after 14 days.
Immunoblotting
Cells were lysed, and total protein was collected with RIPA buffer (25 mm Tris-HCl [pH 7.4], 1 mm EDTA, 150 mm NaCl, 1% NP-40, 1% sodium de-oxycholate, 0.1% SDS, 1× protease and phosphate inhibitor cocktail), and the concentration of protein was determined. 20–30 µg of protein was resolved on 10% SDS-PAGE and blotted onto a nitrocellulose membrane using a semi-dry blotting system (Bio-Rad Laboratories). The membranes were stained with the respective antibodies to detect the expression levels.
Bone marrow-derived osteoblasts
Osteoblasts were obtained by differentiation of bone marrow-derived mesenchymal stem cells (MSCs). MSCs were differentiated into osteoblasts for 7 days in the osteoblast induction media as described previously (Kumar et al. 2018; Somaiah et al. 2018). Differentiation into osteoblasts was detected by staining for alkaline phosphatase (ALP) activity using a membrane ALP kit (Sigma Aldrich).
Data analysis
SPSS software was used to perform statistical analysis, and Student’s t test was used to compare between groups. Primary patient sample data was analyzed by Mann-Whitney non-parametric variables test. The association between the expression levels of different genes in the primary patient samples was determined by bivariate Pearson correlation analysis using SPSS software. p values < 0.05 were considered statistically significant. Colony area, 3D spheroid migration, and the spheroid area were analyzed using Fiji software, and 2D cell migration was analyzed with TScratch software. FlowJo software (Flow-Jo, LLC) was utilized to analyze flow cytometry data.
Results
BMP4 expression correlates with EMT genes
BMPs secreted from the cancer cells and the tumor microenvironment modulates the progression and metastatic behavior of breast cancer cells (Zabkiewicz et al. 2017). To determine the role of the BMP pathway in breast cancer, we examined BMP4 expression in patient tumor samples sub-classified based on the ER (Estrogen receptor), PR (Progesterone receptor), and HER2 (Human epidermal growth factor receptor 2) expression. BMP4 expression was significantly high in ER−/PR− tumors irrespective of HER2 expression (Fig. 1a). BMP4 expression showed a positive correlation with EMT (Epithelial mesenchymal transition) related genes SNAI2 (p = 0.0086), CDH2 (p = 0.049), RHOA (p = 0.006), MMP9 (Matrix metallopeptidase 9), and ALDH1A3 (p = 0.011) in the patient tumor samples (Fig. 1b). To further understand BMP4 mediated signaling in breast cancer, we utilized breast cancer cell lines MCF7, SkBR3, and MDA-MB-231, representing different breast cancer subtypes based on ER/PR/HER2 expression and determined the expression of BMP pathway components. While MCF7 and SkBR3 expressed the BMP receptors BMPRIA and BMPRII (Bone morphogenetic protein type II receptor), MDA-MB-231 predominantly had BMPRII expression, markedly higher than the other cell lines (Fig. 1c). However, at the transcript level, MDA-MB-231 expressed similar levels of BMPRIA but significantly higher BMPRII compared to MCF7 cells (Fig. 1d). Furthermore, phospho SMAD1/5 (pSMAD1/5) and BMP4 protein levels were higher in MDA-MB-231 cells than MCF7 or SkBR3 (Fig. 1c). MDA-MB-231 cells also had high levels of RHOA (Ras homolog family member A) and phospho ERK1/2 (Extracellular signal-regulated kinase 1/2) compared to MCF7 and SkBR3 cells (Fig. 1e).
BMP4 expression in breast cancer cells. a The expression of BMP4 in patient samples classified based on ER, PR, and HER2 expression was analyzed by real-time PCR. Each open circle in the graph represents an individual sample, and the line represents the median value. b Heatmap of bivariate Pearson correlation coefficient values for the indicated genes in patient samples. n = 6 independent samples (ER+PR+ - 3 samples; ER−PR− - 3 samples). c BMP4, BMPRIA, BMPRII, pSMAD1/5 expression was analyzed in MCF7, SkBR3, and MDA-MB-231 cells by immunoblotting analysis. d mRNA levels of BMPRIA and BMPRII were analyzed by real-time PCR in MCF7 and MDA-MB-231, and fold changes in the expression level are shown. Values are mean ± SE, n = 3–4 independent samples. e RHOA, beta-catenin, phospho ERK1/2 (pERK1/2) expression in the breast cancer cell lines was analyzed by immunoblotting. *p < 0.05, **p < 0.005
BMP4 facilitates canonical signaling in breast cancer cells
The effect of BMP signaling in the breast cancer cells was studied by treating the cells with either recombinant BMP4 (BMP4) or inhibitor of BMP receptor LDN193189 (LDN). Upon BMP4 stimulation, an increase in phospho SMAD1/8 (pSMAD1/8) levels in MCF7, MDA-MB-231 cells, and phospho SMAD2/3 (pSMAD2/3) in MDA-MB-231 cells was observed (Fig. 2a, b). Conversely, LDN treatment significantly inhibited SMAD1/5/8 phosphorylation in all the three breast cancer cell lines and SMAD2/3 phosphorylation in MCF7 and MDA-MB-231 cells (Fig. 2a–c). Considering that RHOA and pERK1/2 levels were differentially expressed in the three breast cancer cell lines, we determined whether BMP4 can induce non-canonical pathway in addition to the SMAD dependent pathway. However, pERK1/2 and RHOA levels did not change with either BMP4 or LDN treatment (Fig. 2c).
BMP4 activates SMAD-dependent pathway in breast cancer cells. a Phospho SMAD1/8 (pSMAD1/8), b phospho SMAD 2/3 (pSMAD2/3) expression in MCF7, MDA-MB-231 cells were analyzed by phospho flow cytometry after treatment with BMP4 (10ng/ml) or LDN (1µM). Values are represented as mean (Geometric mean) fluorescence intensity (MFI). c Immunoblotting analysis of pSMAD1/5, pERK1/2, and RHOA expression in MCF7 and MDA-MB-231 after treatment with BMP4, LDN, or BMP4 + LDN (B + L). d-f Control (CON), BMP4, or LDN treated cells were analyzed for the expression of proliferation, self-renewal, and EMT genes by real-time PCR. d Graph showing fold changes in BMP pathway genes in MCF7, e MDA-MB-231 cells. f Heatmap showing fold changes in gene expression in MCF7 and MDA-MB-231 cells. In all the graphs shown, CON represents untreated control. Values are mean ± SE, n = 3–4 independent samples, *p < 0.05, **p < 0.005, ***p < 0.0005
To identify BMP4 regulated genes, we performed extensive gene expression analysis in MCF7 and MDA-MB-231 following treatment with BMP4 or LDN. LDN treatment significantly induced BMP4, BMP6, and BMPRIA expression in MCF7 cells (Fig. 2d) and treatment with BMP4 markedly increased ID1 and ID2 expression while LDN treatment downregulated both the ID genes in MCF7 and MDA-MB-231 cells. Similar to other ID genes, ID4 was upregulated upon BMP4 treatment and downregulated with LDN in MCF7 cells, whereas BMP4 treatment resulted in a moderate decrease in ID4 expression in MDA-MB-231 cells (Fig. 2d, e). In MDA-MB-231 cells, BMP4 treatment downregulated BMP4 transcript levels but upregulated BMP6 (p = 0.0504) expression (Fig. 2e). Further, there was a significant decrease in the expression of proliferation genes, KI67 (p = 0.0203), PCNA (proliferating cell nuclear antigen) (p = 0.0112) in BMP4 treated MCF7 cells. Although BMP4 treatment induced the expression of CCND1 (p = 0.0111) in MCF7 cells, the effect seems to have been nullified by the increase in CDKN1A (Cyclin-dependent kinase inhibitor 1 A) (p = 0.0019) expression. Conversely, LDN treatment increased the expression of KI67 (p = 0.0333) and CCNE2 in MCF7 cells (Fig. 2f). In MDA-MB-231 cells, the expression of CDKN1A (p = 0.0543) level increased after LDN treatment (Fig. 2f). BMP4 markedly upregulated the expression of stem cells related genes CD44 (p = 0.0455), aldehyde dehydrogenase 1A3 (ALDH1A3) (p = 0.0171), NOTCH2 (p = 0.0276), NOTCH3 (p = 0.047) and DNER (Delta and Notch-like epidermal growth factor-related receptor) (p = 0.0003) in MDA-MB-231 cells. Also, the expression of EMT related genes such as SNAI1 (p = 0.048), SNAI2 (p = 0.0031), and FOSL1 (Fos-related antigen 1) (p = 0.0158) increased in BMP4 treated MDA-MB-231 cells. In MCF7 cells, LDN treatment resulted in downregulation of E-cadherin (CDH1) and upregulation of N-cadherin (CDH2, p = 0.0133) transcript levels (Fig. 2f). Thus, the BMP pathway controls the expression of EMT, and stem cell genes in breast cancer cells.
BMP4 regulates proliferation and self-renewal of breast cancer cells
Next, we tested the functional effect of BMP4 on the proliferation and migration of breast cancer cells. BMP4 induction significantly increased the cell number in MDA-MB-231 cells, whereas proliferation was inhibited in MCF7 and SkBR3 cells (Suppl. Figure 1). BMP4 induced cell cycle arrest in MCF7 and SkBR3 cells, with a dramatic increase in the percentage of cells at the G0 stage of the cell cycle. On the contrary, treatment with LDN significantly increased the percentage of cells at G0 in MDA-MB-231 cells and a concomitant decrease in the proliferation index (Fig. 3a-c). We also found that the IC50 value of LDN was similar for all three breast cancer cell lines, and the survival of the cells was not significantly affected until 96 h of treatment at 1µM concentration (Suppl. Figure 2). Considering that in in vivo conditions, the cancer cells proliferate and migrate in a 3D environment, the breast cancer cells were allowed to form spheroids in the presence of BMP4 or LDN. BMP4 significantly reduced the formation of primary spheroids in MCF7 cells, but the cells formed large spheroids in the presence of LDN (Fig. 3d, e), confirming the tumor-promoting effect of LDN in these cell types.
Effect of BMP4 on proliferation and self-renewal. a MCF7, SkBR3, and MDA-MB-231 cells treated with BMP4, LDN, or BMP4 + LDN (B + L) were flow cytometrically analyzed for the G0 phase by KI67/PI staining, and the graph shows the percentage of cells at the G0 stage of the cell cycle. b Proliferation index of MCF7, SkBR3, and MDA-MB-231 cells treated with BMP4, LDN, or B + L was calculated from their cell cycle profile. c Representative flow cytometry plots for KI67/PI staining. d MCF7, SkBR3, and MDA-MB-231 cells were seeded in u-bottom agar coated 96 well plates to form tumor spheroids in the presence of BMP4, LDN, B + L, and the spheroid area was determined for each condition. e Representative images of spheroids. The black line in the images represents the scale bar (200 μm). f One hundred cells of MCF7, SkBR3, and MDA-MB-231 cells were seeded for colony formation and treated with BMP4, LDN, or B + L (BMP4 + LDN) for the initial 48 h (48 hr) or the entire duration of the colony formation assay for 14 days (14D). Normal growth media was added to the cells after 48 h of treatment when the treatment period was indicated as 48 h. The graph shows the number of colonies observed under each condition as the percentage of control (CON) cells. g Representatives images of colonies of MCF7, SkBR3, MDA-MB-231 cells. The black line in the images represents the scale bar (200 μm). h The colony area was determined for the colonies as described in f. i Percentage of CD44+CD24− population after treatment with BMP4, LDN, or B + L in MDA-MB-231 cells was analyzed by flow cytometry. j Graph showing CD49F expression in MDA-MB-231 cells treated with BMP4, LDN, or B + L. Values are represented as mean (Geometric mean) fluorescence intensity (MFI). In all the graphs shown, CON represents untreated control. Values are mean ± SE, n = 3–4 independent samples, *p < 0.05, **p < 0.005, ***p < 0.0005
Further, cancer stem cells contribute to cancer progression and metastasis in several cancers, including breast cancer. Therefore, we examined the effect of BMP4 on the self-renewal of breast cancer cells. For this, we determined the colony formation ability of the treated cells and analyzed the expression of stem cell markers. BMP4 markedly inhibited the colony-forming ability of both MCF7 and SkBR3 cells, with the cells forming fewer and significantly smaller colonies compared to the control cells (Fig. 3f–h). Continuous treatment during the entire duration of the colony formation with BMP4 alone or in combination with LDN further reduced the colony number and size in MCF7 cells (Fig. 3f–h). However, transient treatment of MCF7 with LDN for the initial 48 h of colony formation produced larger colonies (Fig. 3g, h). The inhibitory effect of BMP4 on colony formation was more pronounced in SkBR3 cells, with the treated cells producing fewer and smaller colonies compared to the control cells (Fig. 3f–h). Conversely, in MDA-MB-231 cells, BMP4 increased the colony-forming ability, which was significantly inhibited with LDN. Treatment of MDA-MB-231 cells with LDN for 48 h was sufficient to drastically diminish the colony size, and co-treatment with BMP4 did not restore their colony-forming ability (Fig. 3f–h). Similarly, the cancer stem cell population in MDA-MB-231 as defined by CD44+CD24− cells and CD49F expression was significantly reduced upon treatment with LDN, whereas co-treatment with BMP4 restored CD49F expression (Fig. 3i, j). Taken together, this data indicates that LDN diminishes the self-renewal ability of MDA-MB-231 but not MCF7 or SkBr3 cells.
BMP4 modulates migration and EMT
Migration is an important event during the metastatic transformation of cancer cells and this is accompanied by changes in the actin cytoskeleton. We found that neither BMP4 nor LDN significantly altered the actin cytoskeleton in MCF7 cells. However, MDA-MB-231 cells treated with BMP4 acquired a spindle-shaped morphology with increased polarization as visualized with F-actin staining (Fig. 4b). The migration rate was unaffected in MCF7 cells upon either BMP4 or LDN treatment, but migration was significantly enhanced with BMP4 and inhibited with LDN in MDA-MD-231 cells. Further, co-treatment of BMP4 with LDN reversed the migration speed to its basal levels (Fig. 4c, d). We also tested cancer spheroid migration since it closely resembles the in vivo conditions, and we found that invasion of migrating cells from the cancer spheroids into collagen matrix was enhanced by BMP4 treatment in MDA-MB-231, although it did not reach statistical significance (Fig. 4e, f). In cancer cells, migration induced by EMT is accompanied by downregulation of E-cadherin and a concomitant increase in N-cadherin expression. Consistent with the migration data, in MDA-MB-231, which have high N-cadherin expression, we observed downregulation of N-cadherin with LDN treatment in 2D cultured cells (Fig. 4 g) as well as spheroids (Fig. 4 h) in addition to decreased transcript levels of EMT related genes (Fig. 2f). On the contrary, in MCF7 cells, LDN treatment led to a decrease in E-cadherin expression (Fig. 4i) and an increase in N-cadherin (CDH2) expression (Fig. 2f), indicating induction of EMT.
BMP4 modulates the migration of breast cancer cells. a Experimental design to test the effect of BMP4 and LDN on migration, invasion of breast cancer cells. b Immunocytochemistry images of MCF7, MDA-MB-231 cells left untreated (CON) or treated with BMP4, LDN, and BMP4 + LDN (B + L) and stained for F-actin (red color) with phalloidin-TRITC and the nucleus (blue color) with DAPI. The white arrows indicate polarization of the cells. The white line in the images represents the scale bar (50 μm). c Migration speed in MCF7 and MDA-MB-231 cells treated with BMP4, LDN, or B + L was determined by wound healing assay. d Representative images of wound healing migration in MDA-MB-231 cells at 0 and 30 h. The black line in the images represents the scale bar (200 μm). e, f MCF7 and MDA-MB-231 cells were seeded in u-bottom agar coated 96 well plates and allowed to form tumor spheroids in the growth medium. e The spheroids were transferred to collagen (50 µg/mL) coated wells in the presence of BMP4, LDN, or B + L or untreated (CON) conditions. Invasion into the collagen matrix by the cancer cells from the spheroid was documented at regular intervals, and the migration area was normalized to the spheroid area at t = 0. f Representative images of spheroid migration in MDA-MB-231 cells is shown. The blue line in the images represent the migration front of the cells and the white line represents the scale bar (200 μm). g N-cadherin expression was analyzed by flow cytometry in MDA-MB-231 cells treated with BMP4, LDN, or B + L. Values are represented as mean (Geometric mean) fluorescence intensity (MFI). h, i The tumor spheroids formed in the presence of BMP4, LDN, or B + L conditions were analyzed by immunoblotting for the expression of h N-cadherin and pERK1/2 in MDA-MB-231 cells; i E-cadherin and pERK1/2 in MCF7 cells. In all the graphs shown, CON represents untreated control. Values are mean ± SE, n = 3–6, *p < 0.05, **p < 0.005, ***p < 0.0005
BMP pathway inhibition diminishes anoikis resistance in MDA-MB-231 cells
Anoikis resistance is an important event during metastasis of cancer cells which provides them with survival signals during cancer cells dissemination through the circulatory and lymphatic system. We monitored the effect of BMP4 and LDN on anoikis resistance in breast cancer cells (Fig. 5a); for this, the cells were cultured in anchorage independent suspension culture. Anoikis condition reduced the viability of SkBr3 and MDA-MB-231 cells, which was further reduced by LDN treatment (Fig. 5b). The cells grown in anoikis condition in the presence of BMP4 or LDN were tested for their self-renewal ability by colony formation assay. The cells which were treated with BMP4 or LDN under anoikis conditions are termed as pre-treated cells (PRE-TR), and if the treatment with BMP4 or LDN was repeated again during the colony formation, it is termed as continuously treated cells (CONT). Compared with the control condition (CON), LDN dramatically enhanced the self-renewal ability of SkBR3 cells, and co-treatment with BMP4 (B + L) did not diminish the colony-forming ability (Fig. 5c). On the contrary, LDN abrogated the anoikis resistance and self-renewal of MDA-MB-231 cells in both pre-treated and continuous treated conditions, and BMP4 co-treatment with LDN (B + L) did not reverse the inhibitory effect of LDN. Additionally, BMP4 treatment significantly enhanced the self-renewal ability of anoikis-resistant MDA-MB-231 cells (Fig. 5c). Although LDN treatment enhanced the proliferation of MCF7 cells (Fig. 3c), surprisingly, it diminished anoikis resistance, and the treated cells formed fewer and smaller colonies than control (CON), or BMP4 treated conditions (Fig. 5c-e). LDN treatment induced the formation of larger colonies in SKBr3, whereas, under similar conditions, MDA-MD-231 produced significantly smaller colonies (Fig. 5e).
Modulation of anoikis resistance by BMP4. a Experimental design to test the effect of BMP4 and LDN on anoikis resistance in breast cancer cells. b SkBR3 and MDA-MB-231 cells were cultured under anoikis conditions in the presence of BMP4, LDN, or BMP4 + LDN (B + L), and the live cell number was enumerated microscopically. c The self-renewal ability of MCF7, SkBR3, and MDA-MB-231 cells cultured under anoikis conditions was determined by colony formation assay. The cells were plated in the normal growth media (PRE-TR) or with BMP4, LDN, B + L (CONT) following anoikis culture in the presence of BMP4, LDN or B+L. The number of colonies formed was counted microscopically after 14 days and represented as the percentage of colonies observed with respect to the control untreated cells (CON). d Representative images of colonies formed by the cells cultured under anoikis conditions. The black line in the images represents the scale bar (200 μm). e The colony size was measured for each colony represented in c. f MDA-MB-231 cells cultured under anoikis conditions in the presence of BMP4, LDN, or B + L were analyzed for the expression of beta-catenin, pSMAD1/5, pERK1/2, and BCL2 by immunoblotting. g Graph shows the normalized expression levels of BCL2 protein in MDA-MB-231 cells grown in anoikis conditions. The BCL2 expression level was normalized to its GAPDH level. h The percentage of CD44+CD24−, CD44+EPCAM− population in MDA-MB-231 cells; i CD49F expression levels in MDA-MB-231 cells; j percentage of CD44−CD24+, CD44−EPCAM+ population in SkBR3 cells cultured under anoikis conditions in the presence of BMP4, LDN or B + L was analyzed by flow cytometry. In all the graphs shown, CON represents untreated control. Values are mean ± SE, n = 3–5 independent samples, *p < 0.05, **p < 0.005, ***p < 0.0005
Increased survival and self-renewal ability of MDA-MB-231 cells under anoikis conditions during BMP4 treatment was mediated by a significant increase in BCL2 (B-cell lymphoma 2) expression. However, neither BMP4 nor LDN changed the expression of beta-catenin or phospho ERK1/2 in anoikis resistant MDA-MB-231 cells (Fig. 5f). CD44+CD24−, CD44+EPCAM− and CD49+ cancer stem cell population was reduced in anoikis condition in LDN treated MDA-MB-231 cells (Fig. 5 h, i). Conversely, BMP4 treatment enhanced the CD44−CD24+ population in SkBR3 cells (Fig. 5j), indicating downregulation of self-renewal ability in SkBR3 cells. Thus, taken together, inhibition of BMP signaling with LDN significantly inhibits the self-renewal and anoikis resistance of MDA-MB-231 cells but has a context-dependent role in MCF7 and SKBr3 cells.
LDN diminishes chemoresistance and proliferation on osteoblasts
We reported earlier that LDN treatment chemosensitizes CML cells (Kumar et al. 2017), and here we determined the role of BMP4 and LDN in the chemosensitization of breast cancer cells. For this, breast cancer cells were treated with doxorubicin (dox) in combination with either BMP4 or LDN. Dox treatment reduced the viability of cells, and co-treatment with LDN further diminished the survival of MDA-MB-231 cells (Fig. 6b). Co-treatment with dox and LDN markedly inhibited the proliferation of MDA-MB-231 but not MCF7 or SkBR3 spheroids (Fig. 6c, d). The size of MCF7 spheroids was enhanced upon co-treatment with LDN and dox compared to only dox treated control (CON) condition (Fig. 6c). We observed that MDA-MB-231 spheroids were markedly less sensitive to dox than 2D cultured cells (Fig. 6b, c). We further tested the self-renewal abilityc of breast cancer cells cultured in anoikis condition in the presence of dox along with BMP4 or LDN; that is, for instance, both dox and BMP4 were added together to the cells in anoikis condition. Treatment with LDN in the presence of dox in anoikis condition completely abolished the self-renewal ability of MDA-MB-231 cells, with the treated cells unable to proliferate and form colonies (Fig. 6e–g). However, BMP4 provided a survival advantage to the cells during dox treatment, resulting in the cells forming more and larger colonies compared to the control cells. Further, co-treatment with BMP4 partially reversed the inhibitory effect of LDN during dox treatment in anoikis condition (Fig. 6e–g).
LDN diminishes chemoresistance in MDA-MB-231 cells. a The experimental design to test the effect of BMP4 and LDN on chemoresistance in breast cancer cells. b MDA-MB-231 cells were treated with dox (5 µM) in the presence of BMP4, LDN or BMP4 + LDN (B + L) and the number of live cells was counted in each condition microscopically. UNTR represents untreated cells and CON here represents the cells cultured only with dox. c Spheroids were allowed to form in the presence of dox (5 µM), and BMP4, LDN or B + L by MCF7, SkBR3, and MDA-MB-231 cells and the spheroid area was analyzed. The horizontal line in the graph represents the spheroid area in untreated cells (without Dox, BMP4, or LDN). d Representative images of spheroids of MCF7, SkBR3, and MDA-MB-231 cells as described in c. The white line in the images represents the scale bar (200 μm). e–g MDA-MB-231 cells were cultured under anoikis conditions in the absence (UNTR) or presence of dox and BMP4, LDN, or B + L. CON here represents the cells cultured only with dox. e The self-renewal ability of the cells was analyzed by colony assay, and the number of colonies formed is represented as the percentage of colonies observed with respect to the untreated cells (UNTR); f Graph showing the colony area and g shows the representative colony images. The white line in the images represents the scale bar (200 μm). h, i MCF7 or MDA-MB-231 cells were cultured in anoikis conditions in the presence of BMP4, LDN, or B + L and seeded onto osteoblasts, and their colony-forming ability was analyzed. During colony formation on the osteoblasts, the cells were grown in normal media, and CON represents untreated control cells. i Representative microscopic images showing the proliferation of MCF7 or MDA-MB-231 cells on the osteoblasts. The black line in the images represents the scale bar (200 μm). Values are mean ± SE, n = 3–5 independent samples. *p < 0.05, *** p < 0.0005
The anoikis resistant metastatic cells eventually form colonies at the secondary sites, and bone marrow is the most frequent site of metastasis for breast cancer cells (Awolaran et al. 2016; Hussein and Komarova 2011). Therefore, to evaluate how BMP signaling alters the proliferation of breast cancer cells in the bone marrow, MCF7 or MDA-MB-231 cells were treated with either BMP4 or LDN in anoikis culture conditions and the anoikis resistant cells were seeded on the osteoblasts. Treatment of breast cancer cells with LDN in anoikis condition significantly abrogated the proliferation ability of breast cancer cells on osteoblasts (Fig. 6h, i), suggesting that the BMP pathway plays an essential role in the metastatic growth of breast cancer cells.
Discussion
In the current study, we demonstrate that BMP4 has a context-dependent role in breast cancer progression. We utilized breast cancer cell lines and patient samples to systematically delineate the function of BMP4 and its inhibition on the progression of breast cancer. Through the exogenous addition of BMP4, our study mainly focused on the paracrine effect of BMP4, which might be secreted from the tumor microenvironment of the breast cancer cells. We found increased expression of BMP4 and BMPRII in the aggressive breast cancer cell line MDA-MB-231, which correlates with the finding that elevated BMP4 and BMPRII expression levels are present in the blood of advanced-stage breast cancer patients (Gul et al. 2015). However, in contrast to other studies (Ampuja et al. 2013; Eckhardt et al. 2020), we found that BMP4 enhanced the proliferation and self-renewal of MDA-MB-231 cells. Nevertheless, the effect of BMP4 was cell type specific (Kallioniemi 2012); it drastically reduced the proliferation of MCF7, and SkBR3 by enhancing the percentage of cells at the G0 stage and CDKN1A expression.
We found that BMP4 predominantly signaled through the canonical BMP pathway (Choi et al. 2019; Eckhardt et al. 2020) to modify the proliferation and invasion of breast cancer cells. BMP4 enhanced the migration and invasion of MDA-MB-231 breast cancer cells (Ampuja et al. 2013, 2016; Guo et al. 2012; Owens et al. 2014, 2015) accompanied by increased expression of EMT genes N-cadherin, SNAI1, SNAI2, and FOSL1, which were inhibited on LDN treatment. On the contrary, Cao et al. reported that mouse mammary tumor cells overexpressing BMP4 had lower metastasis due to the inhibition of myeloid-derived suppressor cell activity at the tumor site (Cao et al. 2014). A similar reduction in lung metastasis was also observed in BMP4 overexpressing MDA-MB-231 cells (Eckhardt et al. 2020), and metastasis was inhibited when BMP4 was induced by N-myc downstream-regulated gene 2 (NDRG2) expression (Shon et al. 2009). Given the fact that we observed enhanced migration of MDA-MB-231 cells with BMP4 in wound healing migration assay, it might be possible that BMP4 might have chemoattractant properties. It is possible that when breast cancer cells were modified to overexpress BMP4, it retains the cancer cells at the primary tumor site, inhibits their migration, and reduces lung metastasis. Moreover, several studies have analyzed lung but not bone metastasis, and due to its bone morphogenetic properties (Bessa et al. 2009), increased BMP4 might enhance bone metastasis, a primary metastatic site for breast cancer cells (Hussein and Komarova 2011). We found that BMP4 pre-treatment enhanced the self-renewal of MDA-MB-231 cells, and when MCF7 or MDA-MB-231 cells were pre-treated with LDN, it drastically inhibited their proliferation ability on the osteoblasts, indicating the role of BMP pathway in establishing bone marrow metastasis. Further, anoikis resistance is one of the hallmarks of metastatic cells (Kim et al. 2012), which involves hyperactivation of survival pathways and anti-apoptotic genes (Guadamillas et al. 2011). We found that BMP4 significantly enhanced the survival of metastatic MDA-MB-231 cells under anoikis conditions through BCL2 upregulation, further indicating the positive role of BMP signaling in metastasis formation.
Furthermore, the functional effect of BMP4 extends beyond the migration, invasion, EMT, and in several cancers, the BMP pathway also contributed to chemoresistance (Bach et al. 2018a, b; Kumar et al. 2017). Alarmo et al. reported that high BMP4 expression was associated with a high risk of recurrence (Alarmo et al. 2013), suggesting its association with self-renewal and chemoresistance. In our study, we found that BMP4 enhanced the stem cell properties in MDA-MB-231 cells marked by increased expression of CD44, ALDH1A3 and Notch signaling genes NOTCH2, NOTCH3, and DNER (Choi et al. 2019). Notch signaling enhances migration, metastasis, and therapy resistance of breast cancer cells (Zhang et al. 2019), and Notch inhibitors have anti-tumor properties (Locatelli et al. 2017). Further, BMP4 in the presence of dox enhanced the colony-forming ability, while LDN treatment completely abolished the colony formation in MDA-MB-231 cells. Thus, the presence of BMP4 in the tumor microenvironment might significantly enhance the survival of the breast cancer cells and, subsequently, cancer recurrence (Alarmo et al. 2013). BMP4 indeed has multiple roles in breast cancer (Bach et al. 2018b), and it inhibits proliferation, self-renewal (Shee et al. 2019), and anoikis resistance in breast cancer subtypes represented by MCF7 or SkBR3 cells, and enhancing BMP4 levels in non-expressing cancers might inhibit their metastatic potential. Although studies have suggested that BMP4 might act as a metastatic suppressor (Eckhardt et al. 2020), our data indicate that paracrine BMP4 might enhance the metastatic ability of triple-negative breast cancer cells, especially to the bone (Ampuja et al. 2016). Nevertheless, exogenous BMP4 may have therapeutic benefits in reducing proliferation in luminal-like breast cancers (Shee et al. 2019). Our data also indicate that the response of breast cancer cells to BMP4 might be mediated by the expression levels of the BMP receptors (Lowery and de Caestecker 2010) and the presence of BMP antagonists at the tumor microenvironment.
Thus, BMP4 has a context-dependent effect on breast cancer progression and metastasis. Inhibition of BMP receptors with LDN has an inhibitory effect on anoikis resistance and chemoresistance of triple-negative breast cancer cells represented by MDA-MB-231 cells. Given the limited therapeutic options available for triple-negative breast cancer, and the inhibitory effects of LDN on cancer progression we have observed in our study, future therapeutic options can explore the possibility of interrupting BMP signaling to prevent breast cancer invasion and metastasis.
Availability of data and materials
All data of this study are included in the article and its additional information files.
References
Akrap N, Andersson D, Bom E, Gregersson P, Stahlberg A, Landberg G (2016) Identification of distinct breast cancer stem cell populations based on single-cell analyses of functionally enriched stem and progenitor pools. Stem Cell Rep 6(1):121–136
Alarmo EL, Kallioniemi A (2010) Bone morphogenetic proteins in breast cancer: dual role in tumourigenesis? Endocr Relat Cancer 17(2):R123–R139
Alarmo EL, Huhtala H, Korhonen T, Pylkkanen L, Holli K, Kuukasjarvi T, Parkkila S, Kallioniemi A (2013) Bone morphogenetic protein 4 expression in multiple normal and tumor tissues reveals its importance beyond development. Mod Pathol 26(1):10–21
Ampuja M, Jokimaki R, Juuti-Uusitalo K, Rodriguez-Martinez A, Alarmo EL, Kallioniemi A (2013) BMP4 inhibits the proliferation of breast cancer cells and induces an MMP-dependent migratory phenotype in MDA-MB-231 cells in 3D environment. Bmc Cancer 13
Ampuja M, Alarmo EL, Owens P, Havunen R, Gorska AE, Moses HL, Kallioniemi A (2016) The impact of bone morphogenetic protein 4 (BMP4) on breast cancer metastasis in a mouse xenograft model. Cancer Lett 375(2):238–244
Awolaran O, Brooks SA, Lavender V (2016) Breast cancer osteomimicry and its role in bone specific metastasis; an integrative, systematic review of preclinical evidence. Breast 30:156–171
Bach DH, Luu TTT, Kim D, An YJ, Park S, Park HJ, Lee SK (2018) BMP4 upregulation is associated with acquired drug resistance and fatty acid metabolism in EGFR-mutant non-small-cell lung cancer cells. Mol Therapy-Nucleic Acids 12:817–828
Bach DH, Park HJ, Lee SK (2018) The dual role of bone morphogenetic proteins in cancer. Mol Therapy-Oncolytics 8:1–13
Bessa PC, Cerqueira MT, Rada T, Gomes ME, Neves NM, Nobre A, Reis RL, Casal M (2009) Expression, purification and osteogenic bioactivity of recombinant human BMP-4, -9, -10, -11 and – 14. Protein Expr Purif 63(2):89–94
Cao Y, Slaney CY, Bidwell BN, Parker BS, Johnstone CN, Rautela J, Eckhardt BL, Anderson RL (2014) BMP4 inhibits breast cancer metastasis by blocking myeloid-derived suppressor cell activity. Can Res 74(18):5091–5102
Chau JFL, Jia DY, Wang ZF, Liu Z, Hu YY, Zhang X, Jia H, Lai KP, Leong WF, Au BJ, Mishina Y, Chen YG, Biondi C, Robertson E, Xie D, Liu HJ, He L, Wang XY, Yu Q, Li BJ (2012) A crucial role for bone morphogenetic protein-Smad1 signalling in the DNA damage response. Nat Commun 3
Choi S, Yu JY, Park A, Dubon MJ, Do J, Kim Y, Nam D, Noh J, Park KS (2019) BMP-4 enhances epithelial mesenchymal transition and cancer stem cell properties of breast cancer cells via Notch signaling. Sci Rep 9
Dattachoudhury S, Sharma R, Kumar A, Jaganathan BG (2020) Sorafenib inhibits proliferation, migration and invasion of breast cancer cells. Oncology 98(7):478–486
Eckhardt BL, Cao Y, Redfern AD, Chi LH, Burrows AD, Roslan S, Sloan EK, Parker BS, Loi S, Ueno NT, Lau PKH, Latham B, Anderson RL (2020) Activation of canonical BMP4-SMAD7 signaling suppresses breast cancer metastasis. Can Res 80(6):1304–1315
Feng YX, Spezia M, Huang SF, Yuan CF, Zeng ZY, Zhang LH, Ji XJ, Liu W, Huang B, Luo WP, Liu B, Lei Y, Du S, Vuppalapati A, Luu HH, Haydon RC, He TC, Ren GS (2018) Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis 5(2):77–106
Fukuda T, Fukuda R, Tanabe R, Koinuma D, Koyama H, Hashizume Y, Moustakas A, Miyazono K, Heldin CH (2020) BMP signaling is a therapeutic target in ovarian cancer. Cell Death Discov 6(1)
Gomez-Miragaya J, González-Suárez E (2017) Tumor-initiating CD49f cells are a hallmark of chemoresistant triple negative breast cancer. Mol Cell Oncol 4(4):e1338208
Gomez-Miragaya J, Palafox M, Pare L, Yoldi G, Ferrer I, Vila S, Galvan P, Pellegrini P, Perez-Montoyo H, Igea A, Munoz P, Esteller M, Nebreda AR, Urruticoechea A, Morilla I, Pernas S, Climent F, Soler-Monso MT, Petit A, Serra V, Prat A (2017) Resistance to taxanes in triple-negative breast cancer associates with the dynamics of a CD49f + tumor-initiating population. Stem Cell Rep 8(5):1392–1407
Gomez-Puerto MC, Iyengar PV, de Vinuesa AG, ten Dijke P, Sanchez-Duffhues G (2019) Bone morphogenetic protein receptor signal transduction in human disease. J Pathol 247(1):9–20
Guadamillas MC, Cerezo A, del Pozo MA (2011) Overcoming anoikis - pathways to anchorage-independent growth in cancer. J Cell Sci 124(19):3189–3197
Gul S, Murad S, Ehsan N, Bloodsworth P, Sultan A, Faheem M (2015) Transcriptional up-regulation of BMP-4 and BMPR-II genes in the peripheral blood of breast cancer patients: a pilot study. Cancer Biomarkers 15(5):551–557
Guo D, Huang JY, Gong JP (2012) Bone morphogenetic protein 4 (BMP4) is required for migration and invasion of breast cancer. Mol Cell Biochem 363(1–2):179–190
Hussein O, Komarova SV (2011) Breast cancer at bone metastatic sites: recent discoveries and treatment targets. J Cell Commun Signal 5(2):85–99
Jiramongkolchai P, Owens P, Hong CC (2016) Emerging roles of the bone morphogenetic protein pathway in cancer: potential therapeutic target for kinase inhibition. Biochem Soc Trans 44:1117–1134
Kallioniemi A (2012) Bone morphogenetic protein 4-a fascinating regulator of cancer cell behavior. Cancer Genet 205(6):267–277
Katagiri T, Watabe T (2016) Bone morphogenetic proteins. Cold Spring Harbor Perspect Biol 8(6)
Katsuno Y, Hanyu A, Kanda H, Ishikawa Y, Akiyama F, Iwase T, Ogata E, Ehata S, Miyazono K, Imamura T (2008) Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene 27(49):6322–6333
Kim YN, Koo KH, Sung JY, Yun UJ, Kim H (2012) Anoikis resistance: an essential prerequisite for tumor metastasis. Int J Cell Biol 2012:306879
Kodach LL, Wiercinska E, de Miranda NFCC, Bleuming SA, Peppelenbosch MP, Dekker E, van den Brink GR, van Noesel CJM, Morreau H, ten Dijke P, Offerhaus GJA, Hardwick JCH (2009) The bone morphogenetic protein pathway is inactivated in the majority of sporadic colorectal cancers. Eur J Gastroenterol Hepatol 21(3):A41–A41
Kumar A, Bhattacharyya J, Jaganathan BG (2017) Adhesion to stromal cells mediates imatinib resistance in chronic myeloid leukemia through ERK and BMP signaling pathways. Sci Rep 7(1):9535
Kumar A, Anand T, Bhattacharyya J, Sharma A, Jaganathan BG (2018) K562 chronic myeloid leukemia cells modify osteogenic differentiation and gene expression of bone marrow stromal cells. J Cell Commun Signal 12(2):441–450
Liu Y, Zhang RX, Yuan W, Chen HQ, Tian DD, Li H, Jiang X, Deng ZL, Wang Y (2018) Knockdown of bone morphogenetic proteins type 1a receptor (BMPR1a) in breast cancer cells protects bone from breast cancer-induced osteolysis by suppressing RANKL expression. Cell Physiol Biochem 45(5):1759–1771
Locatelli MA, Aftimos P, Dees EC, LoRusso PM, Pegram MD, Awada A, Huang B, Cesari R, Jiang YQ, Shaik MN, Kern KA, Curigliano G (2017) Phase I study of the gamma secretase inhibitor PF-03084014 in combination with docetaxel in patients with advanced triple-negative breast cancer. Oncotarget 8(2):2320–2328
Lowery JW, de Caestecker MP (2010) BMP signaling in vascular development and disease. Cytokine Growth Factor Rev 21(4):287–298
Neophytou C, Boutsikos P, Papageorgis P (2018) Molecular mechanisms and emerging therapeutic targets of triple-negative breast cancer metastasis. Front Oncol 8
Ouahoud S, Hardwick JCH, Hawinkels LJAC (2020) Extracellular BMP antagonists, multifaceted orchestrators in the tumor and its microenvironment. Int J Mol Sci 21(11)
Owens P, Pickup MW, Novitskiy SV, Giltnane JM, Gorska AE, Hopkins CR, Hong CC, Moses HL (2014) Inhibition of bmp signaling suppresses metastasis in mammary cancer. Cancer Research, 74(19)
Owens P, Pickup MW, Novitskiy SV, Giltnane JM, Gorska AE, Hopkins CR, Hong CC, Moses HL (2015) Inhibition of BMP signaling suppresses metastasis in mammary cancer. Oncogene 34(19):2437–2449
Sharma R, Sharma A, Kumar A, Jaganathan BG (2019) Phospho-protein analysis in adherent cells using flow cytometry. Bio-protocol 9(20):e3395
Shee K, Jiang A, Varn FS, Liu S, Traphagen NA, Owens P, Ma CX, Hoog J, Cheng C, Golub TR, Straussman R, Miller TW (2019) Cytokine sensitivity screening highlights BMP4 pathway signaling as a therapeutic opportunity in ER + breast cancer. Faseb J 33(2):1644–1657
Shon SK, Kim A, Kim JY, Kim KI, Yang Y, Lim JS (2009) Bone morphogenetic protein-4 induced by NDRG2 expression inhibits MMP-9 activity in breast cancer cells. Biochem Biophys Res Commun 385(2):198–203
Somaiah C, Kumar A, Mawrie D, Sharma A, Patil SD, Bhattacharyya J, Swaminathan R, Jaganathan BG (2015) Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PloS One 10(12):e0145068–e0145068
Somaiah C, Kumar A, Sharma R, Sharma A, Anand T, Bhattacharyya J, Das D, Deka Talukdar S, Jaganathan BG (2018) Mesenchymal stem cells show functional defect and decreased anti-cancer effect after exposure to chemotherapeutic drugs. J Biomed Sci 25(1):5–5
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-a Cancer J Clin 71(3):209–249
Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S, Idowu O, Li M, Shen C, Hu A, Haydon RC, Kang R, Mok J, Lee MJ, Luu HL, Shi LL (2014) Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis 1(1):87–105
Ye F, Zhon XR, Qiu Y, Yang LB, Wei B, Zhang Z, Bu H (2017) CD49f can act as a biomarker for local or distant recurrence in breast cancer. J Breast Cancer 20(2):142–149
Yousefnia S, Forootan FS, Forootan SS, Esfahani MHN, Gure AO, Ghaedi K (2020) Mechanistic pathways of malignancy in breast cancer stem cells. Front Oncol 10
Zabkiewicz C, Resaul J, Hargest R, Jiang WG, Ye L (2017) Bone morphogenetic proteins, breast cancer, and bone metastases: striking the right balance. Endocr Relat Cancer 24(10):R349–R366
Zhang LJ, Ye YN, Long XX, Xiao P, Ren XB, Yu JP (2016) BMP signaling and its paradoxical effects in tumorigenesis and dissemination. Oncotarget 7(47):78206–78218
Zhang G, Huang P, Chen A, He W, Li Z, Liu G, Wang J (2018) How BMP-2 induces EMT and breast cancer stemness through Rb and CD44? Cell Death Dis 9(2):20
Zhang Y, Xie ZY, Guo XT, Xiao XH, Xiong LX (2019) Notch and breast cancer metastasis: Current knowledge, new sights and targeted therapy. Oncology Letters 18(3):2743–2755
Funding
RS and A Sharma were supported by Ministry of Education (MoE), Govt. of India. This study was supported by grants from ICMR (05/07/1502/2016-CH), SERB (EMR/2015/002096), Govt. of India to BGJ.
Author information
Authors and Affiliations
Contributions
RS designed, performed the experiments, analyzed, interpreted the data. GG designed the study, performed patient sample analysis, wrote the manuscript. SS performed the experiments on clinical samples. A Sharma analyzed, interpreted the data. DJK was involved in the collection of breast tumor samples. A Sarma did the histopathological analysis of tumor samples. AML supervised the molecular biology aspects of the clinical sample. MKG interpreted the data and wrote the manuscript. JB did clinical sample collection and analysis. BGJ conceptualized, designed the study, analyzed the data, wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval and consent to participate
The study protocol was approved by the Human Ethics committee of the Indian Institute of Technology Guwahati (IITG), Assam Medical College, Dibrugarh, Assam, and Dr. B Borooah Cancer Institute, Guwahati. Written informed consent was obtained from all the patients involved in the study. The study was carried out in accordance with the ethical standards described in the Declaration of Helsinki.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Fig. 1.
MCF7, SkBR3, and MDA-MB-231 breast cancer cells were treated with recombinant human BMP4 (10ng/ml), LDN193189 (LDN, 1µM) or a combination of BMP4 and LDN (B + L) for 48 h, and live cell number was determined. Values are mean ± SE, n = 3, *p < 0.05 (TIF 776 kb)
Supplementary Fig. 2.
Percentage of live cells as analyzed by flow cytometry in MCF7, SkBR3, and MDA-MB-231 cells on treatment with indicated concentrations of LDN for 48 h. Values are mean ± SE, n = 3, *** p < 0.0005 (TIF 854 kb)
Rights and permissions
About this article
Cite this article
Sharma, R., Gogoi, G., Saikia, S. et al. BMP4 enhances anoikis resistance and chemoresistance of breast cancer cells through canonical BMP signaling. J. Cell Commun. Signal. 16, 191–205 (2022). https://doi.org/10.1007/s12079-021-00649-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-021-00649-9