Abstract
Introduction
Recent advancements in the study of nasal chemesthesis have primarily been achieved through in vitro, cellular, and molecular techniques, with a resultant shift of focus away from in vivo experimental methodology. Psychophysical and electrophysiological data derived from long-standing in vivo methods form our core understanding of trigeminal chemoreception, including the functional characterization of responses to many known trigeminal stimuli, across species.
Methods
We selected relevant in vivo data from existing studies relating to nasal trigeminal nerve-mediated chemesthesis and performed a series of inter-species comparisons in cases where the methodological and procedural similarities between studies allowed for a productive analysis.
Results
There was a remarkable similarity between human and rat nasal chemesthesis in terms of comparative sensitivity to select compounds, structure-activity assessments, and mechanisms of action.
Conclusions
The parallels between rat and human nasal chemesthesis suggest that the rat represents an excellent model for the assessment of human trigeminal chemosensitivity. The similarities presented here are not surprising considering the primitive and adaptive nature of the chemesthetic sense.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Activation of chemosensitive somatosensory receptors of the nasal epithelia by endogenous factors or exogenous-inhaled compounds gives rise to the sensory perception of chemesthesis (Keele 1962; Green et al. 1990; Green 1996), resulting in sensations best described using physically or thermally tangible descriptors such as irritating, tingling, stinging, burning, cooling, painful, or pungent. In mediating this sensory experience, chemesthesis serves to signal harmful mucosal or environmental conditions and to trigger reflexive behavioral and physiological responses which act to reduce continued exposure to the tissue-offensive compound and dilute it at the affected region of exposure (Szolcsányi 1996; Holzer 1988; Keverne et al. 1986).
In the nose, chemesthesis is primarily mediated by the trigeminal nerve (TN). The ophthalmic division of the TN innervates the anterior nasal mucosa via the ethmoid nerve, while the posterior portions of the nasal cavity are innervated by the maxillary division via the nasopalatine nerve. Chemosensitive branches of these nerves ramify repeatedly over the course of their extension toward the luminal surface of the nasal cavity and provide ample innervation of the nasal epithelia as they terminate below the line of tight junctions, mere micrometers from the external environment (Silver and Finger 2009; Bryant and Silver 2000; Finger et al. 1990).
Spurred on by a handful of breakthrough findings, investigations into the mechanisms underlying the functionality of these intraepithelial fibers have undergone an unprecedented period of progress over the course of the last decade. The discovery of solitary chemoreceptor cells (SCCs) within the respiratory epithelium, concomitantly exposed to the airway and in direct synaptic communication with peptidergic TN fibers (Finger et al. 2003), redefined our fundamental view of TN-mediated chemesthesis by introducing a specialized sensory cell to a chemosensory system for which no such cellular mechanism had previously been recognized. Utilizing receptors and signaling cascades classically associated with the detection of bitter tastants, SCCs effectively enhance the chemosensory repertoire of the TN, facilitating its indirect activation by hydrophilic compounds that cannot readily cross epithelial layers to reach chemosensitive TN fibers. These specialized cells are present in functionally relevant areas of the respiratory epithelia of humans and rodents, where they respond to irritants and bacterial metabolites, trigger respiratory and other responses, and excite TN fibers upon activation (Saunders et al. 2014; Barham et al. 2013; Tizzano et al. 2010; Finger et al. 2003).
As for molecular mechanisms, cloning of the capsaicin receptor by Caterina et al. (1997) was an early development in a series of discoveries that led to the recognition of the transient receptor potential (TRP) family of ion channels as important polymodal transducers of chemical and physical stimulation in sensory fibers. Subsequent cloning and characterization of various TRP and other ion channels have provided a molecular basis for long-known TN sensitivities to a multitude of compounds (Viana 2011; Kobayashi et al. 2005). TRP channels are found in organisms ranging from worms to fruit flies to humans (Venkatachalam et al. 2014; Harteneck et al. 2000), suggesting TRP-mediated chemesthesis to be a primitive sense. Of the various TRP channels known to be expressed by trigeminal ganglion (TG) neurons, the three most-studied in chemesthesis are the vanilloid-1 (TRPV1), ankyrin-1 (TRPA1), and melastatin-8 (TRPM8) channels, respectively (Roper 2014). Based on its robust expression in sensory neurons and peripheral nerve fibers, TRPV1 appears to play a dominant role in chemesthesis. In addition to sensing elevated temperatures, TRPV1 is activated by a variety of compounds including capsaicin, piperine, allyl isothiocyanate, allicin, ammonia, and acids. In rats, many of the TG neurons that express TRPV1 also express TRPA1. This channel has been implicated to function as a cold sensor and is activated by a diverse group of chemicals including cinnamaldehyde, methyl salicylate, allicin, allyl isothiocyanate, menthol, carbon dioxide, as well as weak organic acids (Roper 2014). In contrast to the co-expression observed with TRPV1 and TRPA1, TRPM8 appears to be expressed by a separate population of TG neurons that respond to cool temperatures and noxious cold. Chemical compounds that activate TRPM8 include menthol, eucalyptol, and other aromatic spices and essential oils (Roper 2014).
As these relatively recent findings and groundbreaking advancements in the cellular and molecular aspects of chemesthesis have been achieved primarily through the use of in vitro techniques, there has been a concurrent shift of focus away from well-established in vivo experimental methodologies. Indeed, our core understanding of TN-mediated chemesthesis is built on a wealth of knowledge acquired through psychophysical, electrophysiological, and other in vivo methods using both human and animal investigative models. Below, we will briefly review the primary in vivo techniques used in the study of nasal chemesthesis and present an analysis of several in vivo-derived findings comparing similarities in trigeminal chemosensitivity between humans and rats.
In Vivo Techniques
Behavior and Physiology
Chemesthetic activation in the mammalian nose can elicit readily observable reflexive behaviors adapted for minimizing continued exposure to tissue-offensive stimuli. These reflexes include familiar movements of aversion, rejection, and withdrawal, some of which, such as blinking and restriction of the nares, provide a non-invasive means of observing and characterizing chemesthetic responses in both human and animal investigative models (Walker et al. 2001). Nasal TN activation may also elicit localized and/or systemic responses affecting cardiovascular physiology. Local vasodilation and extravasation effects associated with neurogenic inflammation are mediated by the so-called sensory-effector function of a specific subset of peptidergic, TRPV1-expressing TN fibers which couple neural activation to a simultaneous and localized release of the vasoactive neuropeptides substance P and calcitonin-gene-related peptide (Saunders et al. 2014; Szolcsányi 1996, 2014; Holzer 1988). Systemic autonomic responses may include increased cortical microvascular blood flow (Major and Silver 1999), decreased respiratory rate, apnea, bradycardia, and shifts in mean arterial blood pressure (Panneton et al. 2012), variables which can be measured using minimally invasive methods.
Psychophysics
Psychophysics is, in essence, the quantification of the relationship between a stimulus and a sensation and as such, plays a prominent role in sensory research. Psychophysical approaches to the evaluation of chemesthetic response utilize various measures to quantitatively judge the presence (threshold) or magnitude (suprathreshold) of a chemical stimulus. Such evaluations can potentially be affected by the psychological and cognitive characteristics of individual test subjects, as well as inter-individual variations in nasal sensitivity. Olfactory-trigeminal interactions may also affect psychophysical evaluations, since most if not all volatile compounds that elicit a TN response also stimulate the olfactory system, albeit at much lower concentrations. Multiple experimental strategies have of course been devised to minimize such confounding variables, including the use of unilateral stimulation of the nares to separate olfactory and trigeminal response, the use of anosmic subjects, and use of the nearly odorless TN irritant, carbon dioxide as a standard test irritant (Dalton 2001).
Electrophysiology
Event-Related Potential
The cortically generated event-related potential (ERP) is a measure of the central nervous system (CNS) activity obtained through non-invasive electroencephalography (EEG) techniques. Cortical ERPs reflect the synchronized activity of large populations of neurons participating in a CNS response to a specific event (Bressler 2002) such as would occur upon activation of chemosensitive receptors of the nose in response to a presented stimulus. TN-mediated ERPs have been used extensively to investigate many different aspects of human nasal chemesthesis, including the spatial heterogeneity of the nasal mucosa as related to perceptual accuracy, as well as sensitivities to different types of stimulation. Interestingly, different components of TN-mediated ERP waveforms have been shown to encode the different characteristics of a given stimulus, including its relative concentration and duration (Frasnelli et al. 2003).
Negative Mucosa Potential
In contrast to the CNS-derived ERP, the so-called negative mucosa potential (NMP) is derived peripherally and measured directly from the inner surface of the nasal epithelia using minimally invasive electrophysiological techniques. Sensitive to pre-treatment of the nasal mucosa with capsaicin or local anesthetics and independent of olfactory activation, the NMP is considered to be a specific peripheral measure of nociception, representing the spatially limited, summated chemesthetic response. NMP assessments have been used extensively in rat and human research to explore many basic aspects of nasal chemesthesis, such as response thresholds and desensitization effects. More complex spatial and temporal chemesthetic characteristics of the nasal mucosa have also been explored using the NMP (Scheibe et al. 2006, 2008; Frasnelli and Hummel 2003).
Whole-Nerve Recording
Arguably, the most exact in vivo measure of nasal chemesthetic response is achieved by directly recording neural activity from an exposed branch of the trigeminal nerve. The highly invasive nature of this technique, requiring surgical exposure and isolation of the nerve, limits its use to animal models of investigation. Although some single-unit recordings from the ethmoid nerves of anesthetized guinea pigs have been reported (Sekizawa and Tsubone 1994), the great majority of available nerve response data are from summated multiunit recordings in rats (Bryant and Silver 2000). Whole-nerve electrophysiology has been used to characterize chemesthetic responses to a wide variety of compounds and pharmacological treatments and is an especially productive technique when used in conjunction with behavioral, physiological, and immunohistochemical assessments.
Methods
In the present analysis, we selected relevant in vivo data from existing studies relating to nasal TN-mediated chemesthesis and performed a series of inter-species comparisons in cases where the methodological and procedural similarities between studies allowed for a productive analysis. Magnitude of chemesthetic response was compared between rats and humans for 19 compounds, based on availability of data (Table 1). For this comparison human response was based on psychometric ratings of perceived intensity in anosmic adults in response to nasal inhalation of stimuli at vapor saturation, as described elsewhere (Doty et al. 1978). In brief, intensity was rated on a nine-point scale with the extremes defined as follows: very weak–very strong, very pleasant–very unpleasant, very cool–very warm, and very safe–very unsafe. Corresponding rat data were based on whole-nerve recordings in response to vapor-saturated air delivery of each compound, as described elsewhere (Silver 1990). In brief, rats were anesthetized and two tracheal cannulae were inserted: a respiratory cannula allowed free breathing of room air, while a nasopharyngeal cannula allowed for controlled airflow through the rat’s nasal cavity. All stimuli were delivered directly to the nares via a computer-controlled air-dilution olfactometer. Multiunit neural activity was recorded from the exposed ethmoid branch of the trigeminal nerve using a pair of platinum-iridium wire hook electrodes, amplified, and integrated for analysis. Maximal response magnitudes were normalized and reported as a percentage value (%) of response to a standard stimulus of 550 ppm cyclohexanone.
In an effort to better visualize overall inter-species similarities and differences in chemesthetic response to the 19 select compounds, relative response magnitude values from Table 1 were replaced by their fractional rank values and sorted in order of increasing rat ethmoid nerve response in Fig. 1. The relationship between human and rat responses to the select stimuli was additionally visualized by plotting the perceived intensity rating for each compound as a function of its corresponding normalized rat response (Fig. 2).
Rank order of response magnitudes for select stimuli. The 19 select stimuli listed in Table 1 were sorted in increasing rank order of rat nerve response magnitude and presented with corresponding rank order values of human psychophysical response
Human psychophysical response in relation to rat nerve response. Individual data points represent the 19 different compounds listed in Table 1
For structure-activity assessments, comparable data from studies investigating the effectiveness of various compounds, including carboxylic acids, lineal 2-ketones, and a homologous series of eight lineal n-alcohols, were combined in Fig. 3 in an effort to illustrate the relationship between carbon chain length and threshold concentration of chemesthetic response. For these comparisons, rat response thresholds were based on electrophysiological recordings obtained directly from the ethmoid branch of the trigeminal nerve as described above and detailed elsewhere (Silver 1988, unpublished data; Silver et al. 1986). Human response thresholds were based on psychophysical assessments in which nasal pungency thresholds were obtained from anosmic subjects using a two-alternative forced-choice presentation via an ascending method of limits, as detailed elsewhere (Cometto-Muñiz and Cain 1990, 1993; Cometto-Muñiz et al. 1998). Additionally, the correlation between human and rat response thresholds to the homologous series of eight lineal n-alcohols (methanol–octanol) was illustrated (Fig. 4), using human response thresholds based on psychophysical assessments of anosmic subjects as described by Cometto-Muñiz and Cain (1990) and rat response thresholds based on whole-nerve recordings as described above and detailed elsewhere (Silver et al. 1986).
Chemesthetic response thresholds decrease with increasing carbon chain length of stimulus compound. Human response threshold concentrations were obtained from psychophysical assessments; rat response threshold concentrations were obtained via electrophysiological recordings from the ethmoid branch of the trigeminal nerve (1Silver et al. 1986; 2Cometto-Muñiz and Cain 1990; 3Cometto-Muñiz and Cain 1993; 4Silver, unpublished data; Silver 1988; 6 Cometto-Muñiz et al. 1998)
Correlation of response to a homologous series of aliphatic alcohols. Individual data points represent methanol (C1) through octanol (C8). Human response threshold concentrations were obtained from psychophysical assessments; rat response threshold concentrations were obtained via electrophysiological recordings from the ethmoid branch of the trigeminal nerve (1Silver et al. 1986; 2Cometto-Muñiz and Cain 1990)
Studies on TN-mediated chemosensitivity to nicotine and carbon dioxide were used to assess inter-species similarities and/or differences relating to mechanisms of action. For nicotine, comparable data were available from studies on the effects of the nicotinic acetylcholine receptor (nAChR) antagonist mecamylamine hydrochloride on nasal chemesthesis (Fig. 5). Human data were obtained via NMP recordings and psychophysical assessments in response to stimulation by nicotine before and after mecamylamine treatment, as described elsewhere (Thuerauf et al. 2006). Rat data were based on the ethmoid nerve response to nicotine as described above and detailed elsewhere (Alimohammadi and Silver 2000). In brief, rats were stimulated with 10 ppm nicotine via an air-dilution olfactometer in 5-min intervals, before and after systemic administration of mecamylamine at 2.5 × 10−5 mol/kg of body weight. Individual responses were normalized by calculating each value as a percentage of the initial recorded response; thus, all nerve response data are reported as relative percentage values.
Reduction of chemesthetic sensitivity to nicotine by mecamylamine. Administration of mecamylamine significantly and similarly reduced response to nicotine in rats and humans. Human response data were obtained via NMP recordings and psychophysical assessments; rat response data were obtained via electrophysiological recordings from the ethmoid branch of the trigeminal nerve (1Alimohammadi and Silver 2000; 2Thuerauf et al. 2006)
For carbon dioxide, comparable data were available from studies on the effects of the carbonic anhydrase inhibitor, acetazolamide, on TN-mediated chemesthetic sensitivities to carbon dioxide and carbonation. Lacking appropriately comparable inter-species data on the effects of acetazolamide on nasal chemesthesis, an indirect comparison between species was instead made, using psychophysical data from a study of acetazolamide on human oral response to carbonated water, as detailed elsewhere (Dessirier et al. 2000). Previously unreported rat data were based on ethmoid nerve response, obtained using the surgical and electrophysiological methods described above. Specifically, carbon dioxide was delivered directly to the nares of anesthetized rats at a final concentration of 50 % via an air-dilution olfactometer. A total of ten rats were stimulated seven times over a 30-min period using a 5-s stimulus duration and a 5-min inter-stimulus interval, during which the olfactometer delivered a steady stream of room air at 2 l/min. Rats in the experimental group (n = 5) received an intraperitoneal injection of acetazolamide at a final concentration of 5 mg/kg, whereas the control group animals (n = 5) received an injection of 0.9 % saline (1 ml/kg body weight, i.p.). All injections were made immediately after the first carbon dioxide presentation was completed and recorded. Response magnitudes were normalized by calculating each value as a percentage of the initial recorded response, as described above for nicotine. Statistical significance of acetazolamide treatment on TN response to carbon dioxide was tested using a two-tailed Mann-Whitney U test to compare the control and experimental groups.
Results
Comparative Sensitivity to Select Compounds
Comparable response magnitude data from human (Doty et al. 1978) and rat (Silver 1990) studies were available for the 19 compounds listed in Table 1. Normalized ethmoid nerve recordings from rats and psychophysical intensity ratings by anosmic human subjects showed agreement in overall patterns of sensitivity, despite apparent inter-species differences in the relative chemesthetic strengths of certain test stimuli. For example, whereas cyclohexanone elicited the largest nerve response in rats, propionic acid was the compound most intensely perceived by humans. Despite this apparent difference, cyclohexanone and propionic acid were among the top three strongest chemesthetic stimuli in both species. Similar patterns emerged for other test stimuli, including heptanoic acid and phenethyl alcohol, which generated the weakest responses from rats and humans, respectively (Table 1).
Overall similarities and specific differences between humans and rats became more apparent when response magnitudes to the select compounds were ranked and sorted in order of increasing rat nerve response (Fig. 1). The largest difference in ranked order of response between humans and rats was found with menthol, which ranked as the third weakest stimulus in rats in contrast to its position as a relatively strong stimulus among human subjects. In general, differences between the rank orders of response between human and rat were more marked among the weaker chemesthetic stimuli. The least difference between humans and rats in rank order of response magnitude was noted with butanol, amyl acetate, and butyric acid.
Plotting the perceived intensity rating (human) for each compound against its corresponding nerve response magnitude (rat) revealed a sigmoidal relationship (Fig. 2, R 2 = 0.8241) highlighting two groupings of compounds. One cluster was comprised of the compounds that elicited the strongest perceived intensity ratings in humans. These were distributed over a relatively limited range of perceived intensity score (7.33–8.73). In the rat, response magnitudes to these same compounds were more variable, being distributed over a relatively large range along the x-axis. The second cluster was comprised of menthol, phenethyl alcohol, α-terpineol, and heptanol, compounds that elicited nearly invariable rat ethmoid-nerve responses. Human intensity ratings for these same compounds were more variable, being distributed over a relatively large range along the y-axis (0.13–6.14).
Structure-Activity Assessments
Comparable data from studies investigating the lipid solubility of stimulus compounds are combined in Fig. 3 and illustrate the relationship between carbon chain length and threshold of chemesthetic response. Detection thresholds in anosmic human subjects and rats generally decreased with increasing molecular size and lipid solubility of the acids, alcohols, and ketones tested. Human and rat response threshold concentration plots for the three acids and two lineal 2-ketones tested were similar and displayed overlap. Less similarity was observed between human and rat response to the series of lineal n-alcohols tested, in that rat nerve response thresholds were found to be consistently lower than human detection thresholds. Despite this apparent difference, human and rat response thresholds to these alcohols displayed a positive correlation (Fig. 4, R 2 = 0.8875).
Mechanisms of Action
Nicotine Sensitivity
In rats, mecamylamine rapidly and significantly reduced ethmoid nerve response to nicotine (Fig. 5). Within 5 min of systemic administration, mean response magnitudes were reduced to levels between 40 and 60 % of the initial response, compared to 90 % in the control group. Within 30 min, nerve response to nicotine had fallen to approximately 17 % whereas response magnitude in the control group remained at approximately 75 % of the initial response (Alimohammadi and Silver 2000). NMP assessments showed a similar effect in human subjects (Fig. 5). Mecamylamine significantly reduced NMPs elicited by nicotine, without affecting response to control stimulation with carbon dioxide. The strong effect of mecamylamine on reducing NMP amplitudes coincided with significant reductions in perceived chemesthetic intensity (Thuerauf et al. 2006).
Carbon Dioxide Sensitivity
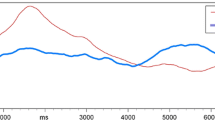
While there were no directly comparable human and rat data describing the effect of the carbonic anhydrase inhibitor, acetazolamide on nasal chemesthetic sensitivity to carbon dioxide, an indirect comparison between species was possible using psychophysical data from studies of human oral response to carbonated water in presence of acetazolamide. Figure 6 summarizes the effect of acetazolamide on rat ethmoid nerve response to inhaled carbon dioxide. Nasal sensitivity to carbon dioxide was significantly reduced, as early as 5 min after systemic administration of acetazolamide (two-tailed Mann-Whitney U tests, P < 0.05). After 30 min, mean nerve response to carbon dioxide dropped to levels only slightly above 10 % of the initial response. Variability in response magnitude was also markedly reduced in acetazolamide-treated rats when compared to the control group (Alimohammadi, unpublished data). Similarly, significant reductions in human oral sensitivity to carbonation were demonstrated in psychophysical studies utilizing dilute solutions of acetazolamide applied directly to the tongues of human subjects, as described by Dessirier and colleagues (2000).
Reduction of chemesthetic sensitivity to carbon dioxide by acetazolamide. Rat ethmoid nerve response to 50 % carbon dioxide significantly decreased over 30 min after intraperitoneal administration of acetazolamide (5 mg/kg) immediately after the first stimulus presentation (two-tailed Mann-Whitney tests, P ≤ 0.05; n = 5 per group). Individual response magnitudes were calculated as a percentage of the initial response. Control group received an intraperitoneal injection of 0.9 % saline (1 ml/kg). Error bars indicate one standard deviation unit (Alimohammadi, unpublished data)
Discussion
It is important to note that the retrospective and observational aspects of the present comparative analyses preclude the formal calculation of most key statistics. The potential for selection and observational biases further hinder the formation of absolute conclusions based on present comparisons. Nonetheless, comparison of these in vivo-derived data do offer useful insights into the remarkable level of fidelity between human and rat nasal chemesthesis and provide a basis for more formal investigations. It should also be noted that this is not the first time that similarities between human and rat TN chemosensitivity have been reported: an early comparison of human and rat responses to a small subset of the compounds included in the present analyses demonstrated a strong correlation between relative ethmoid nerve response magnitudes and anosmic human intensity ratings to nine different stimuli (Silver and Moulton 1982). Those observations lead to the conclusion that the rat represents an excellent model for the assessment of human trigeminal chemosensitivity (Silver and Moulton 1982; Doty et al. 1978). The observed similarities were particularly interesting considering that the measured responses were of a completely different nature, and compared across different mammalian orders (Doty 1995).
In the present analyses, comparative human and rat data were acquired from a more diverse set of studies and included a larger number of stimulus compounds. A comparison of the trigeminal responses to the 19 compounds for which data were available (Table 1) suggests general similarities in chemesthetic response between humans and rats for several compounds. These similarities, as well as some notable differences, became more readily observable when response magnitudes were ranked and ordered in Fig. 1. Inter-species differences in rank order were notably more pronounced among the weaker ranking stimuli, suggesting that trigeminal sensitivity may be more variable between humans and rats for compounds that generate weaker chemesthetic responses. This observation could potentially be an artifact arising from the comparison of psychophysical and electrophysiological data from the lower end of the response spectrum, where perceptual ratings may be relatively less reliable and more variable when compared to direct nerve recordings. Alternatively, this observation could be an indicator of an evolutionary conservation of response to the more potent TN irritants. From an evolutionary perspective, it is plausible that there would be more room for divergence between species in their responses to weaker chemesthetic stimuli, whereas receptor mechanisms for more potent irritants could be expected to be more conserved. The observed inter-species variability in rank order for the weak and strong stimuli seem to at least partially hint at such a possibility, but no formal conclusions can be made based on the existing data.
Interestingly, the largest difference between humans and rats was observed with menthol, which elicited a relatively much larger response in humans than in rats (Fig. 1). While the underlying reasons for this particular discrepancy are not fully clear, such divergences in chemosensitivity may result from the adaptive evolution of the receptor mechanisms involved. In the case of menthol, in vitro studies have shown this compound to have a bimodal effect on the rat TRPA1 channel, activating it at low concentrations and blocking it at high concentrations. In contrast, menthol is thought to solely be an agonist of the human TRPA1, where it displays robust activity at concentrations which in the rat would have an opposite effect (Bianchi et al. 2012; Chen et al. 2013). It is important to note that any inter-species differences in menthol sensitivity that may be attributable to differences between human and rat TRPA1 would likely only affect part of the overall TN response to menthol, since the relative contribution of the prototypical menthol receptor, TRPM8, would likely factor significantly. While the extent of potential inter-species differences for TRPM8 are not fully clear, it is worth noting the differences observed in sequence homology among mammalian TRPA1 and TRPM8 channels: whereas human and rodent TRPA1 are only 79 % identical, TRPM8 sequence homology is much more similar between humans and rodents, at 94 % (Chen and Kym 2009). In regard to the present in vivo observations, it is also plausible that the psychophysical response to menthol, in particular, may have been heightened by subject experience with this widely used and commonly encountered component of toothpastes, chewing gums, cosmetics, ointments, and medications.
Other inter-species differences became apparent when human psychophysical response was plotted as a function of rat nerve response for the 19 select stimuli (Fig. 2). The resulting sigmoidal relationship revealed two clusters that highlight potential inter-species nuances in TN sensitivity. The first cluster, comprised of a set of stimuli which elicited strong human responses of nearly equal magnitude, was found to be distributed over a relatively large range of rat nerve response. A plausible interpretation of this observation may be that the rat ethmoid nerve can generate differing levels of response to certain compounds which generate invariably strong psychophysical responses in humans. This would imply that rats may potentially be finer-tuned to certain compounds such as benzaldehyde, methanol, propionic acid, and butyric acid. The second cluster, corresponding to stimuli which elicited nearly invariable nerve response magnitudes in rats, was conversely distributed over a widely variable range of psychophysical response in human subjects, implying that human nasal chemesthesis is better able to distinguish between certain compounds such as menthol, phenethyl alcohol, α-terpineol, and heptanol. If such differences in acuity do actually exist, they would most likely be based on differential expression of TRP and other receptor channels, resulting from adaptive evolutionary divergences.
Beyond these potential inter-species differences in acuity to specific stimuli, other characteristics of human and rat nasal chemesthetic response were found to be strikingly similar. As for structure-activity assessments of stimulus compounds, lipid solubility was found to be an equally important determinant of stimulus potency in both species, as expected. More lipid soluble molecules, as determined by carbon chain length, generally exhibited lower response thresholds in humans and rats (Fig. 3). This was to be expected, as lipid solubility is known to be related to the effectiveness or potency of many trigeminal stimuli: the more lipid-soluble a compound is, the more easily it can penetrate the epithelial cell membranes and tight junctions which form the barrier between intraepithelial TN fibers and incoming stimuli (Silver 1992).
Humans and rats displayed similar threshold responses to acids and ketones at overlapping stimulus concentrations, suggesting nearly equal chemesthetic sensitivities to the compounds tested. Although rats displayed an overall lower threshold of response to alcohols, suggestive of a potentially higher level of sensitivity (Fig. 3), it is not entirely clear from these data whether or not the noted differences are statistically or biologically significant. Despite the apparent difference, a positive correlation was found between human and rat response thresholds for the homologous series of alcohols tested (Fig. 4), further suggesting lipid solubility to be an equally important determinant of stimulus potency in both species.
As for specific mechanisms of action, inter-species similarities were demonstrated by neural and psychophysical chemesthetic responses to nicotine and carbon dioxide. For nicotine, comparable data from studies utilizing mecamylamine hydrochloride showed clear and significant effects across three different experimental approaches (Fig. 5). The observed reductions in chemesthetic responses to nicotine by this selective neuronal nAChR blocker strongly suggest a shared receptor mechanism in both species involving a functional role for neuronal nAChRs in TN-mediated chemesthesis (Alimohammadi and Silver 2000; Thuerauf et al. 2006).
Mechanistic similarities were also noted between humans and rats in the chemesthetic sensitivity to carbon dioxide, although there were no directly comparable in vivo studies strictly addressing the effect of acetazolamide on nasal chemesthetic response. Acetazolamide is a commonly prescribed sulfonamide derivative used in the treatment of glaucoma, mountain sickness, and certain types of epilepsy and is believed to derive its beneficial effects through its inhibitory effect on carbonic anhydrase, the ubiquitously expressed enzyme which catalyzes the conversion of carbon dioxide to carbonic acid (Hoddevik 2000). Anecdotal reports of side effects associated with acetazolamide therapy commonly include altered taste effects describing the “flat” taste of carbonated beverages (Beck 2007; Martínez-Mir et al. 1997; McMurdo et al. 1990; Graber and Kelleher 1988) which long hinted at a possible role for carbonic anhydrase in the chemesthetic response to carbon dioxide.
Indeed, human studies have shown acetazolamide treatment of the tongue to significantly reduce the sensory perception elicited by carbonated water (Dessirier et al. 2000). In our studies on rats, a significant inhibition of nasal TN response to carbon dioxide by acetazolamide was shown to occur quickly after systemic administration, reaching full effect within 5 min (Fig. 6). The similar effects of acetazolamide on human and rat chemosensitivity to carbonated water and inhaled carbon dioxide suggest a shared enzymatic pathway involving carbonic-anhydrase mediated tissue acidification. It is now understood that high concentrations of carbon dioxide elicit chemesthetic response after diffusion across nociceptor cell membranes, resulting in cytosolic acidification and subsequent activation of TRPA1 (Wang et al. 2010, 2011). This acidification step is presumably similarly mediated by carbonic anhydrase in humans and rats: human studies have documented transient nasal mucosal acidification during phasic carbon dioxide stimulation (Shusterman and Avila 2003), and carbonic anhydrase has been shown to be present in the nasal mucosa of both humans and rats (Tarun et al. 2003; Coates 2001).
Overall, the similarities between humans and rats observed in the available studies were not completely unexpected, considering the primitive and adaptive nature of the chemesthetic sense. Nasal chemesthesis is primarily mediated by TRP and other ion channels expressed on nociceptive fibers originating from the primary sensory neurons of the TG. Functionally, these fibers fall in the same category as those originating from spinal dorsal root ganglia (DRG), with one notable exception: whereas mammalian DRG fibers innervate body areas normally protected from environmental exposure by a cornified epithelium, TG fibers are in regular communication with the external environment via innervation of the airways, cornea, and oronasal cavities. TG nociceptors have thus adapted primitive chemosensory mechanisms, used in the detection of endogenous signals of tissue damage, to function as part of a derived environmental chemosensory system which serves to guard the most sensitive tissues of the airways and face against prolonged exposure to hazardous exogenous factors. The adaptive advantage of such a specialized chemosensory system is clear when considering that the airways and peripheral components of the visual, olfactory, and gustatory systems are in a routine and constant state of exposure to the external environment. The functional roles that vision, olfaction, and gustation play in exploratory and feeding behaviors place the peripheral components of these sensory systems at an elevated level of risk of exposure to potentially dangerous compounds, many of which potently activate the TN and trigger tissue-defensive responses. It is therefore not surprising to observe conserved patterns of TN chemosensitivity across mammalian orders and species.
References
Alimohammadi H, Silver WL (2000) Evidence for nicotinic acetylcholine receptors on nasal trigeminal nerve endings of the rat. Chem Senses 25:61–66
Barham HP, Cooper SE, Anderson CB, Tizzano M, Kingdom TT, Finger TE, Kinnamon SC, Ramakrishnan VR (2013) Solitary chemosensory cells and bitter taste receptor signaling in human sinonasal mucosa. Int Forum Allergy Rhinol 3(6):450–457
Beck KC (2007) Flat beer vs. physiological improvement: effect of acetazolamide during hypoxic exercise. J Physiol 579(Pt 3):568
Bianchi BR, Zhang X-F, Reilly RM, Kym PR, Yao BB, Chen J (2012) Species comparison and pharmacological characterization of human, monkey, rat, and mouse TRPA1 channels. J Pharmacol Exp Ther 341:360–368
Bressler SL (2002) Event-related potentials. In: Arbib MA (ed) The handbook of brain theory and neural networks. MIT Press, Cambridge PA, pp 412–415
Bryant BP, Silver WL (2000) Chemesthesis: the common chemical sense. In: Finger TE, Silver W, Restrepo D (eds) The neurobiology of taste and smell, 2nd edn. Wiley-Liss, New York NY, pp 73–100
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389(6653):816–824
Chen J, Kym PR (2009) TRPA1: the species difference. J Gen Physiol 133(6):623–625
Chen J, Kang D, Xu J, Lake M, Hogan JO, Sun C, Walter K, Yao B, Kim D (2013) Species differences and molecular determinant of TRPA1 cold sensitivity. Nat Commun 4:2501
Coates EL (2001) Olfactory CO2 chemoreceptors. Respir Physiol 129:219–229
Cometto-Muñiz JE, Cain WS (1990) Thresholds for odor and nasal pungency. Physiol Behav 48:719–725
Cometto-Muñiz JE, Cain WS (1993) Efficacy of volatile organic compounds in evoking nasal pungency and odor. Arch Environ Health 48:309–314
Cometto-Muñiz JE, Cain WS, Abraham MH (1998) Nasal pungency and odor of homologous aldehydes and carboxylic acids. Exp Brain Res 118:180–188
Dalton P (2001) Psychophysical methods in the study of olfaction and respiratory tract irritation. AIHAJ 62(6):705–710
Dessirier JM, Simons CT, Carstens MI, O’Mahony M, Carstens E (2000) Psychophysical and neurobiological evidence that the oral sensation elicited by carbonated water is of chemogenic origin. Chem Senses 25:277–284
Doty RL (1995) Intranasal trigeminal chemoreception: anatomy, physiology, and psychophysics. In: Doty RL (ed) Handbook of olfaction and gustation. Marcel Dekker, Inc., New York NY, pp 821–833
Doty RL, Brugger WPE, Jurs PC, Orndorff MA, Snyder PJ, Lowry LD (1978) Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol Behav 20:175–185
Finger TE, St Jeor VL, Kinnamon JC, Silver WL (1990) Ultrastructure of substance P- and CGRP-immunoreactive nerve fibers in the nasal epithelium of rodents. J Comp Neurol 294(2):293–305
Finger TE, Böttger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL (2003) Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci U S A 100(15):8981–8986
Frasnelli J, Hummel T (2003) Age-related decline of intranasal trigeminal sensitivity: is it a peripheral event? Brain Res 987:201–206
Frasnelli J, Lötsch J, Hummel T (2003) Event-related potentials to intranasal trigeminal stimuli change in relation to stimulus concentration and stimulus duration. J Clin Neurophysiol 20:80–86
Graber M, Kelleher S (1988) Side effects of acetazolamide: the champagne blues. Am J Med 84(5):979–980
Green BG (1996) Chemesthesis: pungency as a component of flavor. Trends Food Sci Technol 7:415–420
Green BG, Mason JR, Kare MR (1990) Chemical senses, vol 2: Irritation. Marcel Dekker, New York NY
Harteneck C, Plant TD, Schultz G (2000) From worm to man: three subfamilies of TRP channels. Trends Neurosci 23:159–166
Hoddevik GH (2000) Acetazolamide—are there reasons for its revival in antiepileptic treatment? Tidsskr Nor Laegeforen 120(9):1042-–1045
Holzer P (1988) Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience 24:739–768
Keele CA (1962) The common chemical sense and its receptors. Arch Int Pharmacodyn 139:547–557
Keverne EB, Murphy CL, Silver WL, Wysocki CJ, Meredith M (1986) Non-olfactory chemoreceptors of the nose: recent advances in understanding the vomeronasal and trigeminal systems. Chem Senses 11(1):119–133
Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K (2005) Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol 493(4):596–606
Major DA, Silver WL (1999) Odorants presented to the rat nasal cavity increase cortical blood flow. Chem Senses 24(6):665–669
Martínez-Mir I, Navarro Badenes J, Palop Larrea V (1997) Taste disturbance with acetazolamide. Ann Pharmacother 31:373
McMurdo ME, Hutchison GL, Lindsay G (1990) Taste disturbance with acetazolamide. Lancet 336(8724):1190–1191
Panneton WM, Gan Q, Sun DW (2012) Persistence of the nasotrigeminal reflex after pontomedullary transection. Respir Physiol Neurobiol 180:230–236
Roper SD (2014) TRPs in taste and chemesthesis. In: Nilius B, Flockerzi V (eds) Mammalian transient receptor potential (TRP) cation channels. Handbook of Experimental Pharmacology. Springer International Publishing, Switzerland, pp 827–871
Saunders CJ, Christensen M, Finger TE, Tizzano M (2014) Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc Natl Acad Sci U S A 111(16):6075–6080
Scheibe M, Zahnert T, Hummel T (2006) Topographical differences in the trigeminal sensitivity of the human nasal mucosa. Neuroreport 17:1417–1420
Scheibe M, van Thriel C, Hummel T (2008) Responses to trigeminal irritants at different locations of the human nasal mucosa. Laryngoscope 118(1):152–155
Sekizawa S-I, Tsubone H (1994) Nasal receptors responding to noxious chemical irritants. Respir Physiol 96:37–48
Shusterman D, Avila PC (2003) Real-time monitoring of nasal mucosal pH during carbon dioxide stimulation: implications for stimulus dynamics. Chem Senses 28(7):595–601
Silver WL (1988) Chemoreception by free nerve endings. In: Miller IJ (Ed.) The Beidler Symposium on Taste and Smell: A Festschrift for L.M. Beidler. Book Services Associates, Winston-Salem NC pp.115-125
Silver WL (1990) Physiological factors in nasal trigeminal chemoreception. In: Green BG, Mason JR, Kare MR (eds) Chemical senses, vol 2, Irritation. Marcell Dekker, New York NY, pp 21–41
Silver WL (1992) Neural and pharmacological basis for nasal irritation. Ann NY Acad Sci 641:152–163
Silver WL, Finger TE (2009) The anatomical and electrophysiological basis of peripheral nasal trigeminal chemoreception. Ann NY Acad Sci 1170:202–205
Silver WL, Moulton DG (1982) Chemosensitivity of rat nasal trigeminal receptors. Physiol Behav 28:927–931
Silver WL, Mason JR, Adams MA, Smeraski CA (1986) Nasal trigeminal chemoreception: responses to n-aliphatic alcohols. Brain Res 376:221–229
Szolcsányi J (1996) Capsaicin-sensitive sensory nerve terminals with local and systemic efferent functions: facts and scopes of an unorthodox neuroregulatory mechanism. Prog Brain Res 113:343–359
Szolcsányi J (2014) Capsaicin and sensory neurones: a historical perspective. Prog Drug Res 68:1–37
Tarun AS, Bryant B, Zhai W, Solomon C, Shusterman D (2003) Gene expression for carbonic anhydrase isoenzymes in human nasal mucosa. Chem Senses 28(7):621–629
Thuerauf N, Markovic K, Braun G, Bleich S, Reulbach U, Kornhuber J, Lunkenheimer J (2006) The influence of mecamylamine on trigeminal and olfactory chemoreception of nicotine. Neuropsychopharmacol 31:450–461
Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill MEA, Silver WL, Kinnamon SC, Finger TE (2010) Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci U S A 107:3210–3215
Venkatachalam K, Luo J, Montell C (2014) Evolutionarily conserved, multitasking TRP channels: lessons from worms and flies. Handb Exp Pharmacol 223:937–962
Viana F (2011) Chemosensory properties of the trigeminal system. ACS Chem Neurosci 2:38–50
Walker JC, Kendal-Reed M, Utell MJ, Cain WS (2001) Human breathing and eye blink rate responses to airborne chemicals. Environ Health Perspect 109:507–512
Wang YY, Chang RB, Liman ER (2010) TRPA1 is a component of the nociceptive response to CO2. J Neurosci 30:12958–12963
Wang YY, Chang RB, Allgood SD, Silver WL, Liman ER (2011) A TRPA1-dependent mechanism for the pungent sensation of weak acids. J Gen Physiol 137:493–505
Compliance with Ethics Requirements
This work was supported by funds from the Department of Biology, Wake Forest University.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of Interest
Wayne Silver declares that he has no conflict of interest.
Hessam Alimohammadi declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alimohammadi, H., Silver, W.L. Nasal Chemesthesis: Similarities Between Humans and Rats Observed in In Vivo Experiments. Chem. Percept. 8, 85–95 (2015). https://doi.org/10.1007/s12078-015-9189-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12078-015-9189-4