Abstract
Background & aims
Few studies have investigated the prognosis of patients with non-severe alcoholic hepatitis (Non-SAH). The study aimed to develop a new prognostic model for patients with especially Non-SAH.
Methods
We extracted 316 hospitalized patients with alcoholic cirrhosis without severe alcoholic hepatitis, defined as Maddrey’s discriminant function score lower than 32, from the retrospective Korean Acute-on-Chronic Liver Failure (KACLiF) cohort to develop a new prognostic model (training set), and validated it in 419 patients from the prospective KACLiF cohort (validation set). Prognostic factors for death and liver transplantation were analyzed to construct a prognostic model.
Results
Twenty-one and 24 patients died within 6 months in both sets, respectively. In the training set, the highest area under the curve (AUC) of conventional prognostic models was 0.765, 0.732, and 0.684 for 1-, 3-, and 6-month mortality, respectively. Refractory ascites, vasopressor use, and hyponatremia were independently associated with mortality of cirrhotic patients with Non-SAH. The new model consisted of four variables: past deterioration, neutrophil proportion > 70%, Na < 128 mmol/L, and vasopressor use. It showed the highest accuracy for short-term mortality in the training and validation sets (0.803 and 0.786; 0.797 and 0.776; and 0.789 and 0.721 for 1-, 3-, and 6-month mortality, respectively).
Conclusion
There is a group of patients with high risk among those classified as Non-SAH. The new model will help stratifying cirrhotic patients with Non-SAH more accurately in terms of prognosis. The patients with high Non-SAH score need to monitor closely and might be considered for preemptive liver transplantation.
Trial regestration
ClinicalTrials.gov identifier: NCT02650011.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alcohol-related liver disease (ALD) is one of the main causes of chronic liver disease including liver cirrhosis (LC) and liver cancer. Unlike chronic viral hepatitis, which involves established treatments such as antivirals, ALD became the most common etiology of liver transplantation (LT) owing to the lack of reliable treatments [1]. Therefore, the one-month mortality rate of severe ALD reaches 50% or more, and it is necessary to preemptively prepare LT. Thus, many studies have attempted to differentiate severe alcohol-related liver disease and develop prognostic models including the modified Maddrey’s Discriminant Function (mDF), Age-Bilirubin-INR-Creatinine (ABIC) score, Glasgow Alcoholic Hepatitis Score (GAHS), and Lille model. For patients with cirrhosis, Child–Pugh, Model for End-stage Liver Disease (MELD), and albumin-bilirubin (ALBI) scores were clinically used to stratify severity of liver disease. Among them, mDF has been widely used, and if its score is 32 or higher, clinicians should consider steroid treatment and LT [2].
However, recent studies have shown that patients with non-severe alcoholic hepatitis (Non-SAH) with mDF score of <32 could die in a short duration. In a meta-analysis [3], 6%, 7%, and 13% of patients with non-severe alcoholic hepatitis died within 28 days, 90 days, and 1 year, respectively. More than half of the deaths within one year occurred within a few months. In addition, alcohol abstinence influences the long-term prognosis, while the degree of liver injury affects the short-term prognosis of patients with severe alcoholic hepatitis [4]. However, few studies have been conducted on patients with Non-SAH.
Thus, it is necessary to evaluate the predictors for the short-term prognosis of patients with Non-SAH and to identify high-risk patients using these predictors.
Materials and methods
Study population
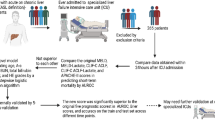
This multicenter observational study, the Korean Acute-on-Chronic Liver Failure (KACLiF) study, had two cohorts. The retrospective cohort consisted of 1,470 patients who were admitted to the liver unit due to acute deterioration of either chronic liver disease or LC between January 2013 and December 2013 at 21 referral hospitals in Korea. Patients who met any of the following criteria were excluded: (1) age < 19 years, (2) presence of hepatocellular carcinoma, (3) severe chronic extrahepatic disease, (4) admission due to other chronic illness, and (5) human immunodeficiency virus infection [5]. Their survival data were collected until September 2015. The prospective cohort consecutively enrolled 1498 patients who were hospitalized with the same criteria as the retrospective cohort for the first time from October 2015 to December 2018 at 31 referral hospitals in Korea [6].
We extracted patients with active alcoholism and excluded those with mDF scores ≥32 and missing laboratory finding in each cohort (Fig. 1). To reduce the heterogeneity of cohorts, we then included patients with alcoholic cirrhosis and without viral hepatitis from the retrospective cohort as a training set, and those from the prospective cohort as a validation set.
Definitions
We collected demographic and clinical data, and laboratory results such as age, sex, liver function, past deterioration, vital signs at admission, alcohol intake, precipitants of acute deterioration, and all components of organ failure from the patients in the two cohorts. Serum C-reactive protein (CRP) and lactate levels were collected in the prospective cohort, but there were considerable missing data. In the KACLiF studies, acute deterioration was defined as having one or more conditions, such as overt ascites, hepatic encephalopathy (HE), gastrointestinal bleeding (including variceal bleeding), bacterial infection, and hyperbilirubinemia (serum bilirubin ≥3 mg/dL) within four weeks prior to admission [7]. LC was defined based on liver’s histologic confirmation or radiological features on ultrasonography or computed tomography scans (e.g., undulating liver surface, compensatory left lobe hypertrophy, splenomegaly, ascites, and portosystemic shunts) [8]. Drinking an average of >40 g/day in men and >20 g/day in women within 3 months prior to admission was considered active alcoholism [9]. ALD with mDF score of ≥32 was considered as SAH, and the opposite case was defined as Non-SAH [2]. Thus, we defined alcoholic cirrhosis with mDF score of <32, which means “without severe alcoholic hepatitis”, as ALC-nSAH. Chronic viral hepatitis indicated the comorbidity of chronic hepatitis B or C infection. Systemic inflammatory response syndrome (SIRS) was evaluated using the four signs of the American College of Chest Physicians/Society of Critical Care Medicine [10]. Refractory ascites was indicated when it was uncontrollable even with high-dose diuretics or intractable due to diuretic-induced complications, requiring repeated paracentesis [8]. Hepatic failure as a death cause was decided based on the definition of acute on chronic liver failure (ACLF) of Asian Pacific Association for the Study of the Liver [11]. The prognostic models, such as Child–Pugh, MELD, and mDF scores, were assessed based on clinical variables within 24 hours of admission.
Study endpoints
The primary endpoint was overall survival for up to 6 months. In addition, we analyzed the associated variables and constructed a new prognostic model. The secondary endpoint was to compare the predictive performances of the prognostic models for mortality and LT within 6 months.
Statistical analyses
Statistical analyses were performed using the R software (version 4.1.2; http://cran.r-project.org/). Statistical significance was defined as p <0.05.
Continuous variables were expressed as mean ± standard deviation or median (interquartile range [IQR]) according to the normal distribution, and those were compared using Student’s t-test or the Mann–Whitney U test. Categorical variables are presented as numbers (%) and were compared using the chi-square test.
The cumulative overall survival was estimated using the Kaplan–Meier method, and the differences between the groups were assessed using the log-rank test. Patients lost to follow-up were censored on the date of their occurrence. A Cox proportional hazard regression model was established to analyze the factors associated with the primary outcome. and all variables were subjected to multivariable analyses with backward stepwise elimination to construct a new prognostic model, whereas only variables with p < 0.05 in univariate analyses were included in the multivariable analysis to identify independent risk factors. Only the original variables (not composite models such as mDF, MELD, and so on) were included in the multivariable analyses to avoid multicollinearity. For easy use of the new model, continuous variables were converted to categorical variables by cutoffs settled according to the distribution and receiver operating characteristic (ROC) of variables in the training set.
The accuracy of the prognostic models was evaluated using time-varying ROC curves, and the area under the curve (AUROC) was calculated. DeLong’s test was used to assess the statistical differences between the ROC curves. In addition, the concordance between the prognostic models was calculated and compared using Harrell’s method.
Results
Patient characteristics
As 1154 and 1079 patients were excluded from the retrospective and prospective cohorts, respectively, 316 and 419 patients were enrolled in the training and validation sets for the present study, respectively (Fig. 1). The median ages were 52.9 and 55.0 years, and males were predominant (85.4% and 85.4%) in the training and validation sets, respectively. The main features of acute deterioration are ascites, jaundice, and gastrointestinal bleeding. There were different in the presence of ascites, serum albumin level, and albumin-bilirubin (ALBI) scores between two sets (Table 1).
Overall survival
During the follow-up of 6 months, 21 and 24 patients died in the training and validation sets, respectively, but none received LT. The causes of death were hepatic failure (29% and 32%), variceal bleeding (29% and 16%), sepsis (24% and 4%), and hepatorenal syndrome (5% and 16%) in the training and validation sets, respectively.
Overall survival was not significantly different between the training and validation sets (96.5% vs. 98.4%, 94.8% vs. 95.1%, and 92.9% vs. 92.8% at 1-, 3-, and 6-month between training and validation sets, p = 0.940; Supplementary Figure 1). In the training set, refractory ascites (adjusted hazard ratio [aHR] = 4.17), vasopressor use (aHR = 5.02), and serum sodium level (aHR = 0.92) were independent risk factors for 6-month overall survival in the Cox regression model (Supplementary Table 1).
Predictive performance of conventional prognostic models
Time-varying ROC curves of the conventional models showed fair predictive performances for 6-month mortality in the order of MELD 3.0, ALBI, and GAHS scores (AUROCs of 0.684, 0.651, and 0.642) at 6-months, respectively). These performances were not poor, but were sharply reduced in the validation sets (0.637, 0.545, and 0.505, respectively; Table 2). Owing to these limitations, we attempted to establish a new prognostic model for patients with ALC-nSAH.
Development and performance of the new prognostic model
Using backward stepwise selection, we constructed a new model, namely the Non-SAH score, with four binary variables including neutrophil proportion, serum sodium level, vasopressor use, and past acute deterioration experience for the 6-month survival (Table 3). These factors were also significantly associated with 3- and 6- month survival in the logistic regression analyses (Supplementary Table 2). Patients with these factors showed significantly higher short-term mortality rates than those factors without in the training set and even in the validation set (Supplementary Figure 2). Non-SAH scores were simplified to an integer scoring system with the sum of each score, ranging from 0 to 4.
Applying the Non-SAH score to the training set, the time-varying AUROC values for 1-, 3-, and 6- month mortalities were 0.803, 0.797, and 0.789, respectively, which were the highest compared with those of the conventional models. The superiority of this model was also maintained in the validation set, and the AUROCs for 1-, 3-, and 6-month mortality were 0.786, 0.776, and 0.721, respectively. Although the superiority of the new model was not statistically significant compared to some conventional prognostic models, it showed the highest predictive performance throughout 1-, 3-, and 6-month in the validation set (Table 2).
In addition, the concordance of the Non-SAH score was higher than that of other prognostic models and was significantly different in both sets (0.775 and 0.716 in the training or validation sets, respectively; p < 0.05 when comparing with other models), except for MELD 3.0 score in the validation set (Table 4).
Three risk groups for mortality stratification
The Non-SAH scores, ranged 0–4, were stratified into three categories according to each probability of death: 0–2, 3, and 4. The cumulative probabilities of overall survival for three categories were 97.1%, 77.4%, and 65.6% of overall survival for 6-month in patients with the Non-SAH score of 0–2, 3, and 4 in the training set, respectively, and 95.5%, 88.4%, and 34.6% in the validation set, respectively. The risk of the three categories was significantly stratified in both sets (p < 0.001, Figure 2).
Discussion
To date, there are no established standard treatments for alcohol-related liver disease other than abstinence, steroid treatment, and LT [12]. Therefore, it is important to select and monitor high-risk patients early, and conventional prognostic models have been used as criteria for this selection and aggressive treatment, such as steroid administration and LT. However, conventional models generally select patients with severely compromised. In practice, even in patients excluded from conventional selections, a substantial number of patients die in the short term, [3, 13]; therefore, it is necessary to screen them for close monitoring to prevent further progression. In the present study, even in patients with ALC-nSAH classified by the mDF score, more than 50% had ascites. In addition, more than 60% of deaths within 6 months were directly attributed to liver-related causes. Thus, patients with ALC-nSAH are not free from short-term progression or mortality. Therefore, the mDF score is not a reliable scale to set a cutoff value for constructing a short-term prognostic score, given that most patients with ALC-nSAH that meet the criteria are eventually going to die within the 6 months with a fairly high probability.
In the present study, vasopressor use, refractory ascites, and hyponatremia were independent prognostic factors for short-term mortality. These results were different from those of a previous study of patients with decompensated alcoholic steatohepatitis, which presented HE at baseline and lack of alcohol abstinence as prognostic factors [13]. This difference is attributed to the differences in the characteristics of the study subjects, variables investigated, and follow-up period. Degré et al. [13] recruited subjects who were diagnosed with alcoholic steatohepatitis by biopsy and who had more HE (32%), respectively, than those in the present study. In addition, the study focused on long-term survival of up to 5 years and investigated alcohol abstinence, but the present study explored short-term survival of up to 6 months and inspected organ failures at baseline instead of abstinence. Vasopressor use reflects an unstable hemodynamic status, which can be followed by ischemic organ injuries. In addition, it is an important factor in circulatory dysfunction in ACLF [14]. Accompanying ascites in patients with chronic liver disease reduces the 1- and 2-year survival rates by 30–50% [15]. Ascites is not only a component of the Child–Pugh score but also a prognostic predictor of cirrhotic patients independent of the MELD score [16]. Hyponatremia in LC is caused by solute-free water retention or diuretic use, which makes it difficult to control ascites with diuretics and acts as a precipitant for HE. Based on these mechanisms, it has been proven to be a significant predictor of poor prognosis in many studies on patients with LC and ACLF; therefore, the serum sodium level was incorporated into the MELD score. [17, 18]
Based on these factors, the new prognostic model included the blood neutrophil proportion, serum sodium level, and the presence of past acute deterioration. The Non-SAH score targeted cohorts that excluded patients with severe liver function impairment based on the mDF score using bilirubin and INR. Thus, it consisted of systemic inflammation and extrahepatic findings that accelerated deterioration rather than factors associated with liver function.
Systemic inflammation is one of the main mechanisms for underlying LC progression [19]. Claria, et al. showed that systemic inflammation is strongly related to the severity of ACLF and short-term mortality in patients with ACLF by measuring the levels of various cytokines. In addition, the relationship was stronger than that between systemic circulatory dysfunction and ACLF [20]. Neutrophil proportion are representative markers of systemic inflammation. The neutrophil-to-lymphocyte ratio (NLR) has been proven to be a prognostic marker in various liver diseases, including alcoholic hepatitis, ACLF, and decompensated LC without ACLF [21,22,23,24]. These studies showed that a high NLR is independently associated with high mortality. Considering that NLR is a ratio of neutrophils and lymphocytes and that neutrophils and lymphocytes make up the majority of WBC, the neutrophil proportion used in the present study could replace NLR.
Acute decompensation in patients with chronic liver disease reduces the underlying hepatic reserve, leading to end-stage liver disease. The PREDICT study also showed that patients with unstable decompensated LC, who were readmitted due to acute decompensation, had higher mortality, bacterial infections, and complications of portal hypertension than those with stable decompensated cirrhosis [25]. A previous study by our group showed that past acute deterioration significantly increased long-term mortality (HR = 1.62) in patients with ALC-nSAH [7].
Based on the systemic inflammation–organ failure framework, we developed a model with high statistical accuracy to predict short-term mortality in hospitalized patients with ALC-nSAH. The concordance index of the Non-SAH score in the validation set was lower than that in the training set but the highest than that of the other 7 conventional prognostic models. In addition, these conventional scores have some limitations. The Child-Pugh score has subjective components and has a lump sum scoring method. The MELD score series have the disadvantage that creatinine, a component of those models, is influenced by various circumstances and the calculation of scores is very complicated. However, the components of the Non-SAH score are only four objective binary factors, so can be simply applied and summed. The Non-SAH score will help distinguish high-risk patients with a 6-month mortality of over a third from those with a 6-month mortality just below 5% and enable individually tailored therapy in patients with ALD.
Despite these meaningful findings, the present study had several limitations. First, we used a retrospective observational cohort for the training set; therefore, we inevitably faced bias and confounding factors. To address this issue, we conducted subgroup and multivariable analyses, and validated the Non-SAH score in a separate prospective cohort. Due to the small number of outcomes, many parameters were applied in analyses but did not show statistical significance (data not shown). Second, due to missing data on several variables, we had to use only blood WBC count and neutrophil proportion as inflammatory markers, not the CRP or NLR, which are more specific markers. However, CRP is influenced by the liver and may not be objective in studies on liver disease. Third, because two cohorts were not alcohol-specific, many factors that should be considered in ALD, such as drinking behavior, prior attempts to stop drinking, and household income, were not included. We look forward to apply these factors in a well-designed future study.
In conclusion, using the KACLiF cohorts, we constructed a new, evidence-based, and simple model to stratify the risk of short-term mortality in patients with ALC-nSAH who were hospitalized due to acute deterioration. The Non-SAH score significantly improved the predictive ability compared to other conventional models including mDF, Child–Pugh, and MELD scores in both the training and validation sets. These findings warrant further validation in a large cohort of patients with Non-SAH but without cirrhosis.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due personal medical records, but are available from the corresponding author on reasonable request and approval of IRB.
References
Wong RJ, Singal AK. Trends in lliver ddisease eetiology aamong aadults aawaiting lliver ttransplantationliver disease etiology among adults awaiting liver transplantation in the United States, 2014–2019. JAMA Netw Open. 2020;3(2): e1920294
European Association for the Study of the Liver (EASL). EASL clinical practice guidelines: management of alcohol-related liver disease. J Hepatol. 2018;69(1):154–181
Bennett K, Enki DG, Thursz M, Cramp ME, Dhanda AD. Systematic review with meta-analysis: high mortality in patients with non-severe alcoholic hepatitis. Aliment Pharmacol Ther. 2019;50:249–257
Louvet A, Labreuche J, Artru F, et al. Main drivers of outcome differ between short term and long term in severe alcoholic hepatitis: a prospective study. Hepatol. 2017;66:1464–1473
Shin J, Yu JH, Jin Y-J, et al. Acute-on-chronic liver failure as a major predictive factor for mortality in patients with variceal bleeding. Clin Mol Hepatol. 2020;26:540–553
Kim JH, Kim SE, Song DS, et al. Platelet-to-white blood cell ratio is associated with adverse outcomes in cirrhotic patients with acute deterioration. J Clin Med. 2022;11:2463
Yoon EL, Kim TY, Song DS, et al. The impact of previous acute decompensation on the long-term prognosis of alcoholic hepatitis in cirrhotic patients. J Clin Med. 2019;8:1600
Kim TH, Um SH, Lee YS, et al. Determinants of re-compensation in patients with hepatitis B virus-related decompensated cirrhosis starting antiviral therapy. Aliment Pharmacol Ther. 2022;55:83–96
Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines: management of alcoholic liver disease. Clin Mol Hepatol. 2013;19:216–254
Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655
Sarin SK, Choudhury A, Sharma MK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353–390
Tornai D, Szabo G. Emerging medical therapies for severe alcoholic hepatitis. Clin Mol Hepatol. 2020;26:686–696
Degré D, Stauber RE, Englebert G, et al. Long-term outcomes in patients with decompensated alcohol-related liver disease, steatohepatitis and Maddrey’s discriminant function <32. J Hepatol. 2020;72:636–642
Moreau R. Acute-on-chronic liver failure: a new syndrome in cirrhosis. Clin Mol Hepatol. 2016;22:1–6
Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for liver cirrhosis: ascites and related complications. Clin Mol Hepatol. 2018;24:230–277
Kim TH, Ku DH, Um SH, et al. How can we improve the performance of model for end-stage liver disease sodium score in patients with hepatitis B virus-related decompensated liver cirrhosis commencing antiviral treatment? J Gastroenterol Hepatol. 2018;33:1641–1648
Kim WR, Mannalithara A, Heimbach JK, et al. MELD 3.0: the model for end-stage liver disease updated for the Modern Era. Gastroenterology. 2021;161:1887–1895
Cárdenas A, Solà E, Rodríguez E, et al. Hyponatremia influences the outcome of patients with acute-on-chronic liver failure: an analysis of the CANonIC study. Crit Care. 2014;18:700
Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359–1376
Clària J, Stauber RE, Coenraad MJ, et al. Systemic inflammation in decompensated cirrhosis: characterization and role in acute-on-chronic liver failure. Hepatol. 2016;64:1249–1264
Forrest EH, Storey N, Sinha R, et al. Baseline neutrophil-to-lymphocyte ratio predicts response to corticosteroids and is associated with infection and renal dysfunction in alcoholic hepatitis. Aliment Pharmacol Ther. 2019;50:442–453
Chen L, Lou Y, Chen Y, Yang J. Prognostic value of the neutrophil-to-lymphocyte ratio in patients with acute-on-chronic liver failure. Int J Clin Pract. 2014;68:1034–1040
Cai YJ, Dong JJ, Dong JZ, et al. A nomogram for predicting prognostic value of inflammatory response biomarkers in decompensated cirrhotic patients without acute-on-chronic liver failure. Aliment Pharmacol Ther. 2017;45:1413–1426
Sun J, Guo H, Yu X, et al. A neutrophil-to-lymphocyte ratio-based prognostic model to predict mortality in patients with HBV-related acute-on-chronic liver failure. BMC Gastroenterol. 2021;21:422
Trebicka J, Fernandez J, Papp M, et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73:842–854
Acknowledgments
We thank the Collaborators of the Korean Acute-on-Chronic Liver Failure (KACLiF) study group, and Editage (www.editage.co.kr) for English language editing.
Funding
This work was supported partly by Korea University Research Grants (No. K2119231) and partly by Basic Science Research Program (National Research Foundation of Korea), No. 2020R1A6A1A03043026.
Author information
Authors and Affiliations
Consortia
Contributions
Study conception and design: Yim HJ, Kim DJ; Acquisition of data: Yoon EL, Kang SH, Kim HY, Chang Y, Lee SW, Yoo JJ, Baek GJ, Park JK, Kim TY, Song DS, Park JW, Kim SE, Jeong SW, Suk KT, Jung YK, Kim MY, Kim SG, Jang JY, Kim W and Yang JM.; Data analysis and interpretation: Kim TH and Yim HJ.; Draft writing: Kim TH.; Review and final approval of the submission of the manuscript: Yim HJ, Jang JY and Kim DJ.
Corresponding authors
Ethics declarations
Conflict of interest
The authors Tae Hyung Kim, Hyung Joon Yim, Young Kul Jung, Do Seon Song, Eileen L. Yoon, Hee Yeon Kim, Seong Hee Kang, Young Chang, Jeong-Ju Yoo, Baek Gyu Jun, Sung Won Lee, Jung Gil Park, Ji Won Park, Sung-Eun Kim, Tae Yeob Kim, Soung Won Jeong, Ki Tae Suk, Moon Young Kim, Sang Gyune Kim, Won Kim, Jae Young Jang, Jin Mo Yang, Dong Joon Kim have no relevant financial or non-financial interests to disclose.
Ethical approval
The present study was approved by the institutional review board of each participating center (approval nos. 2014AS0123 and 2015AS0090 from the center of the corresponding author). All authors had access to the study data and had reviewed and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, T.H., Yim, H.J., Jung, Y.K. et al. New prognostic model for hospitalized patients with alcoholic cirrhosis and Maddrey’s discriminant function <32. Hepatol Int 18, 500–508 (2024). https://doi.org/10.1007/s12072-023-10582-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-023-10582-1