Abstract
Background and Aim
Cirrhosis is a controversial determinant of mortality in HBV-related acute-on-chronic liver failure (HBV–ACLF). The present study aimed to explore the effects of cirrhosis and the associated risk factors, especially its complications, on the outcome of HBV–ACLF.
Methods
A prospective–retrospective cohort of 985 patients was identified from the APASL–ACLF Research Consortium (AARC) database and the Chinese Study Group. Complications of ACLF (ascites, infection, hepatorenal syndrome, hepatic encephalopathy, upper gastrointestinal bleeding) as well as cirrhosis and the current main prognostic models were measured for their predictive ability for 28- or 90-day mortality.
Results
A total of 709 patients with HBV–ACLF as defined by the AARC criteria were enrolled. Among these HBV–ACLF patients, the cirrhotic group showed significantly higher mortality and complications than the non-cirrhotic group. A total of 36.1% and 40.1% of patients met the European Association for the Study of Liver (EASL)–Chronic Liver Failure consortium (CLIF-C) criteria in the non-cirrhotic and cirrhotic groups, respectively; these patients had significantly higher rates of mortality and complications than those who did not satisfy the CLIF-C criteria. Furthermore, among patients who did not meet the CLIF-C criteria, the cirrhotic group exhibited higher mortality and complication rates than the non-cirrhotic group, without significant differences in organ failure. The Tongji prognostic predictor model score (TPPMs), which set the number of complications as one of the determinants, showed comparable or superior ability to the Chinese Group on the Study of Severe Hepatitis B–ACLF score (COSSH–ACLFs), APASL–ACLF Research Consortium score (AARC–ACLFs), CLIF-C organ failure score (CLIF–C OFs), CLIF-C–ACLF score (CLIF-C–ACLFs), Model for End-Stage Liver Disease score (MELDs) and MELD–sodium score (MELD–Nas) in HBV–ACLF patients, especially in cirrhotic HBV-–ACLF patients. Patients with two (OR 4.70, 1.88) or three (OR 8.27, 2.65) complications had a significantly higher risk of 28- or 90-day mortality, respectively.
Conclusion
The presence of complications is a major risk factor for mortality in HBV–ACLF patients. TPPM possesses high predictive ability in HBV–ACLF patients, especially in cirrhotic HBV–ACLF patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute-on-chronic liver failure (ACLF) is a complex clinical syndrome with high morbidity and mortality [1]. HBV infection is the main etiology of ACLF in the Asia–Pacific region, whereas alcohol and HCV infection are the main etiologies in Europe and North America [1]. Most ACLF patients in the Asia–Pacific region have ACLF that was precipitated by hepatic insults, while extrahepatic insults are the precipitants in Europe and North America [1, 2]. Hepatic or extrahepatic insults in HBV–ACLF could result in differences in clinical manifestations and disease prognosis [3].
Complications of ACLF, including ascites, infection, hepatorenal syndrome, hepatic encephalopathy and gastrointestinal bleeding, constitute the main risk factors for disease progression, triggering multi-organ dysfunction and failure in ACLF [4,5,6,7,8,9] Cirrhosis is a late phase in chronic liver disease. Previous studies have indicated controversial results regarding the use of cirrhosis as a determinant of mortality in HBV–ACLF.
Several prognostic systems were established to evaluate the mortality due to short-term progression in end-stage liver disease regardless of etiology, such as the APASL–ACLF Research Consortium score (AARC–ACLFs), Chronic Liver Failure Consortium organ failure score (CLIF-C OFs), CLIF-C acute-on-chronic liver failure score (CLIF-C ACLFs), Model for End-Stage Liver Disease score (MELDs) and MELD–sodium score (MELD–Nas) [10,11,12]. Recently, the Tongji prognostic predictor model score (TPPMs) and Chinese Group on the Study of Severe Hepatitis B-ACLF score (COSSH–ACLFs) were developed specifically in HBV–ACLF patients and showed excellent predictive values [5,6,7].
The TPPM scoring system was established and validated in HBV–ACLF patients, and it showed superior predictive value compared with the MELD system. Furthermore, so far, it is the only model that includes complications as risk factors. However, the differential effectiveness of the predictive ability of cirrhosis between current models has not yet been fully elucidated.
In the present study, we evaluated ACLF-associated complications and cirrhosis as key determinants in disease progression and compared the predictive values of the TPPMs, AARC–ACLFs, COSSH–ACLFs, CLIF-C OFs, CLIF-C ACLFs, MELDs and MELD–Nas for short-term mortality in an Asia–Pacific multi-national cohort diagnosed with ACLF according to the APASL–ACLF research consortium (AARC) criteria.
Patients and methods
Subjects
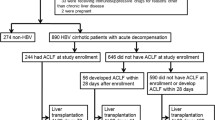
A total of 985 patients from the AARC database and the Chinese Study Group who were diagnosed with “chronic severe hepatitis B” or “HBV–ACLF” between 2006 and 2018 were prospectively and retrospectively identified. A total of 709 patients who fulfilled the 2014 AARC ACLF criteria were enrolled, of whom 620 were enrolled between 2014 and 2018, and 89 were enrolled before 2014 [13]. The data were collected using a pre-defined, web-based proforma in the AARC database (http://www.aclf.in). Approval from the institutional ethics committees was obtained. The data were annotated and encrypted before analysis. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Asia–pacific Association for the Study of Liver ACLF Research Consortium) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study. The members of the AARC working party assumed full responsibility for the accuracy and completeness of the data and subsequent analyses. All authors had access to the study data and reviewed and approved the final manuscript.
The country/region and the number of patients contributed are listed as follows:
Country/region | Patients |
|---|---|
China | 639 |
India | 153 |
Bangladesh | 117 |
Malaysia | 33 |
Pakistan | 19 |
Singapore | 11 |
Armenia | 8 |
HongKong, China | 3 |
Turkey | 1 |
Indonesia | 1 |
Total | 985 |
Complete data for analysis | 709 |
In detail, the HBV–ACLF diagnostic criteria mainly included jaundice (serum total bilirubin ≥ 5 mg/dl) and coagulopathy (INR ≥ 1.5 or prothrombin activity < 40%), accompanied by ascites and/or encephalopathy within 4 weeks. The screening and enrolment processes are shown in Fig. 1.
Screening and enrollment of patients with HBV–ACLF as defined by AARC criteria. A total of 985 patients who were diagnosed with “chronic severe hepatitis B” or “HBV–ACLF” from the AARC database and the Chinese Study Group between 2006 and 2018 were screened. A 709 of patients who fulfilled the 2014 AARC ACLF criteria were enrolled
Complications of HBV–ACLF included ascites, hepatorenal syndrome (HRS), hepatic encephalopathy (HE), infection, and upper gastrointestinal bleeding [8, 9]. The diagnosis of ascites relied on the patient’s history, physical examination, imaging evidence and laboratory assessment of liver function. Ascites was classified into grade 1 or mild ascites, grade 2 or moderate ascites and grade 3 or large ascites [7, 14, 15]. HRS was mainly defined by serum creatinine level (Cr > 1.5 mg/dl) regardless of cirrhosis. It was graded as two types: type 1 HRS, manifested as a rapid and progressive impairment of kidney function, and type 2 HRS, manifested as a stable or less progressive impairment of kidney function [14, 15]. HE was defined by the West Haven criteria. It was graded as 4 levels: I, II, III and IV [15, 16]. Infection was defined by physical examination, laboratory tests, imaging evidence and clinical manifestations [4, 7]. Upper gastrointestinal bleeding was defined by the bleeding history and endoscopic findings [7, 17].
Nucleot(s)ide analogues (NAs) were prescribed according to HBV–DNA levels and patient willingness. A total of 688 patients (97.04%) received oral antiviral treatment: 503 patients (70.94%) were treated with entecavir, 95 patients (13.40%) with lamivudine, 17 patients (2.40%) with adefovir, 16 patients (2.26%) with telbivudine, 10 patients (1.41%) with tenofovir, and 47 patients (6.63%) with a combination of two NAs. All patients were treated with standard medical therapy during their hospital stays. At the attending physicians’ discretion, this included but was not limited to glutathione; compound glycyrrhizin; transmetil; hepatocyte growth-promoting factors; vitamin K1; sodium restriction; diuretics and paracentesis combined with albumin infusion for ascites; artificial liver support system (ALSS) with indications; lactulose and l-ornithine aspartate for hepatic encephalopathy; prophylactic antibiotics for bacterial infections and renal replacement for hepatorenal syndrome and uremic symptoms; and coagulation factor supplementation with fresh plasma and cryoprecipitates. Patients were closely monitored during treatment for clinical manifestations, and laboratory examinations were performed as needed.
Study design
A total of 709 AARC HBV–ACLF patients were sub-divided into non-cirrhotic and cirrhotic groups with or without complications. Cirrhosis was diagnosed based on radiological imaging and endoscopy results regarding liver nodularity and/or portal hypertension or the clinical evidence of previous hepatic decompensation and laboratory tests and/or liver biopsy in patients with CHB [3]. According to the CLIF-C criteria [18], patients in the non-cirrhotic and cirrhotic groups were further sub-divided into non-CLIF-C ACLF and CLIF-C ACLF groups for subgroup analysis. Organ failure was diagnosed by the CLIF-C SOFA criteria [18]. Clinical characteristics and short-term mortality rates were analysed in all patients according to their definitions. The area under the receiver operating characteristic curve (AUROC) was used to compare the superiority of the models among the TPPM, AARCs, COSSH–ACLF, CLIF-C OF, CLIF-C ACLF, MELD, and MELD–Na.
The TPPMs uses the TBIL, INR, HBV–DNA and number(s) of complications as parameters [5, 6], which showed good prognostic ability in patients with HBV–ACLF. The AARC–ACLFs include the TBIL, HE grade, PT-INR, lactate and creatinine as parameters that showed adequate prognostic value in the overall AARC database [19]. The COSSH–ACLF was created based on the TBIL, INR, age and HBV–SOFA, and it was modified according to the CLIF–SOFA criteria and verified in a Chinese HBV–ACLF cohort [7]. The CLIF-C OF score was established based on the sequential organ failure assessment (SOFA), and it showed comparable ability to the CLIF–SOFA and was superior to the MELDs and MELD–Nas in ACLF patients in intensive care units [12]. The CLIF-C OFs and two other independent predictors of mortality (age and white blood cell count) were combined to develop a specific prognostic score for ACLF, the CLIF Consortium–ACLF score (CLIF-C ACLFs) [12]. The MELD score (serum bilirubin, serum creatinine and international normalized ratio for prothrombin time) and MELD–Na score (Meld score and serum sodium) are scoring systems used to assess the severity of chronic liver disease [10, 11]. They are prognostic models that are used to determine the severity and extent of liver disease to support decisions regarding specialist medical interventions, such as specific medical treatments and liver transplantation.
Data collection
The following clinical data were collected at enrolment: demographic data, vital signs, physical examination results, history, complications, precipitating events, antiviral treatment plan, and laboratory measurements, e.g., white blood cell (WBC) count, hemoglobin (Hb) level, platelet (PLT) count, alanine aminotransferase (ALT) level, aspartate aminotransferase (AST) level, serum albumin (ALB) level, total bilirubin (TBIL) level, serum sodium (Na) level, serum creatinine (Cr) level, the international normalized ratio (INR), pulse oximetry, HBV infection biomarker levels, and HBV–DNA levels. Telephone follow-up calls helped confirm the prognosis at 28 and 90 days and information regarding liver transplantation after hospital discharge.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation, and they were compared by Student’s t tests and/or the Mann–Whitney U test. Categorical variables are presented as frequencies and percentages, and they were compared by the Chi square test or Fisher’s exact test. The AUROCs were used to compare the predictive ability of the TPPMs, AARCs, COSSH–ACLFs, CLIF-C OFs, CLIF-C ACLFs, MELDs and MELD–Nas with regard to the short-term mortality rate. Values were also compared by DeLong’s test. A p value less than 0.05 was considered statistically significant, and all statistical analyses were performed with SPSS release 23.0 for Windows (SPSS, Inc., Chicago, IL) and MedCalc software (version 11.4, Ostend, Belgium).
Results
More complications and high mortality in cirrhotic HBV–ACLF
Among a total of 709 HBV patients who fulfilled the AARC ACLF criteria, 446 (62.90%) patients had cirrhosis. HBV–DNA, ALT, AST, ALB, TBIL, Hb, PLT, and serum Na levels were significantly higher in the non-cirrhotic group than in the cirrhotic group. However, age and Cr, AFP, and CRP levels were significantly higher in the cirrhotic group. Patients in the cirrhotic group had more complications, including ascites, bacterial or fungal infections and upper gastrointestinal bleeding. Similarly, a significantly higher 90-day mortality rate was observed in cirrhotic patients, although there was no difference in 28-day mortality between the cirrhotic and non-cirrhotic groups (Table 1). However, organ failure as defined by the CLIF-C criteria in patients with and without cirrhosis was not significantly different (Supplementary Table 1).
High mortality in cirrhotic HBV–ACLF patients who did not meet the CLIF-C criteria
In total, 709 patients had ACLF according to the AARC criteria, and 274 patients were diagnosed with ACLF according to the CLIF-C criteria. Patients from the CLIF-C ACLF group exhibited more severe laboratory indexes regardless of the presence of cirrhosis when compared with those from the non-CLIF-C ACLF group. CLIF-C ACLF patients had worse levels of ALB, TBIL, Cr, serum Na, INR, WBC; a higher incidence of complications (especially HRS and HE); a higher incidence of organ failure (liver, coagulation, kidney and cerebral); and eventually, higher 28-day and 90-day mortality rates (Table 2).
The 274 patients who met the CLIF-C ACLF criteria included 95 non-cirrhotic ACLF patients (non-cirrhotic CLIF-C ACLF group) and 179 cirrhotic ACLF patients (cirrhotic CLIF-C ACLF group). When compared with non-cirrhotic patients, the cirrhotic CLIF-C ACLF group had lower HBV–DNA, ALT, AST, Hb, PLT and INR levels and a higher incidence of complications (ascites, bacterial or fungal infection and upper gastrointestinal bleeding), while there was no difference in the incidence of organ failure or the short-term mortality rate (Table 2).
However, in the majority of patients (63.9% in the non-cirrhotic group and 59.9% in the cirrhotic group) who did not meet the CLIF-C criteria, cirrhotic patients exhibited higher mortality rates and incidence of complications than non-cirrhotic patients, without a significant difference in organ failure (Table 2).
TPPMs shows adequate predictive value in HBV–ACLF patients
Among all patients, the predictive abilities of the TPPMs (0.847, 0.804) and COSSH–ACLFs (0.851, 0.804) were superior to those of the CLIF-C OFs (0.835, 0.783, p = 0.472, 0.221), AARC–ACLFs (0.790, 0.766, p = 0.096, 0.219),CLIF-C ACLFs (0.773, 0.738, p = 0.001, 0.005), MELDs (0.789, 0.748, p = 0.001, 0.002) and MELD–Na (0.783, 0.756, p < 0.001, 0.013) in predicting 28-day and 90-day mortality, respectively. Notably, the TPPMs (0.847, 0.804) and COSSH–ACLFs (0.851, 0.804) showed equivalent effectiveness in predicting short-term mortality in the entire cohort of patients (Table 3).
We then stratified HBV–ACLF patients with and without cirrhosis. In the cirrhotic HBV–ACLF group, the TPPMs (0.870, 0.792) had the highest predictive ability compared with the COSSH–ACLFs (0.843, 0.773, p = 0.135, 0.354),CLIF-C OFs (0.819, 0.753, p = 0.025, 0.072), AARC–ACLFs (0.807, 0.759, p = 0.013, 0.074), CLIF-C ACLFs (0.735, 0.698, p < 0.0001, 0.002), MELDs (0.784, 0.727, p < 0.001, 0.008) and MELD–Nas (0.773, 0.733, p < 0.001, 0.026)in predicting day 28 and day 90, respectively (Table 3).
Cirrhotic HBV–ACLF patients with complications exhibited a significantly higher mortality rate than those without complications
To understand the contributions of complications to mortality in HBV–ACLF patients, patients were stratified according to the occurrence of complications. As shown in Fig. 2, cirrhotic HBV–ACLF patients with complications had a significantly higher mortality rate than those without complications. However, no significant difference in mortality was found between non-cirrhotic patients with or without complications.
Complication is an important risk factor for short-term mortality rate in patients with HBV–ACLF. a Comparison of 28-day and 90-day transplant-free mortality in all the non-cirrhotic patients with or without complication. b Comparison of 28-day and 90-day transplant-free mortality in all the cirrhotic patients with or without complication
Two and more than two complications were independent risk factors for mortality in cirrhotic HBV–ACLF patients
Risk factors associated with transplant-free 28- or 90-day mortality were evaluated according to a multivariate logistic regression model in cirrhotic HBV–ACLF patients. Odds ratios of two (OR 4.701, 1.881) or three (OR 8.266, 2.648) complications showed a significantly higher risk of 28-day (Supplementary Table 2) or 90-day (Supplementary Table 3) mortality, which were four- and eightfold higher, respectively.
Discussion
In recent decades, the APASL (AARC) and EASL (CLIF-C) consecutively defined ACLF with independent criteria based on the Eastern and Western populations, respectively.[13, 18] Each definition may apply to different patient populations. Understanding ACLF patient characteristics in various definitions is essential for management consensus. Although ACLF etiology may have changed in recent years, with trends towards more alcohol insults and fewer chronic HBV infections, the latter is still the leading cause of ACLF in many Asian countries [20]. The AARC criteria cover the early stage of ACLF, with a golden window for the reversal of disease progression.
The impact of cirrhosis on the mortality of ACLF patients remains controversial. Several studies have indicated that cirrhosis is not an independent risk factor for ACLF mortality [5, 21, 22]. However, some studies demonstrated that cirrhosis independently predicted 3-month mortality [23, 24]. Cirrhosis is still a controversial determinant of mortality in HBV–ACLF. Our data from patients across 10 Asian countries revealed that patients with cirrhotic HBV–ACLF exhibited significantly higher 90-day transplant-free mortality than those without cirrhosis, indicating that cirrhosis could be regarded as a risk factor for disease progression and could perhaps be used for patient stratification. According to the COSSH criteria, cirrhotic HBV–ACLF patients had a significantly higher risk (1.02- to 1.94-fold) than non-cirrhotic patients for 28-day mortality [7], and this result further confirmed the important role of cirrhosis in HBV–ACLF. Further analyses demonstrated that cirrhotic patients have more complications than non-cirrhotic patients, mainly presenting as ascites, bacterial or fungal infections and upper gastrointestinal bleeding. However, there was no difference in extrahepatic organ failure in cirrhotic versus non-cirrhotic HBV–ACLF patients. These data suggested that complications, ascites, bacterial or fungal infections and upper gastrointestinal bleeding are major risk factors for the mortality of cirrhotic HBV–ACLF patients. When the CLIF-C criteria were applied to Asian HBV–ACLF patients, they only captured 36.1% in the non-cirrhotic patients and 40.1% in the cirrhotic patients, with no significant differences in 28-day and 90-day mortality. This indicated that cirrhosis was not a risk factor for short-term mortality based on the CLIF-C criteria in HBV–ACLF patients. Second, the 63.9% of non-cirrhotic and 59.9% of cirrhotic HBV–ACLF patients who were not captured by the CLIF-C criteria had short-term mortality rates of 7.7% and 25.6%, respectively.
To evaluate the disease severity and predict the mortality of ACLF, several models have been explored. Initially, the MELDs and MELD–Nas were established based on patients with end-stage liver disease and have traditionally been used as prognostic assessments [10, 11]. Nevertheless, there are approximately 15–20% of patients whose survival could not be accurately predicted by the MELD score. Recently, the CLIF-C OFs and CLIF-C ACLFs were developed based on the CLIF-C ACLF in cirrhosis (CANONIC) studies, regardless of etiology [12], which were shown to be poor at predicting effectiveness in HBV–ACLF [7]. Generally, the main limitations of the existing predictive models were not etiology-specific; thus, they might vary significantly with different causes such as HBV versus alcoholic ACLF.
The effects of complications (ascites, infection, hepatorenal syndrome, hepatic encephalopathy and gastrointestinal bleeding) on 28-day and 90-day TFM were assessed by univariate analysis. As shown in Supplementary Table 4, HE, HRS, GI bleeding and infection were independent risk factors for 28-day and 90-day TFM in all and cirrhotic HBV–ACLF patients. Moreover, HE and HRS were observed to be the most crucial risk factors for short-term TFM.
Several predictive models were well developed in HBV–ACLF patients. We recently established and evaluated the TPPM model in HBV–ACLF patients from a large single-centre cohort, and it has a superior predictive ability when compared with the MELD and MELD–Na models [5, 6]. The TPPMs used TBIL, INR, HBV–DNA and complications as parameters. Recently, the COSSH–ACLF was created based on the TBIL, INR, age and HBV–SOFA and was modified according to the CLIF–SOFA criteria [7]. These models are superior to the MELD in HBV–ACLF patients. Nevertheless, no detailed comparisons and subgroup analyses have been performed with the present models.
Based on the current multi-national cohort, the TPPMs was shown to be comparable to the COSSH–ACLFs and superior to the AARC–ACLFs, CLIF-C OFs, CLIF-C ACLFs, MELDs and MELD–Nas in predicting 28-day and 90-day mortality in HBV–ACLF patients, with the highest predictive value when compared with the existing models in cirrhotic HBV–ACLF patients. Our data also indicated that cirrhotic HBV–ACLF patients had a significantly higher mortality rate and more complications than non-cirrhotic patients. Complications usually occur earlier than organ failure [2]. Thus, a prompt recognition and intervention in complications are vital for cirrhotic HBV–ACLF patients. This may explain the higher predictive value of the TPPMs in all patients diagnosed by the AARC criteria, particularly in cirrhotic individuals.
HBV genotypes are associated with disease progression and the long-term outcome of HBV infection [25]. They may serve as viral genetic markers for the risk stratification of chronic hepatitis B patients in clinical practice. HBV genotypes C, D and F carry a higher lifetime risk of cirrhosis and HCC development than genotype A. However, the relationship between HBV genotypes and ACLF development was not involved in the current study, and it needs to be fully examined in future studies.
In conclusion, the presence of complications is a major risk factor for mortality in HBV–ACLF patients, particularly in cirrhotic individuals. The TPPMs possesses high predictive ability for mortality in HBV–ACLF patients. The importance of complications as an early risk factor is worth exploring in alcohol- and autoimmune hepatitis-related ACLF.
Abbreviations
- ACLF:
-
Acute-on-chronic liver failure
- HBV–ACLF:
-
HBV-related acute-on-chronic liver failure
- AARC:
-
Asia-pacific association for the study of liver ACLF research consortium
- APASL:
-
ACLF research consortium score (AARC–ACLFs)
- CLIF-C OF:
-
Chronic liver failure consortium organ failure
- CLIF-C ACLF:
-
CLIF-C acute-on-chronic liver failure
- MELD:
-
Model for end-stage liver disease
- MELD–Na:
-
MELD–sodium score
- TPPM:
-
Tongji prognostic predictor model
- COSSH–ACLF:
-
Chinese Group on the Study of Severe Hepatitis B-ACLF
- AUROC:
-
Area under the receiver operating characteristic curve
References
Hernaez R, Solà E, Moreau R, Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66(3):541–53.
Kumar SD, Vadiraja PK, Nayak B, Thakur B, Das P, et al. Acute on chronic liver failure because of acute hepatic insults: etiologies, course, extrahepatic organ failure and predictors of mortality. J Gastroenterol Hepatol. 2016;31(4):856–64.
Shi Y, Yang Y, Hu Y, Wu W, Yang Q, Zheng M, et al. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology. 2015;62(1):232–42.
Bajaj JS, O’Leary JG, Reddy KR, Wong F, Biggins SW, Patton H, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60(1):250–6.
Wang J, Ma K, Han M, Guo W, Huang J, Yang D, et al. Nucleoside analogs prevent disease progression in HBV-related acute-on-chronic liver failure: validation of the TPPM model. Hepatol Int. 2014;8(1):64–71.
Ma K, Guo W, Han M, Chen G, Chen T, Wu Z, et al. Entecavir treatment prevents disease progression in hepatitis B virus-related acute-on-chronic liver failure: establishment of a novel logistical regression model. Hepatol Int. 2012;6(4):735–43.
Wu T, Li J, Shao L, Xin J, Jiang L, Zhou Q, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2017;2017:gutjnl-2017-314641.
Wang B, Jin G, Li L, Chen T. Other precipitating factors for AECHB. Acute exacerbation of chronic hepatitis B[M]. Chapter 2, volume I. Qin Ning, Chief editor. Germany: Springer; 2019. pp. 215–368.
Song J, Zhu L, Zhu C, Hu J. Main complications of AECHB and severe hepatitis B (liver failure). Acute exacerbation of chronic hepatitis B[M]. Chapter 6, volume II. Qin Ning, Chief editor. Germany: Springer; 2019, pp. 227–272.
Kamath PS, Kim WR. The model for end-stage liver disease (MELD). Hepatology. 2007;45(3):797–805.
Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018–26.
Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Gines P, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61(5):1038–47.
Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8(4):453–71.
European Association For The Study Of The Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53(3):397–417.
Zheng MH, Shi KQ, Fan YC, Li H, Ye C, Chen QQ, et al. A model to determine 3-month mortality risk in patients with acute-on-chronic hepatitis B liver failure. Clin Gastroenterol H. 2011;9(4):351–356.e3.
Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–35.
Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65(1):310–35.
Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426–37 (1437.e1–9).
Choudhury A, Jindal A, Maiwall R, Sharma MK, Sharma BC, Pamecha V, et al. Liver failure determines the outcome in patients of acute-on-chronic liver failure (ACLF): comparison of APASL ACLF research consortium (AARC) and CLIF-SOFA models. Hepatol Int. 2017;11(5):461–71.
Wang X, Sarin SK, Ning Q. Definition of ACLF and inclusion criteria for extra-hepatic organ failure. Hepatol Int. 2015;9(3):360–5.
Yang WB, Chen EQ, Bi HX, Bai L, Chen XB, Feng P, et al. Different models in predicting the short-term prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure. Ann Hepatol. 2012;11(3):311–9.
Li N, Huang C, Yu KK, Lu Q, Shi GF, Zheng JM. Validation of prognostic scores to predict short-term mortality in patients with HBV-related acute-on-chronic liver failure: the CLIF-C OF is superior to MELD, CLIF SOFA, and CLIF-C ACLF. Med (Baltim). 2017;96(17):e6802.
Shi Y, Zheng M, Yang Y, Wei W, Yang Q, Hu A, et al. Increased delayed mortality in patients with acute-on-chronic liver failure who have prior decompensation. J Gastroen Hepatol. 2015;30(4):712–8.
Zhao RH, Shi Y, Zhao H, Wu W, Sheng JF. Acute-on-chronic liver failure in chronic hepatitis B: an update. Expert Rev Gastroenterol Hepatol. 2018;12(4):341–50.
Lin CL, Kao JH. Natural history of acute and chronic hepatitis B: the role of HBV genotypes and mutants. Best Pract Res Clin Gastroenterol. 2017;31(3):249–55.
Acknowledgements
The authors would like to thank Professor Osamu Yokosuka, Prof. Sombat Treeprasertsuk and Prof. Gamal Shiha for their support in valuable discussion and editing.
Funding
This study was partially supported by the National Thirteenth “Five Years” Project in Science and Technology of China (2017ZX10202201, 2018ZX10302-206).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest about this work.
Informed consent in studies with human subjects
The data were collected using a pre-defined, web-based proforma in the Asia–pacific Association for the Study of Liver ACLF Research Consortium (AARC) database (http://www.aclf.in). Approval from the institutional ethics committees was obtained. The data were annotated and encrypted before analysis. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Asia–pacific Association for the Study of Liver ACLF Research Consortium) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, T., Yang, Z., Choudhury, A.K. et al. Complications constitute a major risk factor for mortality in hepatitis B virus-related acute-on-chronic liver failure patients: a multi-national study from the Asia–Pacific region. Hepatol Int 13, 695–705 (2019). https://doi.org/10.1007/s12072-019-09992-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-019-09992-x