Abstract
Tinnitus is a symptom of various disorders that affects the quality of life of millions people. Given the significance of the access to an objective and non-invasive method for tinnitus detection, in this study the auditory brainstem response (ABR) electrophysiological test was used to diagnose salicylate-induced tinnitus, in parallel with common behavioral tests. Wistar rats were divided into saline (n = 7), and salicylate (n = 7) groups for behavioral tests, and salicylate group (n = 5) for the ABR test. The rats were evaluated by pre-pulse inhibition (PPI), gap pre-pulse inhibition of the acoustic startle (GPIAS), and ABR tests, at baseline, 14 and 62 h after salicylate (350 mg/kg) or vehicle injection. The mean percentage of GPIAS test was significantly reduced following salicylate administration, which confirms the induction of tinnitus. The ABR test results showed an increase in the hearing threshold at click and 8, 12, and 16 kHz tones. Moreover, a decline was observed in the latency ratio of II-I waves in all tone burst frequencies with the highest variation in 12 and 16 kHz as well as a decrement in the latency ratio of III-I and IV-I only in 12 and 16 kHz. ABR test is able to evaluate the salicylate induced tinnitus pitch and confirm the results of behavioral tinnitus tests. GPIAS reflexive response is dependent on brainstem circuits and the auditory cortex while, ABR test can demonstrate the function of the auditory brainstem in more details, and therefore, a combination of these two tests can offer a more accurate tinnitus evaluation.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tinnitus or illusive perception of sounds affects 15–17% of the population, 2% of them experience severe and disabling tinnitus [1, 2]. It can affect the daily life and decrease the quality of life [1, 3, 4]. Despite the growing knowledge in the latest decades, the neurological and biological mechanisms of tinnitus are still not well understood. In this regard, the animal studies can play a decisive role in understanding tinnitus generation and development; however, displaying and evaluation tinnitus in animals is a challenging issue [2, 5, 6]. Two common method for tinnitus induction includes salicylate injection and exposure to noise. High doses of salicylate have been widely employed in animal studies due to its rapid and reversible effect on tinnitus induction [7,8,9]. Moreover, in contrast to noise with the induction rate of 30–80%, salicylate can induce tinnitus with a higher efficiency [1]. Following oral and parenteral salicylate, tinnitus will occur in several minutes and will fade within 72 h after the last dosage [2, 7, 10].
One of the main concerns in tinnitus assessment is the lack of an objective and reliable evaluation method [1, 2]. Introduction of a cost-effective and objective method could be a good step in facilitating the assessment of the neural mechanisms underlying tinnitus [11]. Turner et al. (2006) proposed a behavioral assessment method based on the acoustic startle reflex. This approach does not require water and food deprivation, negative shock, months of training, and memory performance [2, 10, 12]. Acoustic startle reflex occurs in all mammalians, which is created by the contraction of the main muscles following unpredictable intense sounds. This reflex can be attenuated by embedding a silence gap in the background noise [10, 12, 13]. The ratio of the startle stimulus amplitude in the un-gapped trials to those of the gapped trials can be calculated as the ratio of the gap pre-pulse inhibition of the acoustic startle (GPIAS) test. Despite the significance of the GPIAS test in tinnitus evaluation, there are some doubts about its validity [6, 14], for example due to the variety of responses in different studies [4, 6, 12]. Moreover, there is a possibility of a significant decrease in the startle amplitudes and false-positive results of tinnitus because of reduced response dynamic range [14].
Although recent advances in brain imaging techniques are largely useful in identifying neural correlations of tinnitus, their validity, efficiency, and, above all, cost-effectiveness for tinnitus assessments have not yet been established. These tests also lack the necessary temporal sensitivity to the degree of neural synchronization [11]. The auditory brainstem response (ABR) test is an early auditory evoked potential that exhibits cochlear, auditory nerve, and brainstem activity. This test is commonly used to assess the auditory function of animals in various studies. In this study, we tracked the changes in the latency and amplitude of ABR waves and; introduce it as a supplementary test for behavioral assessments of tinnitus.
Materials and Methods
Animals and Experimental Design
Male Wistar rats (weighing, 250–320 g, 3 months old) were purchased from Iran University of Medical Sciences. All rats were maintained under standard laboratory conditions including 12-h light/dark cycle (lights on at 7:00 a.m.), the temperature of 22 ± 2 °C and humidity of 53 ± 2, and free access to food and water. All experiments were carried out based on the guide for the care and use of laboratory animals (National Institutes of Health Publication No. 80–23, revised 1978) and confirmed by the Ethics Committee of Iran University of Medical Sciences (IR.IUMS.REC.1399.402).
Nineteen rats, with a normal hearing threshold and the mean percentage of GPIAS response of ≥ 25 were included in this study. The rats were randomly allocated into saline group (n = 7), and salicylate group (n = 7 for behavioral tests and n = 5 for the ABR test) and were handled for 5 days before conducting the ABR test and habituated to the test environment (10 min/day, total of 5 days, without stimulus presentation) before behavioral assessments [15, 16]. The tests were performed at baseline, 14 and 62 (wash-out) hours post salicylate or saline injection (Fig. 1a, 2a).
The effect of salicylate administration on GPIAS and PPI tests. a Schematic illustration of experimental design of behavioral tests. Rats were handled and hearing threshold was evaluated by the ABR test 5 days before behavioral tests. Animals were placed in the behavioral test box (10 min/day, total of 5 days, without stimulus presentation) for habituation. Tinnitus evaluation was performed by the GPIAS and PPI tests on baseline, 14 and 62 (wash-out) hours after salicylate or saline injection. b Schematic illustration of PPI and GPIAS tests stimuli. c, d The effect of saline and salicylate administration (350 mg/kg) on the GPIAS and PPI tests are presented as mean ± SEM. The differences between groups were determined by two way RM ANOVA followed by Tukey test. *P < .05 and **P < .01
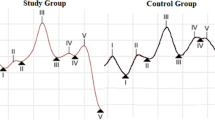
The effect of salicylate administration (350 mg/kg) on the ABR test. a Schematic illustration of experimental design of the ABR test. Animals were handled and hearing threshold and tinnitus evaluations was performed by the ABR test using click and tone burst (4, 8, 12, 16 kHz) stimuli at 70 dBSPL on baseline, 14 and 62 (wash-out) hours after salicylate injection. b The effect of salicylate administration on hearing threshold. c Representative waveform of ABR befor and after salicylate injection. d–g Latency ratio of ABR waves (d; II-I, e; III-I, f; IV-I, g; V-I). The data are presented as mean ± SEM. The differences between groups were determined by one way RM ANOVA followed by Tukey test. *P < .05, **P < .01 and, ***p < .001 vs. baseline, #P < .05 and ##P < .01 vs. post 14-h
Drug Administration
Sodium Salicylate (Merck, CAS 54–21-7) was freshly dissolved in 0.9% saline at a concentration of 175 mg/ml and intraperitoneally injected at the dose of 350 mg/kg. The rats in the saline group received the same volume of saline (2 ml/kg). After salicylate injection, the rat’s behavior was aggressive as mentioned in previous studies [1, 17]. To reduce the effect of rat’s aggressive behavior on the results of the pre-pulse inhibition (PPI) and GPIAS tests, in the pilot study (data not shown), the tests were repeated at different time points after injection and the best time point of 14 h post injection was used for the rest of experiments.
Behavioral Assessments
Tinnitus induction in animals was evaluated by PPI and GPIAS behavioral tasks. The tests were performed using the SR-LAB system (San Diego Instrument, San Diego, CA) inside a Plexiglas chamber, mounted on a platform with a sensitive piezoelectric transducer inside an acoustic chamber. Moreover, a secondary acoustic chamber was used to reduce the background noise level in the test chamber. All stimuli were generated using a high frequency speaker inside the chamber located in the center of the Plexiglas. The speaker output was calibrated using sound level meter (SLM, Bruel-kjaer 2230, Denmark) before behavioral assessments. The stimuli were performed according to the study by Turner and Parrish [12]. The startle stimulus was a 20 ms broadband noise (BBN) at 115 dBSPL. The pre-pulse signal was a 50 ms BBN at 60 dBSPL, with a 50% probability, 100 ms before the startle stimulus. The GPIAS test was performed in the presence of continuous background noise at 60 dBSPL, 50% of trials included a gap (50 ms duration, 1 ms rise/fall times), 100 ms before the startle stimulus (Fig. 1b). Each PPI or GPIAS test session started with a two-minute acclimatization period, followed by two startle stimulus trials, which the data of these two first trials were not used in the analysis of the results [18]. Then, 20 trials were presented in a quasi-random manner with stimulus intervals of 15 to 20 s. To eliminate the possible effect of the test sequence in each group, evaluations were started with the PPI test for half of the rats and with GPIAS for the next half. The amplitude of the startle response (SR) will be reduced if the animal can detect the pre-pulse (SRpp) or gap (SRg); while the presence of tinnitus probably prevents the gap detection and decreases the startle response [18]. Finally, the inhibition percentage was extracted according to the following formula: ((SR-SRg or pp)/SR×100).
ABR Test
The ABR test was performed using an electrophysiological device (Biologic / Navigator pro, USA) in an acoustic chamber under anesthesia by ketamine (80 mg/kg i.p) and xylazine (10 mg/kg i.p). A high frequency speaker was placed in the acoustic chamber at a distance of 3 cm from the right ear of the rat. Prior to the test, the speaker output was calibrated using SLM (Bruel and Kjar type 2209, Denmark). Acoustic stimuli consisted of click and tone bursts (4, 8, 12, and 16 kHz with a duration of 5 ms, rise/fall time of 2 ms) from the intensity of 70 dBSPL to the threshold level. The average of ABR waves (n = 1024 sweep) were obtained in a 10.56 ms time window with a 30–2000 Hz band pass filter, a rate of 13.1/s, alternate polarity, and × 5000 amplification. The ABR waves were recorded by subcutaneous needle electrodes (positive electrode on vertex, reference electrode under the right earlobe, ground electrode under the left earlobe). Before the test, the impedance of the electrodes was checked and kept below 5 k \(\Omega\) and the difference between electrodes was less than 2 k \(\Omega\). During the test, the rat's rectal temperature was controlled and maintained at 37 ± 1 °C with a non-electric heating pad.
The hearing threshold, the lowest intensity level that causes a repetitive response wave, for all rats was first tracked by the ABR test (data not shown). Wave II, as the predominant ABR wave in Wistar rats (19), was used to determine the threshold. The latency (ms) and amplitude (µV) of waves (I to V) were measured at 70 dBSPL. Since wave I is the only ABR that is generated from a specified and approved generator and in order to reduce the variability of the waves (especially their amplitude), in all analyzes, the amplitude and latency of wave I was considered as a reference. The amplitude and latency of other waves (II to V) were reported and analyzed relative to wave I.
Statistical Analysis
Statistical analysis was carried out using Prism v9 software. The repeated measures ANOVA (RM ANOVA) was employed for analysis. The normal distribution of the data was verified by D'Agostino-Pearson omnibus (K2); while their homoscedasticity was explored by Bartlett and Spearman’s test. When the hypothesis did not hold for the parametric tests, non-parametric test (Friedman test) was applied. Tukey's multiple comparisons test was utilized to analyze the significance in various subgroups. P < 0.05 was considered statistically significant. The results were reported as mean ± SEM.
Results
The Effect of Salicylate Administration on Behavioral Tests
As shown in Fig. 1c, RM ANOVA analysis of the GPIAS test results showed significant differences in salicylate group responses at baseline and 14 h post salicylate injection, as well as 14 and 62 (wash-out) hours post salicylate injection (p = 0.0295 and, p = 0.0099, respectively); while the PPI test results were almost constant (Fig. 1d, p > 0.05). In the saline group, GPIAS and PPI tests results were not significant (Fig. 1c,d, p > 0.05); but the GPIAS response of salicylate group was significantly reduced in compared with saline group at 14 h post injection (Fig. 1c, p = 0.0239).
The Effect of Salicylate Administration on the ABR Test
As shown in Fig. 2b, RM ANOVA analysis of hearing threshold results indicated significant differences between baseline and 14 h post salicylate injection at click and 8, 12, and 16 kHz tones (p = 0.0067, p = 0.0088, p = 0.0071 and, p = 0.0006, respectively); as well as at click, 8 and 16 kHz tones in 14 and 62 h post injection (p = 0.0371, p = 0.05 and p = 0.0043, respectively). The latency ratio of II-I waves was significantly different in baseline and 14 h post salicylate injection at all frequencies of tones (more profound at 12 and 16 kHz, Fig. 2c,d, p = 0.0287, p = 0.0155, p = 0.0043 and p = 0.0081, respectively); also at 12 kHz tone in 14 and 62 h post salicylate injection (p = 0.0129). Additionally, a significant difference was observed in the latency ratio of III-I waves in baseline and 14 h post salicylate injection at 12 kHz tone (Fig. 2e, p = 0.0025); as well as at 12 and 16 kHz tones in 14 and 62 h post injection (p = 0.0077 and p = 0.0105, respectively). The latency ratio of IV-I waves showed significant difference in baseline and 14 h post salicylate injection at 12 and 16 kHz tones (Fig. 2f, p = 0.019 and p = 0.0012, respectively); moreover in 14 and 62 h post salicylate injection at 12 kHz tone (p = 0.0056). The latency ratio of V-I waves and amplitude ratio of none of the ABR waves (II/I, III/I, IV/I, and V/I) was not significant (p > 0.05).
Discussions
The results of the current study showed that the inhibition percentage of the GPIAS test was significantly different in the salicylate group baseline and 14 h post injection, which almost returned to baseline at 62 h post injection. These results are consistent with previous studies on salicylate-induced tinnitus [2, 12, 20]. Some animal studies have suggested that salicylate administration induces tonal tinnitus [2, 4, 13], while others, like the current study, have demonstrated an induction of pseudo-noise tinnitus [6, 12, 20, 21]. However, in studies relying on tonal tinnitus, different pitches of salicylate-induced tinnitus (e.g. 9, 12, 13, 16, 17, 20, 24, 32 kHz) [2,3,4, 6, 13, 22, 23] (Table1) have been shown. According to Turner hypothesis, if animals or humans suffering from tinnitus constantly hear an imaginary sound, that partially fills the gap in a background noise, the gap must still be detectable; therefore, the GPIAS deficit can be only observed if the background noise is matched with the intensity and pitch of the tinnitus [10, 12]. This hypothesis may be best relevant to humans but is under question for the animals due to their lack of awareness of the characteristics of tinnitus processing. Contrary to general belief, animal tinnitus rarely encompasses only one frequency and often includes a wide range of frequencies [10]. These discrepancies have also been observed in human studies; despite the high-pitch of tinnitus (11 and 16 kHz) estimated by psychoacoustic measurements in individuals, the GPIAS deficit was observed in both low and high-frequency ranges (500 and 4 kHz) [10]. Moreover, an evidence suggests that at very high doses of aspirin, the tinnitus perception changed logically from tonal to cricket-like [7].
The mechanism of salicylate-induced tinnitus is not well understood and appears to be multifactorial [13]. The variety of behavioral responses in different studies could be attributed to many factors like; the use of different animal species and breeds, interval between gap and startle, duration, depth and On-set / Off-set time of the gap, long evaluation times, the dose of salicylate treatment and low sample volume in each group (according to the high SD of test) [4, 6, 10, 12, 13, 15, 20]. In the present study, in contrast to the study by Yi et al. (2016), acute salicylate-induced tinnitus was detected by the GPIAS test. In their study, the baseline evaluation was not performed and only intergroup comparisons were done [4], which might be the main reason for showing no GPIAS deficit in their study in response to acute salicylate injection. Other studies have also emphasized the need to achieving stable baseline suppression responses before assessment of tinnitus in animals when using the GPIAS test [15, 24]. Considering the high SD and SEM and the wide variety in animal responses of one species and the possibility of the negative startle response is of great importance in the evaluation of baseline responses [16, 18, 24]. In our study, in addition to the baseline evaluation, only the animals with the startle inhibition response of ≥ 25% were included [10, 15].
In the present study, a temporary threshold shift at ~ 5–20 dBSPL, as in other studies [11, 13, 25], was observed in individual rats using click and tone burst stimuli (4, 8, 12 and 16 kHz) following salicylate injection, and almost returned to baseline at 62 h post injection. The results revealed that the latency ratio of II-I, III-I and IV-I waves were significantly reduced with prominent differences at the frequencies of 12 and 16 kHz. The significant results appeared at specific frequency and it was in accordance with the possible pitch of the salicylate-induced tinnitus. Moreover, these changes were not affected by hearing loss, as decreased sensory input to the auditory nerve must be accompanied by increased latency. Therefore, such a reduction in central wave latency probably indicates the compensation of central structures associated with tinnitus. In the study of Duron et al. (2020), after injecting a moderate dose of salicylate (150 mg/kg), the latency of waves III and IV (at 6, 10 and 12 kHz) was significantly decreased [26]. While in Castañeda et al. (2019) study, the latency of waves II and IV decreased in response to tone bursts (8, 16 and 24 kHz) after salicylate administration (350 mg/kg), the waves latency remained significantly reduced until 1-day post-administration [27]. Lowe and Walton (2015) employed the modified gap in noise paradigm in the ABR test to evaluate salicylate-induced tinnitus (250 mg/kg). They reported a reduction in P4 (IV) latency at high-intensity levels [11]. In the study by Liu and Chen (2015), salicylate injection (300 mg/kg) reduced the latency ratio of waves II-I and IV-II [22]. The variability between results could be assigned to the use of tones with different frequencies and differences in the type of analysis, as some studies have only examined the latency of each wave, while our study compared the latency ratio of the waves before and after salicylate injection. Reduction of the ABR waves latency has been rarely considered because most hearing diseases delay these waves [26]. The waves latency provides evidence of brainstem function and the alterations of neurotransmitters after tinnitus induction [11, 19]. The decline in central waves’ latency implies overstimulation of neural circuits and increased transmission time, synchronization, and firing of neurons in the auditory brainstem following the salicylate-induced tinnitus [11].
In our study, the amplitude ratio of the ABR waves was not different before and after salicylate injection. Since the ABR responses are recorded from a far-field using relatively high averaging of test stimuli, high variability can be observed in the waves amplitude. Such variability in amplitude is well visible even in two consecutive records of a wave at a supra-threshold intensity. This variability has also been observed in other studies; the ABR waves amplitude after salicylate injection was increased in some studies [11, 22, 27, 28], while others indicated a decline in this parameter [3, 8]. In line with our results, some studies also reported unchanged amplitude of ABR waves [26] (Table2).
In rats, wave I is associated with peripheral auditory nerve activity, while waves II to V are thought to originate from central auditory system [1, 11, 19, 27] (Fig. 3). The ABR waves’ analysis provides the possibility to study the neural correlations of tinnitus to the auditory nerve, cochlear nucleus, and middle auditory brain [11].
Schematic illustration of the neuronal pathways of the ABR and GPIAS tests responses. CN Cochlear nucleus; SOC Superior olivery complex; LL Lateral lemniscus; IC Inferior colliculus; MGB Medial geniculate body; AC Auditory cortex; PPTg Pedunculopontine tegmental nucleus; LDTg Laterodorsal tegmental nucleus; PnC Pontine reticular nucleus caudal. Created in Biorender.com
Conclusion
The nature of the startle reflex response causes behavioral assessment to be affected by many variables including animal stress level, baseline evaluation, motor functions and sample volume. Given that the ABR test can be a good complement to behavioral tests as it is an integral part of assessments in most animal studies to determine the hearing threshold. It is independent to the animal cooperation, insensitive to anesthesia, cost-effective and the specific frequency method for assessing pitch of tinnitus in all animals. Moreover, although some of the GPIAS and ABR generators are the same, but the ABR test shows the function of the auditory brainstem structures in more details and the GPIAS test depends on the auditory cortex; and thus, they can be used to complete each other (Fig. 3).
References
Domarecka E, Olze H, Szczepek AJ (2020) Auditory Brainstem Responses (ABR) of rats during experimentally induced tinnitus: literature review. Brain Sci 10(12):901. https://doi.org/10.3390/brainsci10120901
Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R et al (2007) Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res 226(1–2):244–253. https://doi.org/10.1016/j.heares.2006.06.013
Fang L, Fu Y, Zhang T-y (2016) Salicylate-Induced hearing loss trigger structural synaptic modifications in the ventral cochlear nucleus of rats via medial olivocochlear (MOC) feedback circuit. Neurochem Res 41(6):1343–1353. https://doi.org/10.1007/s11064-016-1836-x
Yi B, Hu S, Zuo C, Jiao F, Lv J, Chen D et al (2016) Effects of long-term salicylate administration on synaptic ultrastructure and metabolic activity in the rat CNS. Sci Rep 6(1):1–11. https://doi.org/10.1038/srep24428
Ralli M, Troiani D, Podda MV, Paciello F, Eramo S, De Corso E et al (2014) The effect of the NMDA channel blocker memantine on salicylate-induced tinnitus in rats. Acta Otorhinolaryngol Ital 34(3):198 (PMID: 24882929)
Berger JI, Coomber B, Shackleton TM, Palmer AR, Wallace MN (2013) A novel behavioural approach to detecting tinnitus in the guinea pig. J Neurosci Methods 213(2):188–195. https://doi.org/10.1016/j.jneumeth.2012.12.023
Stolzberg D, Salvi RJ, Allman BL (2012) Salicylate toxicity model of tinnitus. Front Syst Neurosci 6:28. https://doi.org/10.3389/fnsys.2012.00028
Sawka B, Wei S (2014) The effects of salicylate on auditory evoked potential amplitwde from the auditory cortex and auditory brainstem. J Otol 9(1):30–35. https://doi.org/10.1016/S1672-2930(14)50006-2
Rezapour M, Moossavi A (2019) Tinnitus induction in animals and its impact on auditory system structure. Auditory Vestibular Res; 28(4):204–16. https://doi.org/10.18502/avr.v28i4.1455
Galazyuk A, Hébert S (2015) Gap-prepulse inhibition of the acoustic startle reflex (GPIAS) for tinnitus assessment: current status and future directions. Front Neurol 6:88. https://doi.org/10.3389/fneur.2015.00088
Lowe AS, Walton JP (2015) Alterations in peripheral and central components of the auditory brainstem response: a neural assay of tinnitus. PLoS ONE 10(2):e0117228. https://doi.org/10.1371/journal.pone.0117228
Turner JG, Parrish J (2008) Gap Detection Methods for Assessing Salicylate-Induced Tinnitus and Hyperacusis in Rats. Am J Audiol 17(2):S185–S192. https://doi.org/10.1044/1059-0889(2008/08-0006)
Martel DT, Pardo-Garcia TR, Shore SE (2019) Dorsal cochlear nucleus fusiform-cell plasticity is altered in salicylate-induced tinnitus. Neuroscience 407:170–181. https://doi.org/10.1016/j.neuroscience.2018.08.035
Berger JI, Owen W, Wilson CA, Hockley A, Coomber B, Palmer AR et al (2018) Gap-induced reductions of evoked potentials in the auditory cortex: a possible objective marker for the presence of tinnitus in animals. Brain Res 1679:101–108. https://doi.org/10.1016/j.brainres.2017.11.026
Longenecker R, Galazyuk A (2012) Methodological optimization of tinnitus assessment using prepulse inhibition of the acoustic startle reflex. Brain Res 1485:54–62. https://doi.org/10.1016/j.brainres.2012.02.067
Moreno-Paublete R, Canlon B, Cederroth CR (2017) Differential neural responses underlying the inhibition of the startle response by pre-pulses or gaps in mice. Front Cell Neurosci 11:19. https://doi.org/10.3389/fncel.2017.00019
Lauer AM, Larkin G, Jones A, May BJ (2018) Behavioral animal model of the emotional response to tinnitus and hearing loss. J Assoc Res Otolaryngol 19(1):67–81. https://doi.org/10.1007/s10162-017-0642-8
Turner J, Larsen D, Hughes L, Moechars D, Shore S (2012) Time course of tinnitus development following noise exposure in mice. J Neurosci Res 90(7):1480–1488. https://doi.org/10.1002/jnr.22827
Alvarado JC, Fuentes-Santamaría V, Jareño-Flores T, Blanco JL, Juiz JM (2012) Normal variations in the morphology of auditory brainstem response (ABR) waveforms: a study in Wistar rats. Neurosci Res 73(4):302–311. https://doi.org/10.1016/j.neures.2012.05.001
Sun W, Doolittle L, Flowers E, Zhang C, Wang Q (2014) High doses of salicylate causes prepulse facilitation of onset-gap induced acoustic startle response. Behav Brain Res 258:187–192. https://doi.org/10.1016/j.bbr.2013.10.024
Rüttiger L, Ciuffani J, Zenner H-P, Knipper M (2003) A behavioral paradigm to judge acute sodium salicylate-induced sound experience in rats: a new approach for an animal model on tinnitus. Hear Res 180(1–2):39–50. https://doi.org/10.1016/S0378-5955(03)00075-3
Liu X-P, Chen L (2015) Forward acoustic masking enhances the auditory brainstem response in a diotic, but not dichotic, paradigm in salicylate-induced tinnitus. Hear Res 323:51–60. https://doi.org/10.1016/j.heares.2015.01.013
Su Y-Y, Luo B, Jin Y, Wu S-H, Lobarinas E, Salvi RJ et al (2012) Altered neuronal intrinsic properties and reduced synaptic transmission of the rat’s medial geniculate body in salicylate-induced tinnitus. PLoS ONE. https://doi.org/10.1371/journal.pone.0046969
Leggett K, Mendis V, Mulders W (2018) Divergent responses in the gap prepulse inhibition of the acoustic startle reflex in two different guinea pig colonies. Int Tinnitus J 22(1):1–9. https://doi.org/10.5935/0946-5448.20180001
Radziwon KE, Stolzberg DJ, Urban ME, Bowler RA, Salvi RJ (2015) Salicylate-induced hearing loss and gap detection deficits in rats. Front Neurol 6:31. https://doi.org/10.3389/fneur.2015.00031
Duron J, Monconduit L, Avan P (2020) Auditory brainstem changes in timing may underlie hyperacusis in a salicylate-induced acute rat model. Neuroscience 426:129–140. https://doi.org/10.1016/j.neuroscience.2019.11.038
Castañeda R, Natarajan S, Jeong SY, Hong BN, Kang TH (2019) Electrophysiological changes in auditory evoked potentials in rats with salicylate-induced tinnitus. Brain Res 1715:235–244. https://doi.org/10.1016/j.brainres.2019.04.004
Liu X-P, Chen L (2012) Auditory brainstem response as a possible objective indicator for salicylate-induced tinnitus in rats. Brain Res 1485:88–94. https://doi.org/10.1016/j.brainres.2012.04.048
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All the authors have read the manuscript and have approved this submission. We further confirm that any aspect of the work covered in this manuscript that has involved experimental animals has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics Approval
This study is part of Mitra Rezapour's Ph.D dissertation in the field of audiology at Iran University of Medical Sciences. All experiments were carried out based on the guide for the care and use of laboratory animals (National Institutes of Health Publication No. 80–23, revised 1978) and confirmed by the Ethics Committee of Iran University of Medical Sciences (IR.IUMS.REC.1399.402).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rezapour, M., Akbari, M., Dargahi, L. et al. The Auditory Brainstem Response (ABR) Test, Supplementary to Behavioral Tests for Evaluation of the Salicylate-Induced Tinnitus. Indian J Otolaryngol Head Neck Surg 75 (Suppl 1), 6–15 (2023). https://doi.org/10.1007/s12070-022-03117-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-022-03117-x