Abstract
Cyclin D1 and p53 play an important role in tumorigenesis of human cancers. The present study aims to evaluate cyclin D1 and p53 expression in resectable OSCC, and to determine their prognostic significance at the end of 5 year follow-up: A total of 100 patients aged 31–74 years, stage 3/4 were recruited. Cyclin D1 and p53 expression in the tumour tissue was estimated by IHC and was statistically correlated with demographic and clinicopathological data and prognosis was evaluated at the end of 5 year outcome. The positive expression rate of cyclin D1 was 50% and p53 it was 40% and they neither showed any statistical significant correlation with each other nor with demographic or clinicopathological data. The OS was 32%.Negative and weak expression predicted better outcomes with regard to DFS and OS. DFS and OS were significantly worse in patients of overexpressed cyclin D1 (p < 0.001) and p53 (p = 0.008). Cyclin D1 is a better prognostic marker as compared to p53 for both DFS and OS. p53 expression (high versus low) for disease free non-survival and overall nonsurvival showed an OR of 3.576 (p = 0.003) and 8.803(p < 0.001) respectively for strong expression while in case of cyclin D1 it showed an OR of 13.067(p < 0.001) and 37.465(p < 0.001) for strong expression.So higher the level of expression of tumour markers higher is the odds ratio so poorer is the prognosis. Overexpression of cyclin D1 and p53 was significantly associated with poor prognosis in terms of DFS and OS
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral squamous cell carcinomas (OSCC) remain a significant cause of morbidity and mortality with 300,000 new cases worldwide each year [1, 2]. The primary treatment for OSCC is radical surgery with or without post -operative chemo-radiation.Despite technological advances in the detection and management of oral cancer during the last few decades, many centres still report low survival rate(50% to 60%).The poorer outcome is mainly noted in those with loco-regionally advanced disease [3, 4].
The cyclin D1 gene is a proto-oncogene located on chromosome 11q13, is a positive cell-cycle regulator. Cyclin D1 binds and activates CDK4 and CDK6, forming a complex that phosphorylates and inactivates retinoblastoma (Rb) protein, resulting in the release of transcriptional regulators E2F from Rb. This promotes cell-cycle progression from G1 to S-phase [5]. The increased expression of cyclin D1 causes potential for growth advantage and enhances tumorigenesis [6, 7], but still there are controversial data on the prognostic value of cyclin D1 overexpression in OSCC. Some studies suggest that increased expression of cyclin D1 is associated with poor survival [8,9,10,11,12], whereas other studies show that cyclin D1overexpression provides little prognostic information for patients with OSCC [13, 14].
p53 is a tumour suppressor gene located on chromosome 17p, encodes a 53 kDa, 393 aminoacid nuclear phosphoprotein known to regulate cell regulation and proliferation. Alteration or mutation of p53 gene is one of the most common events in human carcinogenesis and as mutated protein is not easily digestible, therefore it accumulates inside the cancer cell leading to immune-histochemical over expression which is considered a marker of poor prognosis. Moreover, p53 over expression may result in decreased sensitivity of tumour cells to chemotherapeutic drugs [15].
The aim of this study is to evaluate cyclin D1 and p53 expression in the preoperative/postoperative biopsy samples from patients with resectable OSCC (stage 3 and stage 4), and to investigate the correlation of cyclin D1 and p53 expression with clinic-pathological parameters in 100 OSCC patients and to determine their prognostic significance at the end of 5 year follow-up. We hypothesized that higher levels of expression of cyclin D1 will be associated with shortened DFS and OS. Therefore is important to identify and stratify patients with greater precision to the most appropriate choice of a treatment plan, avoiding excessive treatment in patients with low risk of recurrence and excessively conservative treatments in patients with high risk of recurrence.
Materials and Methods
This longitudinal prospective study was conducted after due ethical clearance from the institutional ethical committee of a tertiary care centre in North India. The cohort was assembled from patients with primary OSCC (stage-3 and stage-4) treated during 2008–2015.The cohort was followed up for a period of 5 years till 2020. All patients gave informed consent for participation. All patients undergoing surgical resection as a primary treatment modality were included in the study. Patients with non-resectable disease, recurrence, distant metastasis, histology other than OSCC, death due to comorbidity (cardiac, pulmonary, systemic, renal) and those with inadequate follow-up data were excluded from the study. Tumour stage was classified according to the 7th edition of the classification of malignant tumours of American Joint Committee on Cancer [16]. Surgical margins were classified according Sutton et al. [17] Surgical resection consisted of radical resection of the primary lesion with full neck dissection (functional/radical) with appropriate reconstruction (pedicle or free flap). Postoperative radiotherapy was commenced 4–6 weeks after surgery. Patients with positive surgical margins, high-grade histology, aggressive biologic behaviour, or advanced staged disease underwent adjuvant radiotherapy (external-beam radiotherapy, 55–66 Gy) / chemotherapy (5-fluorouracil and cisplatin) whatsoever was indicated. After treatment patients were monitored every 2 months for 1 year and every 6 months in subsequent 5 years. Cohort consisted of 128 patients out of which 13 defaulted complete treatment and another 15 patients were lost to follow-up, hence were excluded from the cohort.
Immunohistochemical Analysis
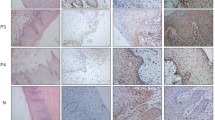
For histopathological and immunohistochemical studies, tumour samples from the lesion site were fixed in 10% buffered formalin and then embedded in paraffin. Paraffin embedded formalin fixed tissues were processed and routine H and E stained sections were evaluated to confirm the diagnosis of squamous cell carcinoma and to grade the lesion. Further sections were processed for Cyclin-D1and p53 biomarkers by immunohistochemistry (IHC) using primary monoclonal antibodies and a polymer based secondary anti-body detection kit from Diagnostic Biosystem. Standard immune-histochemistry protocol was used. In short deparaffinized rehydrated sections were blocked for endogenous peroxidases in 0.3% hydrogen peroxide in methanol, followed by a rinse in distil water. Antigen re-trieval was achieved at 121 °C in 10 mM citrate buffer (pH 6.0) for 10 min using Pascal retrieval system from Diagnostic Biosystem. Slides were cooled to room temperature were washed thrice with TBS and there-after incubated overnight at 4 °C with Primary Antibodies to Cyclin- D1 and p53. After washing with Tris-buffered saline, the sections were incubated for 30 min with secondary antibody. Cyclin-D1 and p53 were visualized with DAKO Liquid Diamino-benzidine substrate chromogen and counterstained with diluted Mayer's hematoxylin. Sections mounted with DPX were inspected under magnification. Brown nuclei were taken as positive for p53 and cyclin D1. Cytoplasmic brownish discoloration was ignored. The scoring of p53 was.
% positivity | Proportion score | Staining intensity | Intensity score |
|---|---|---|---|
0% | 0 | Nil | 0 |
1–24% | 1 | Mild | 1 |
25–49% | 2 | Moderate | 2 |
50–74% | 3 | Severe/strong | 3 |
75–100% | 4 |
A final immunoscore was calculated adding scores of % and intensity and categorised as weak (2 to 3), moderate (4 to 5) and strong (6 to 7) [18].
The scoring of Cyclin D1 was.
Positive cell% | Proportion score | Staining intensity | Intensity score |
|---|---|---|---|
< 5% | 0 | No staining | 0 |
5–25% | 1 | Mild | 1 |
26–50% | 2 | Moderate | 2 |
51–75% | 3 | Strong | 3 |
> 75% | 4 |
Total score = proportion score + intensity score. Negative expression- total score of 0. Weak expression- total score of 1–4. Strong expression- total score of 5–7.
Statistical Analysis
Data was represented as number and percentage. Association among categorical variables was calculated using Chi-Square test. Local recurrence was defined as time from day of diagnosis to development of locally recurrent disease Time interval (expressed in years) between primary treatment and last follow-up or death of the patient corresponded to overall survival (OS). Time interval (expressed in years) between primary treatment and the first recurrence (whether local, regional or distant) corresponded to disease-free survival (DFS). Predictors of DFS and OS were assessed using Cox’s univariate and multivariate regression analysis. P value < 0.05 was taken as statistically significant and p < 0.001 as highly significant. All analysis was done using SPSS 23.0 version.
Results
Demographic and Clinic-Pathological Variable Analysis
This longitudinal prospective study consisted a study cohort of 100 cases that met the inclusion criteria.80% were males while 20% were females with age ranging from 31 to 74 years. 48% were at TNM stage 3 while 52% were at TNM stage 4. Major primary sites included buccal mucosa (26%), buccogingival sulcus (2%), mandible (26%), maxilla (6%), palate (4%) and tongue (36%). As per histological grade, 16% tumours were well differentiated, 84 were moderately differentiated. 28% showed vascular invasion, 14% showed perineural invasion while 38% showed muscle invasion on histopathology. Demographic and clinicopathological variables of the cohort are summarized in Table 1.
Cyclin D1 and p53 Expression and Correlation
Expression of cyclin D1 and p53 markers and their correlation in OSCC patients are summarized in Table 2 and 3.The positive expression rate of cyclin D1 was 50% (38 samples were weak positive, 12 samples were strong positive) and for p53 it was 40% (10 samples were weak positive,8 were moderate positive while 22 samples were strong positive). Expression of cyclin D1 and p53 showed no statistical significant correlation with each other (p = 0.427).
Association of Expressions with Demographic and Clinicopathological data
Association of cyclin D1 and p53 with demographic and clinicopathological data is summarized in Table 4.Cyclin D1 showed no significant association with age (p = 0.225), sex (p = 0.973), histological grade (p = 0.471), stage of disease (p = 0.491), vascular invasion (p = 0.380), perineural invasion (p = 0.457) and muscle invasion (p = 0.198). p53 also showed no significant association with age (p = 0.373), sex(p = 0.328), histological grade (p = 0.191), stage of disease(p = 0.718),vascular invasion(p = 0.493) and muscle invasion(p = 0.892). However, it showed statistical significant correlation with perineural invasion (p = 0.034).
Association of expressions and coexpressions with survival status at the end of 5 year followup
The cohort of 100 patients was observed for a period of 5 years after treatment or until their death. The OS was 32% Negative and weak expression predicted better outcomes with regard to DFS and OS.DFS and OS were significantly worse in patients of overexpressed cyclinD1(p < 0.001) and p53(p = 0.008) when compared to those who showed weak expression of the tumour markers. If we compare both the markers then cyclin D1 is a better prognostic marker as compared to p53 for both DFS and OS (Table 5). To assess the effect of demographic and clinicopathological factors on DFS and OS, a Cox model was carried out using univariate analysis. (Table 6), and none of them showed any significant correlation with either DFS or OS.
Using the Cox regression analysis, a univariate and multivariate analysis was done to assess the independent predictive value of cyclin D1 and p53 expression groups (high versus low) for disease free non-survival and overall nonsurvival. The univariate Cox regression analysis of p53 for disease free non survival concluded that the odds ratio (OR) for weak expression was 1.701(p = 0.348), moderate expression OR was 8.72 (p < 0.001) and for strong OR was 3.576(p = 0.003), while in the multivariate Cox regression for the same the OR for weak expression was 1.987(p = 0.247), for moderate expression OR was 20.012(p < 0.001) and for strong OR was 8.803(p < 0.001). The univariate Cox regression analysis of cyclin D1 for disease free non survival concluded that the odds ratio (OR) for weak expression was 4.189(p < 0.001), and for strong OR was 13.067(p < 0.001), while in the multivariate Cox regression for the same the OR for weak expression was 6.811(p < 0.001), and for strong OR was 37.465(p < 0.001), thus concluding that the molecular marker expressions of cyclin D1 and p53 were significant predictors of DFS. The univariate Cox regression analysis of p53 for overall nonsurvival concluded that the odds ratio (OR) for weak expression was 1.851(p = 0.274), moderate expression OR was 17.405(p < 0.001) and for strong OR was 3.771(p = 0.002), while in the multivariate Cox regression for the same the OR for weak expression was 2.575(p = 0.108), moderate expression OR was 40.254(p < 0.001) and for strong OR was 9.272(p < 0.001). The univariate Cox regression analysis of cyclin D1 overall non survival concluded that the odds ratio (OR) for weak expression was 4.438(p < 0.001), and for strong OR was 10.286(p < 0.001), while in the multivariate Cox regression for the same the OR for weak expression was 7.167(p < 0.001), and for strong OR was 28.958(p < 0.001), thus concluding that the molecular marker expressions of cyclin D1 and p53 were significant predictors of OS.(Table 7 and 8).
Taken together these data show that, higher the level of expression of molecular tumour markers poorer is the prognosis in patients with OSCC. (Figs. 1–4).
Discussion
Deregulation and aberrations of cell cycle-related cyclins have been implicated in the tumour growth and progression of several cancers [19, 20]. The understanding of these alterations in tumourigenesis may identify new proteins that may serve as important cancer diagnostic and prognostic indicators as well as potential targets for therapeutic approaches in patients with OSCC [21, 22]. With this in mind, we conducted this study to evaluate the influence of the expression of the cyclin D1, and p53 status on clinical, pathological, and prognostic characteristics of patients with OSCC. In this study we found that positive expression rate of cyclin D1 was 50% and for p53 it was 40% in a cohort of 100 patients. Pillay M et al. in his study of 110 cases of OSCC reported an expression rate of 36% of p53,while Saawarn S et al. reported an expression rate of 45% of cyclin D1 [23, 24]. In the present study expression of cyclin D1 and p53 neither showed any statistical significant correlation with each other, nor did the molecular marker expressions showed any significant correlation with demographic or clinicopatholoical profile of the patients except in patients with perineural invasion who showed significant correlation with high expression of p53 (p = 0.034).Similar results have been reported by Zhong et al. and H Khan et al. [25, 26].
Cyclin D1 plays an important role in cell-cycle regulation by forming a complex and functioning as a regulatory subunit of CDK4/CDK6, whose activity is required for cell-cycle G1–S transition through phosphorylation of the Rb protein [27]. Therefore, cyclin D1 overexpression promotes cell growth and tumorigenesis. Cyclin D1 amplification is one of the most frequent molecular alterations in (HNSCC) Head and Neck Squamous cell carcinoma [28]. Several studies have examined the prognostic significance of cyclin D1 overexpression in HNSCC. Dong et al. [29], found that coexpression of cyclin D1 and CDK4 by immunohistochemistry was associated with the poorest overall survival in a cohort of 102 patients with laryngeal carcinoma. Pignataro et al. [30] found that cyclin D1 overexpression by immunohistochemistry was an independent predictor of adverse disease-free survival in a cohort of 149 patients with laryngeal carcinomas treated with surgery and radiotherapy. Bova et al. [8] also reported that cyclin D1 overexpression is an independent predictor for disease-specific death in a cohort of 148 patients with carcinoma of the anterior tongue. In a similar fashion, Kyomoto et al. [31] reported that cyclin D1 amplification by differential PCR method and protein overexpression by immunohistochemistry was associated with poor outcome in 45 paraffin-embedded sections from HNSCC. In the present study we found a significant reduced overall survival (p < 0.001) and disease free survival (p < 0.001) in cyclin D1 positive patients than in cyclinD1 negative patients.
p53 plays an important role in apoptosis, genomic stability, and inhibition of angiogenesis and thus functions as a key tumour suppressor. However, mutant p53 proteins gain oncogenic properties favoring the insurgence, the maintenance, and the spreading of malignant tumors [32]. 40% of our OSCC samples were p53 positive which is consistent with the results described in the literature [33]. Many immunohistochemical studies in literature have failed to detect any correlation between p53 expression and clinical outcome [34,35,36,37,38] however, they do have reported reduced survival [39,40,41,42] while in some it was observed to be associated with prolonged survival [43]. We found a significant reduced overall survival (p = 0.008) and disease free survival (p = 0.008) in p53 positive patients than in p53 negative patients. The expression of p53 can serve a prognostic role for disease recurrence and disease specific mortality in HNSCC [44], whereas no association of p53 expression serve as a marker for poor prognosis [45]. Our findings of present study suggest that p53 expression serves as a marker for poor prognosis in OSCC patients. When we compare the DFS and OS of cyclinD1 with p53, cyclinD1 proves to be a better prognostic marker with a survival rate of 60% as compared to p53 which shows a DFS and OS of 50%. Cyclin D1 overexpression has also been found to be site-specific [46]. However, we studied tumour marker expression in limited number of OSCC cases so studies based on large sample size can provide more insight into possible biological and clinical relevance of cyclin D1 and p53 in OSCC.
Conclusion
We report high expression of cyclinD1 and p53 in OSCC. Overexpression of cyclin D1 and p53 was significantly associated with poor prognosis in terms of DFS and OS, hence should be considered as prognostic biomarkers of poor prognosis in patients of resectable OSCC. When compared to p53, cyclin D1 is a better marker for OS.
References
Kademani D (2007) Oral cancer. Mayo Clin Proc 82:878–887
Petersen PE (2003) The world oral health report: continuous improvement of oral health in the 21st century—the approach of WHO global oral programme. Community Dent Oral Epidemiol 31:3–23
Neville BW, Day TA (2002) Oral cancer and precancerous lesions. CA Cancer J Clin 52:195–215
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Pestell RG, Albanese C, Reutens AT, Segall JE, Lee RJ, Arnold A (1999) The cyclins and cyclin-dependent kinase inhibitors in hormonal regulation of proliferation and differentiation. Endocr Rev 20:501–534
Hinds PW, Dowdy SF, Eaton EN, Arnold A, Weinberg RA (1994) Function of a human cyclin gene as an oncogene. Proc Natl Acad Sci USA 91:709–713
Jiang W, Kahn SM, Zhou P, Zhang YJ, Cacace AM, Infante AS, Doi S, Santella RM, Weinstein IB (1993) Overexpression of cyclin D1 in rat fibroblasts causes abnormalities in growth control, cell cycle progression and gene expression. Oncogene 8:3447–3457
Bova RJ, Quinn DI, Nankervis JS, Cole IE, Sheridan BF, Jensen MJ et al (1999) Cyclin D1 and p16INK4A expression predict reduced survival in carcinoma of the anterior tongue. Clin Cancer Res 5:2810–2819
Carlos de Vicente J, Herrero-Zapatero A, Fresno MF, Lopez-Arranz JS (2002) Expression of cyclin D1 and Ki-67 in squamous cell carcinoma of the oral cavity: clinicopathological and prognostic significance. Oral Oncol 38:301–308
Feng Z, Guo W, Zhang C, Xu Q, Zhang P, Sun J et al (2011) CCND1 as a predictive biomarker of neoadjuvant chemotherapy in patients with locally advanced head and neck squamous cell carcinoma. PLoS ONE 6:e26399
Kaminagakura E, Werneck da Cunha I, Soares FA, Nishimoto IN, Kowalski LP (2011) CCND1 amplification and protein overexpression in oral squamous cell carcinoma of young patients. Head Neck 33:1413–1419
Huang SF, Cheng SD, Chuang WY, Chen IH, Liao CT, Wang HM et al (2012) Cyclin D1 overexpression and poor clinical outcomes in Taiwanese oral cavity squamous cell carcinoma. World J Surg Oncol 10:40
Shah NG, Trivedi TI, Tankshali RA, Goswami JV, Jetly DH, Shukla SN et al (2009) Prognostic significance of molecular markers in oral squamous cell carcinoma: a multivariate analysis. Head Neck 31:1544–1556
Perisanidis C, Perisanidis B, Wrba F, Brandstetter A, El Gazzar S, Papadogeorgakis N et al (2012) Evaluation of immunohistochemical expression of p53, p21, p27, cyclin D1, and Ki67 in oral and oropharyngeal squamous cell carcinoma. J Oral Pathol Med 41:40–46
Khan H, Gupta S, Husain N et al (2014) Correlation between expressions of cyclin-D1, EGFR and p53 with chemo-radiation response in patients of locally advanced oral squamous cell carcinoma. BBA Clin 3:11–17
Brandwein-Gensler M, Smith RV (2010) Prognostic indicators in head and neck oncology including the new 7th edition of the AJCC staging system. Head Neck Pathol 4:53–61
Sutton DN, Brown JS, Rogers SN, Vaughan JA, Woolgar JA (2003) The prognostic implications of the surgical margin in oral squamous cell carcinoma. Int J Oral Maxillofac Surg 32:30–34
Raju K, Punnayanapalya S, Mariyappa N (2015) Significance of p53, pRb and Ki-67 Markers in Cervical Intraepithelial Lesion and Malignancy. Biomed Res Ther 2(10):374–384
Hahn WC, Weinberg RA (2002) Rules for making human tumor cells. N Engl J Med 347(20):1593–1603
Schmitz S, Machiels JP (2010) Molecular biology of squamous cell carcinoma of the head and neck: relevance and therapeutic implications. Expert Rev Anticancer Ther 10(9):1471–1484
Monteiro LS, Diniz-Freitas M, Garcia-Caballero T, Warnakulasuriya S, Forteza J, Fraga M (2012) Combined cytoplasmic and membranous EGFR and p53 overexpression is a poor prognostic marker in early stage oral squamous cellcarcinoma. J Oral Pathol Med 41(7):559–567
Mishra R (2013) Cell cycle-regulatory cyclins and their deregulation in oral cancer. Oral Oncol 9(6):475–481
Pillay M, Vasudevan DM, Rao CP, Vidya M (2003) p53 expression in oral cancer: observations of a South Indian study. J Exp Clin Cancer Res 22(3):447–451
Saawarn S, Astekar M, Saawarn N, Dhakar N (2012) Cyclin D1expression and its correlation with histopathological differentiation in oral squamous cell carcinoma. Sci World J 65:1–5
Zhong L-P (2013) Elevated cyclin D1 expression is predictive for a benefit from TPF induction chemotherapy in oral squamous cell carcinoma patients with advanced nodal disease. Mol Cancer Ther 12(6):1112–1120
Khan H et al (2014) Correlation between expressions of Cyclin-D1, EGFR and p53with chemoradiation response in patients of locally advanced oral squamous cell carcinoma. BBA Clinical 3:11–17
Marx J (1994) How cells cycle toward cancer. Science 263:319–321
Callender T, el Naggar AK, Lee MS, Frankenthaler R, Luna MA, Batsakis JG (1994) PRAD-1 (CCND1)/cyclin D1 oncogene amplification in primary head and neck squamous cell carcinoma. Cancer 74:152–158
Dong Y, Sui L, Sugimoto K, Tai Y, Tokuda M (2001) Cyclin D1-CDK4 complex, a possible critical factor for cell proliferation and prognosis in laryngeal squamous cell carcinomas. Int J Cancer 95:209–215
Pignataro L, Pruneri G, Carboni N et al (1998) Clinical relevance of cyclin D1 protein overexpression in laryngeal squamous cell carcinoma. J Clin Oncol 16:3069–3077
Namazie A, Alavi S, Olopade OI et al (2002) Cyclin D1 amplification and p16(MTS1/CDK4I) deletion correlate with poor prognosis in head and neck tumors. Laryngoscope 112:472–481
Strano S, Dell’Orso S, Mongiovi AM et al (2007) Mutant p53 proteins: between loss and gain of function. Head Neck 29(5):488–496
Abrahao AC, Bonelli BV, Nunes FD, Dias EP, Cabral MG (2011) Immunohistochemical expression of p53, p16 and hTERT in oral squamous cell carcinoma and potentiallymalignant disorders. Brazilian Oral Res 25(1):34–41
Grabenbauer GG, Muhlfriedel CH, Rodel F, Niedobitek G (2000) Squamous cell carcinoma of the oropharynx: Ki-67 and p53 can identify patients at high risk for local recurrence after surgery and postoperative radiotherapy. Int J Radiat Oncol Biol Phys 48:1041–1050
Field JK, Zomupourlis V, Spandidos DA, Jones AS (1994) P53 expression and mutation in squamous cell carcinoma of head and neck: expression correlates with the patients’ use of tobacco and alcohol. Cancer Detect Prev 18(3):197–208
Sisk EA, Soltys SG, Zhu S, Fisher SG, Carey TE, Bradford CR (2002) Human papillomavirus and p53 mutational status as prognostic factors in head and neck carcinoma. Head Neck 24:841–849
Bosch FX, Ritter D, Enders C, Flechtenmacher C, Abel U, Dietz A, Hergenhahn M, Weidauer H (2004) Head and neck tumor sites differ in prevalence and spectrum of p53 alterations but these have limited prognostic value. Int J Cancer 111:530–538
Vlachtsis K, Nikolaou A, Markou K, Fountzilas G, Daniilidis I (2005) Clinical and molecular prognostic factors in operable laryngeal cancer. Eur Arch Otorhinolaryngol 262:890–898
Waitzberg AF, Nonogaki S, Nishimoto IN, Kowalski LP, Miguel RE, Brentani RR, Brentani MM (2004) Clinical significance of c-Myc and p53 expression in head and neck squamous cell carcinomas. Cancer Detect Prev 28(3):178–186
Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, Ridge JA, Goodwin J, Kenady D, Saunders J, Westra W, Sidransky D, Koch WM (2007) TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med 357:2552–2561
Shin DM, Lee JS, Lippman SM, Lee JJ, Tu ZN, Choi G, Heyne K, Shin HJ, Ro JY, Goepfert H, Hong WK, Hittelman WN (1996) p53 expression: predicting recurrence and secondary primary tumors in head-and-neck squamous-cell carcinoma. J Nat Cancer Inst 88:519–529
Smith EM, Wang D, Rubenstein LM (2008) WA Morris, Turek LP, Haugen TH: Association between p53 and human papillomavirus in head and neck cancer curvival. Cancer Epidemiol Biomark Prev 17:421–427
Hirvikoski P, Kumpulainen E, Virtaniemi J, Johansson R, Haapasala R, Marin S, Halonen P, Helin H, Raitiola H, Pukander J, Kosma VM, Kellokumpu-Lehtinen P (1997) p53expression and cell proliferation as prognostic factors in laryngeal squamous-cell carcinoma. J clin Oncol 15:3111–3120
Blons H, Laurent-Puig P (2003) TP53 and head and neck neoplasms. Hum Mutat 21:252–257
Almangush A, Heikkinen I, Mäkitie AA, Coletta RD, Läärä E, Leivo I, Salo T (2017) Prognostic biomarkers for oral tongue squamous cell carcinoma: a systematic review and meta-analysis. Br J Cancer 117(6):856–866
Freier K, Bosch FX, Flechtenmacher C et al (2003) Distinct site-specific oncoprotein overexpression in head and neck squamous cell carcinoma: a tissue microarray analysis. Anticancer Res 23:3971–3977
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kakkar, V., Sarin, V., Chatterjee, A. et al. Expression of Cyclin-D1 and p53 as Prognostic Markers in Treatment of Oral Squamous Cell Carcinoma. Indian J Otolaryngol Head Neck Surg 74 (Suppl 3), 6136–6145 (2022). https://doi.org/10.1007/s12070-021-02716-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-021-02716-4