Abstract
This narrative review compares the advantages and drawbacks of imaging and other investigation modalities which currently assist with lung cancer diagnosis and staging, as well as those which are not routinely indicated for this. We examine plain film radiography, computed tomography (CT) (alone, as well as in conjunction with positron emission tomography (PET)), magnetic resonance imaging (MRI), ultrasound, and newer techniques such as image-guided bronchoscopy (IGB) and robotic bronchoscopy (RB). While a chest X-ray is the first-line imaging investigation in patients presenting with symptoms suggestive of lung cancer, it has a high positive predictive value (PPV) even after negative X-ray findings, which calls into question its value as part of a potential national screening programme. CT lowers the mortality for high-risk patients when compared to X-ray and certain scoring systems, such as the Brock model can guide the need for further imaging, like PET-CT, which has high sensitivity and specificity for diagnosing solitary pulmonary nodules as malignant, as well as for assessing small cell lung cancer spread. In practice, PET-CT is offered to everyone whose lung cancer is to be treated with a curative intent. In contrast, MRI is only recommended for isolated distant metastases. Similarly, ultrasound imaging is not used for diagnosis of lung cancer but can be useful when there is suspicion of intrathoracic lymph node involvement. Ultrasound imaging in the form of endobronchial ultrasonography (EBUS) is often used to aid tissue sampling, yet the diagnostic value of this technique varies widely between studies. RB is another novel technique that offers an alternative way to biopsy lesions, but further research on it is necessary. Lastly, thoracic surgical biopsies, particularly minimally invasive video-assisted techniques, have been used increasingly to aid in diagnosis and staging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, with over 1.7 million deaths in 2020 [1]. Smoking is the greatest risk factor for developing lung cancer. Radon [2] exposure and asbestos [3] exposure are also major risk factors, showing synergistic effects with cigarette use. The 5-year survival rate of lung cancer varies from 3 to 63%, depending on histological classification and staging [4].
Early detection of lung cancers facilitates curative treatment by surgical resection ± chemotherapy [5]. Advances in imaging modalities may be reflected in increased rates of lung cancer resection [6]. This review considers imaging modalities (including chest radiography, computed tomography (CT), and positron emission tomography (PET)) used in current lung cancer care. Additionally, novel techniques such as computer-aided detection/diagnosis and the use of robotics are included in this review to explore innovative approaches involved in the future of lung cancer.
Lung cancer across the globe: incidence and screening
There were approximately 2 million new cases of lung cancer globally, making it the most common cancer (2018). Ninety percent of lung cancer cases worldwide are caused by smoking which means many cases are preventable [7].
Incidence and death rates are higher in developing countries compared to developed countries due to increased use of tobacco. In the UK, the incidence of lung cancer is higher in the most deprived areas compared to the least deprived areas, highlighting an urgent need for appropriate screening and monitoring pathways taking into consideration socioeconomic factors in these areas [7].
The survival rate of lung cancer in the USA has increased due to screening using CT screening and novel treatment options. The US Preventive Services Task Force recommends screening with low-dose CT in adults aged 50 to 80 years with significant smoking history [8]. In the UK, there is no national screening programme for lung cancer. The Accelerate, Coordinate, Evaluate (ACE) programme piloted several projects to improve the lung cancer pathway. This included direct access to CT scans by general practitioners for patients who had an abnormal chest X-ray or those who met the symptoms criteria [9].
Chest X-ray
In many countries, chest X-ray (CXR) is the first-line investigation in the workup diagnosis of suspected lung cancer. In the UK, the National Institute for Health and Care Excellence (NICE) guidelines for suspected malignancy advise that patients over the age of 40 presenting with two or more symptoms such as cough, dyspnoea, haemoptysis, or weight loss should have an urgent CXR. Patients with positive findings suggestive of lung cancer are then referred through the suspected cancer pathway for an appointment within 2 weeks [10]. The sensitivity and specificity of CXR in detecting lung cancer are well researched. A systematic review that included 3 main databases showed that CXR has a sensitivity of just below 80% and a specificity of more than one-fifth [11]. In addition, another study has shown that CXR has a negative predictive value of approximately 99% when used to screen for lung cancer in patients who present with the symptoms mentioned by the NICE guidelines [12]. Table 1 gives a summary of the findings in the cohort study mentioned.
Although CXRs are still first line for screening of lung pathology, its diagnostic accuracy is very limited and caution should be taken when using them to guide surgical intervention. For thoracic surgeons, CXRs are useful during the postoperative period to assess patients’ progress, recovery, and any gross abnormalities. Furthermore, CXRs can be used in postoperative follow-up clinics to assess for effusions and other parenchymal changes such as consolidation or collapse.
Summary
-
CXRs are easily available and are used as first-line investigations for lung diseases; however, small lesions can be missed using this investigation.
-
CXRs have a good value in screening programmes prior to CT scans.
Computerized tomography (CT) scan
Following chest radiograph, CT scan with contrast is the most common next imaging modality for furthering the diagnosis and staging in patients with suspected lung cancer. CT uses a series of X-rays to produce a three-dimensional (3D) image, presented as cross-sectional slices providing far greater detail than plain film radiography. Low-dose CT (LDCT) uses reduced radiation exposure (1–4 mSv) making it safer for repeated imaging. CT may be used for staging; however, PET-CT scanning has higher sensitivity and specificity than conventional CT (77% vs 55% and 86% vs 81%, respectively) [13].
Two large-scale randomised trials, with a long-term follow-up have demonstrated the efficacy of LDCT imaging for screening of people deemed at high risk of developing lung cancer. Across all studies, there was a reduction in mortality in the screened groups [14]. Although useful, LDCT screening was shown to have a false positive rate of 96.4% when using a nodule size threshold of 4 mm, placing patients at risk of unnecessary investigation [15].
Predictive models, such as the Brock model, may be used to predict the likelihood of a nodule detected on CT being malignant [16]. In this model, predictive indicators included female sex, increasing size of nodule, location of nodule (upper lung), nodule spiculation, and family history of lung cancer, emphysema, part-solid nodule type, and older age [16]. More recent studies, while validating the Brock model, have shown nodule size to be the most important predictor, with sex being less useful as a risk predictor [17].
CT is the main imaging modality for guiding transthoracic needle biopsy of lung lesions [18], with favourable diagnostic yield and sample adequacy when compared to other imaging modalities [19]. CT guidance may be conventional (CCT-guided) or used in combination with fluoroscopy (CTF-guided) [18]. In a meta-analysis of 9 studies, CTF-guided biopsy yielded higher diagnostic accuracy, without differences in incidence of complications, or radiation dose, when compared to CT-guided biopsy [20]. The most common complication is pneumothorax, with incidence of 20–64% in all CT-guided biopsies [21], although incidence of pneumothorax can be reduced through adopting a biopsy-side down patient position [22]. Another complication is haemorrhage from lung parenchyma commonly due to the needle track traversing a pulmonary vessel [23]. Table 2 highlights the main findings seen in the studies reviewed.
CT scans offer the ability to assess the size of the primary tumour and level of spread. It also helps in identifying any chest wall invasion or pleural nodularity. This helps the thoracic surgeons to identify the best surgical approach for resection as minimally invasive techniques will not be suitable for large tumours and those with local spread that need to be resected en-bloc. Planning for chest wall resection and reconstruction with help from colleagues can be done with pre-surgical CT reconstructions. CT scans also help in further staging of the tumour by identifying metastasis, commonly seen in the adrenals or brain.
Summary
-
Conventional CT is a useful modality for furthering diagnosis of lung cancer, although is less sensitive and specific than PET-CT for staging.
-
LDCT represents a novel means of screening for lung cancer in those at risk.
-
CT is the favoured modality for guiding transthoracic needle biopsies.
Positron emission tomography (PET)
It is important that all individuals with lung cancer for treatment with curative intent are offered positron emission tomography CT (PET-CT) as it is useful in the assessment of lung nodules [26]. PET-CT is a form of nuclear imaging which uses radionuclides such as fluorodeoxyglucose (FDG). These radionuclides are emitted and then combine with electrons. The gamma-ray which is emitted in the process is detected by a PET camera to generate images [27]. This is combined with CT to allow accurate localisation of uptake. Tumour cells have an increased uptake of radionuclides such as FDG through the Warburg effect [28]. A systematic review and meta-analysis found that PET-CT was both sensitive and specific [29]. As seen in Table 1, another study demonstrated the usefulness of PET-CT for the detection of extensive disease in patients with small cell lung cancer (SCLC) [30]. During evaluation of a solitary pulmonary nodule, Brock’s score is used initially to calculate the probability of malignancy. If Brock’s score shows high probability, patients are referred on to have further staging investigations such as PET-CT. The probability of malignancy following PET-CT is calculated using the Herder model. The Herder model takes into consideration (a) patient characteristics like age, smoking status, and any history of other cancers and (b) nodule characteristics such as size, presence of nodule in the upper lobe, speculation, and FDG uptake on PET-CT. This model was based on a previous work by Swensen which was shown to have external validity but thought to underestimate the probability of malignancy [31]. However, the additional consideration of FDG uptake on PET was shown to be more accurate in estimating the probability of malignancy [32].
One of the limiting factors of PET imaging is that false positive results can often be seen when patients have diseases such as tuberculosis and sarcoidosis. This is due to the increased uptake of FDG in these areas where there is inflammation and increased metabolic activity as a result [33]. A study explored the accuracy of FDG-PET CT in areas with infectious lung disease and found that there was reduced specificity of PET-CT in regions with endemic infectious lung disease compared with non-endemic regions. However, sensitivity was comparable [34]. Therefore, PET-CT serves as a useful tool in countries like India alongside the use of video-assisted thoracoscopic surgery (VATS) biopsy and tissue diagnosis.
In terms of the economic case for the role of PET-CT in the management of cancer, previous studies have shown that PET-CT provides more accurate staging and reduces the number of surgeries such as thoracotomies required, lowering the overall cost of lung cancer management [35]. Table 3 gives a summary of the benefits identified in the various studies for PET-CT scans.
In staging for lung cancers, PET-CT scans play an invaluable role in identifying nodal spread, distant metastasis, and local invasion. Apart from staging, PET scans help the surgeon in multidisciplinary discussions regarding any neoadjuvant treatments if needed to downstage tumours prior to surgery. It also helps the surgeons to identify patients that may not benefit from surgical resection due to the spread of their disease.
Summary
-
PET-CT serves as a useful tool to demonstrate cellular activity in lung nodules which can fit into the Herder model to calculate probability of malignancy.
-
PET-CT may not be the best imaging modality in areas endemic to infectious lung disease, and hence histological diagnosis is needed for confirmation.
Magnetic resonance imaging (MRI)
MRI is the gold standard for the diagnosis and staging of brain and prostate malignancy, as well as monitoring the response of these tumours to treatment [36]. Yet, when it comes to lung malignancy, the role of MRI remains the subject of debate, as there are several challenges in MRI signal acquisition that are unique to the lung. MRI currently plays a limited role in the assessment of non-small cell lung cancer (NSCLC) which accounts for approximately 85% of all lung malignancies [37]. NICE guidelines advise against routine use of MRI to assess the T-stage of the primary tumour, yet they recommend it as one of the possible first-line options for the assessment of isolated metastases, especially in the brain, for patients who are having treatment with curative intent [10]. Additionally, a recent multicentre trial (Streamline-L) found no significant differences between per-patient sensitivity and specificity for detection of NSCLC metastasis, when comparing whole-body MRI (WB-MRI) to standard staging pathways [38].
MRI has also been routinely used for many years as an adjunct to CT for staging rarer types of lung malignancy, such as malignant pleural mesotheliomas and superior sulcus (Pancoast) tumours [39]. The excellent soft tissue contrast provided by MRI makes it a highly appropriate modality for assessing local invasion of the cancer into surrounding structures, including the mediastinum and the chest wall [40].
Finally, recent literature suggests that novel, functional MRI sequences, such as diffusion-weighted imaging (DWI) can provide information about features of the cancer that may not be evident with CT, such as pleural effusions and vasculature of the tumour [36]. Recent evidence from Zhang and colleagues also suggests that DWI and PET/CT are equally accurate when differentiating between the gross target volume (GTV) of a lung tumour in patients with atelectasis, a crucial step in the planning of targeted radiotherapy [41].
MRI scans give further information in staging the lung cancers by identifying metastasis in brain as well as in assessing the degree of chest wall invasion in locally advanced tumours. MRI scans of the abdomen are used at times to investigate liver lesions to rule out metastasis. In Pancoast tumours, they help surgeons to plan surgical margins and reconstruction [42]. If neoadjuvant treatment is given, MRI scans help to assess degree of tumour shrinkage.
Summary
-
MRI is used more in diagnosis of brain metastasis as mentioned in the guidelines.
-
It is also used in evaluating the operability of Pancoast tumour by assessing the brachial plexus and chest wall involvement.
Ultrasound-guided modalities
The use of ultrasound techniques such as endobronchial ultrasonography with transbronchial needle aspiration (EBUS-TBNA) and endoscopic ultrasound-guided fine needle aspiration (EUS‑FNA) are not used in the diagnosis of lung cancer. However, in the staging assessment, they provide valuable information to the assessment of the chest wall, parietal pleura, and lymph node involvement [42]. Current evidence in the literature recommends a combined approach using EBUS-TBNA and EUS-FNA to increase the sensitivity by reaching and sampling more lymph nodes that are difficult to sample using EBUS-TBNA or surgical sampling alone. In addition, a combined approach is more comfortable to the patient and more cost-effective [42, 43].
In patients with enlarged PET avid mediastinal lymph nodes, EBUS-TBNA helps in giving pathological confirmation of nodal involvement. This further helps in staging of lung cancer and in decision-making regarding need for neoadjuvant treatments. Patients who have been down staged following neoadjuvant treatment benefit from EBUS-TBNA to facilitate in decision for surgery if the biopsy is negative.
Summary
-
Ultrasound can be used in assessing pleural effusions and taking samples for cytology investigations.
-
Ultrasound is also part of EBUS and EUS examination and biopsies of lymph nodes to stage the lung cancers.
Novel imaging modalities
Image-guided bronchoscopy (IGB)
IGB techniques are most frequently used to biopsy peripheral pulmonary lesions (PPLs) as these are often difficult to reach and have a lower diagnostic yield with conventional bronchoscopy alone.
Electromagnetic navigation (ENB), a type of IGB, is used as a guidance tool in the diagnosis of solitary pulmonary nodules (SPNs) and masses [44]. ENB is a real-time navigation system that combines three-dimensional CT imaging with real-time bronchoscopy, using a low-frequency electromagnetic field locator to guide the bronchoscope to a target lesion near the bronchial tree [45].
A multicentre cohort (NAVIGATE) study [46] of 1157 lung lesion biopsy cases using ENB had a 12-month diagnostic yield of 72.9%. ENB diagnosed malignancy in 44.3% of the successful biopsies (484 of 1092) and were negative in 55.7% (608 of 1092) [46] (Table 4). A 12-month follow-up showed that 46% of the initial negative outcomes were considered true negative and 36% were false negative. Evidence suggests the diagnostic accuracy for ENB and radial endobronchial ultrasound (R-EBUS) approach only 50% [47].
Robotic bronchoscopy
Robotic bronchoscopy (RB) is a relatively new technology which may offer an alternative approach to tackle the clinical challenges presented with diagnosing PPLs. There are two available RB platforms; The Monarch™ Robotic Endoscopy System (Auris Robotics, Redwood City, CA, USA) and The Ion™ Robotic Endoluminal System (Intuitive Surgical, Sunnyvale, CA, USA).
The BENEFIT study of robotic-assisted bronchoscopy using The Monarch™ system [48] was the first feasibility study using this platform (Table 4).
A diagnosis was obtained in 40 of 54 patients (74.1%) out of which malignancy accounted for 33 of 40 patients (82.5%). The diagnostic yield for peripheral lesions with a concentric view was 80.6% (25/31 lesions) compared with 70% for eccentric lesions (14/20 lesions) [48].
The three key components of successful peripheral lung lesion biopsy using guided bronchoscopy are navigation to the lesion, confirmation of successful navigation, and precise tissue acquisition [49]. The main findings of the NAVIGATE and BENEFIT studies are highlighted in Table 4.
These newer modalities incorporating bronchoscopy help in getting tissue diagnosis from lesions that are difficult to biopsy by conventional methods such as CT-guided biopsies. They help surgeons in getting a preoperative diagnosis in cases where a VATS wedge excision might not be feasible and the patient may need a lobectomy to get tissue samples. In addition, they have been used in clinical practice to help localise lesions for surgeons to perform minimally invasive surgeries such as segmentectomies.
Convoluted neural networks (CNNs)
CNNs, a type of deep learning algorithm, are applied to the detection and diagnosis of pulmonary nodules; the CT image is inputted into CNN, which filters the pixelated data against the programmed criteria to categorise the image [25]. CNN is utilised in computer-aided detection (CADe) to locate pulmonary nodules, while computer-aided diagnosis (CADx) uses CNN to classify nodules [24] (Fig. 1). CT slice thickness of 1.25–2.5 mm is shown to be optimal for CNN processing. The image below shows how the CNNs are used in assessment.
Summary
-
Image-guided and robotic bronchoscopies enable biopsies to be taken from lung lesions that cannot be obtained by current standard procedures like CT-guided biopsies or EBUS.
-
CNNs will help to eliminate human errors when reading scans.
Surgical biopsy
Surgical biopsy for lung cancer diagnosis may be divided into 2 categories: (1) open lung biopsy (via thoracotomy) or (2) VATS biopsy.
VATS biopsy involves the use of fibre-optic cameras, and traditionally, 3–4 incisions arranged in a triangular configuration [50]. In the past decade, the use of uniportal VATS has increased due to the advantages it offers in reducing postoperative pain [51].
VATS is currently recommended when there is high clinical suspicion of mediastinal lymph node involvement, despite negative needle biopsy. Additionally, its use as a surgical technique for resection of stage I NSCLC is recognised by the American Association of Chest Physicians [52]. Another possible application of VATS is enabling intraoperative biopsy and diagnosis. VATS can be used to collect a wedge section or core biopsy which is then immediately analysed histologically as a frozen section — the resulting diagnosis determining the procedure undertaken within the same session [53].
VATS has been shown to have a reduced need for blood transfusion (5.5% vs 1.4%) and reduced incidence of pneumonia postoperatively (5.5% vs 0.6%) when compared to open biopsy [54], while also reducing the proportion of patients reporting pain from 24 h to 52 weeks post-procedure [55]. A recent multicentre RCT from the UK further highlighted the advantages of VATS over open lobectomy, reporting improved physical function at 5 weeks in the VATS arm, with fewer complications (RR 0.74) and reduced visual analogue pain scores (MD − 0.54) during the hospital stay [56].
For the purposes of mediastinal staging in NSCLC, in cases where PET-CT has identified positive nodal involvement, but EBUS fails to show malignant lymph node involvement, cervical mediastinoscopy may be performed, permitting biopsy of the paratracheal nodes [57].
Summary
-
Surgical biopsies are required when non-invasive methods such as CT-guided or EBUS biopsies are inconclusive, and the PET scans are very suspicious with high Herder scores.
-
VATS wedge biopsies and frozen section enable for diagnosis and treatment in the same setting without the need for a second operation.
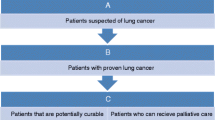
Recommendation
Following the above review on present-day imaging modalities, we suggest a brief algorithm for diagnosis and staging of lung cancers (Fig. 2).
Consequently, depending on clinical tumor, node, and metastasis (TNM) staging, further investigations may be undertaken based on tumour stage. We recommend Table 5 for perusal at multidisciplinary meetings. These investigations may vary slightly depending on trust recommendations and resources available at local thoracic units.
Conclusion
Multiple imaging modalities have been studied for the diagnosis and staging in lung cancer assessment. CXR has been the prevalent first-line investigation being a good modality for diagnosis but not for screening of lung cancer. LDCT is shown to be more effective than CXRs alone in reducing mortality. CTF-guided biopsies produce a higher diagnostic accuracy than CCT-guided biopsies. PET-CT scan is mainly used for evaluation of incidental small pulmonary nodules or for SCLC. Low proton density of lung tissue makes MRI signal acquisition challenging and limits its use in diagnosis and screening of lung cancer. Nonetheless, MRI is appropriately used for assessing local invasions into surrounding structures like the mediastinum or chest wall. Ultrasound-guided modalities provide valuable information about chest wall, parietal pleura, and lymph node involvement in the staging assessment. EBUS-TBNA and EUS-FNA are used in combination to assess metastasis to mediastinal lymph nodes. Despite advances in novel image modalities such as ENB, diagnostic yield remains highly variable. A feasibility study using robotic-assisted bronchoscopy such as The Monarch™ system yielded high diagnostic results, but the small sample size highlights the need for further studies in this area. In situations where radiological modalities and guided biopsies are unable to give a clear diagnosis in highly suspicious cases, the surgical biopsy techniques are the best diagnostic tools.
References
World Health Organization International Agency for Research on Cancer, 2020. GLOBOCAN 2020: estimated cancer incidence, mortality and prevalence. 2020. https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf. Accessed 14 December 2021
Samet JM, Brenner D, Brooks AL, et al. Health effects of exposure to radon. Washington, D.C.: National Academy Press; 1999.
Gilham C, Rake C, Burdett G, et al. Pleural mesothelioma and lung cancer risks in relation to occupational history and asbestos lung burden. Occup Environ Med. 2016;73:290–9. https://doi.org/10.1136/oemed-2015-103074.
American Cancer Society. Lung cancer survival rates. 2019. https://www.cancer.org/content/dam/CRC/PDF/Public/8705.00.pdf. Accessed 14 December 2021.
Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e278S–e313s. https://doi.org/10.1378/chest.12-2359.
Royal College of Physicians. National Lung Cancer Audit annual report 2016. 2017. https://www.rcplondon.ac.uk/file/5794/download. Accessed 14 December 2021.
Thandra KC, Barsouk A, Saginala K, Aluru JS, Barsouk A. Epidemiology of lung cancer. Contemp Oncol 2021;25:45–52. https://doi.org/10.5114/wo.2021.103829.
Krist AH, Davidson KW, Mangione CM, et al. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:962–70. https://doi.org/10.1001/jama.2021.1117.
NHS England- National Cancer Programme. Targeted screening for lung cancer with low radiation dose computed tomography. 2019. https://www.england.nhs.uk/wp-content/uploads/2019/02/targeted-lung-health-checks-standard-protocol-v1.pdf. Accessed 9 December 2021.
Scenario: Referral for suspected lung or pleural cancer | Management | Lung and pleural cancers - recognition and referral | CKS | NICE [Internet]. Cks.nice.org.uk. 2021. https://cks.nice.org.uk/topics/lung-pleural-cancers-recognition-referral/management/referral-for-suspected-lung-or-pleural-cancer/. Accessed 13 December 2021.
Bradley SH, Abraham S, Callister ME, et al. Sensitivity of chest X-ray for detecting lung cancer in people presenting with symptoms: a systematic review. Br J Gen Pract. 2019;69:e827–e835. https://doi.org/10.3399/bjgp19X706853.
Bradley SH, Hatton NLF, Aslam R, et al. Estimating lung cancer risk from chest X-ray and symptoms: a prospective cohort study. Br J Gen Pract. 2021;71:e280–e286. https://doi.org/10.3399/bjgp20X713993.
Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2013;143:e211S-e250S. https://doi.org/10.1378/chest.12-2355.
de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503–13. https://doi.org/10.1056/NEJMoa1911793.
Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. https://doi.org/10.1056/NEJMoa1102873.
McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369:910–9. https://doi.org/10.1056/NEJMoa1214726.
Winkler Wille MM, van Riel SJ, Saghir Z, et al. Predictive accuracy of the PanCan Lung cancer risk prediction model -external validation based on CT from the Danish Lung Cancer Screening Trial. Eur Radiol. 2015;25:3093–9. https://doi.org/10.1007/s00330-015-3689-0.
Birchard KR. Transthoracic needle biopsy. Semin Intervent Radiol. 2011;28:87–97. https://doi.org/10.1055/s-0031-1273943.
Tsai P-C, Yeh Y-C, Hsu P-K, Chen C-K, Chou T-Y, Wu Y-C. CT-guided core biopsy for peripheral sub-solid pulmonary nodules to predict predominant histological and aggressive subtypes of lung adenocarcinoma. Ann Surg Oncol. 2020;27:4405–12. https://doi.org/10.1245/s10434-020-08511-9.
Fu Y-F, Li G-C, Cao W, Wang T, Shi Y-B. Computed tomography fluoroscopy-guided versus conventional computed tomography-guided lung biopsy: A systematic review and meta-analysis. J Comput Assist Tomogr. 2020;44:571–7. https://doi.org/10.1097/RCT.0000000000001044.
Sabatino V, Russo U, D’Amuri F, et al. Pneumothorax and pulmonary hemorrhage after CT-guided lung biopsy: incidence, clinical significance and correlation. Radiol Med. 2021;126:170–7. https://doi.org/10.1007/s11547-020-01211-0.
Appel E, Dommaraju S, Camacho A, et al. Dependent lesion positioning at CT-guided lung biopsy to reduce risk of pneumothorax. Eur Radiol. 2020;30:6369–6375. https://doi.org/10.1007/s00330-020-07025-y.
Nour-Eldin NE, Alsubhi M, Naguib NN, et al. Risk factor analysis of pulmonary hemorrhage complicating CT-guided lung biopsy in coaxial and non-coaxial core biopsy techniques in 650 patients. Eur J Radiol. 2014;83:1945–52. https://doi.org/10.1016/j.ejrad.2014.06.023.
Firmino M, Angelo G, Morais H, Dantas RM, Valentim R. Computer-aided detection (CADe) and diagnosis (CADx) system for lung cancer with likelihood of malignancy. Biomed Eng Online. 2016;15:2. https://doi.org/10.1186/s12938-015-0120-7.
Gu Y, Chi J, Liu J, et al. A survey of computer-aided diagnosis of lung nodules from CT scans using deep learning. Comput Biol Med. 2021;137:104806. https://doi.org/10.1016/j.compbiomed.2021.104806.
Al-Jahdali H, Khan AN, Loutfi S, Al-Harbi AS. Guidelines for the role of FDG-PET/CT in lung cancer management. J Infect Public Health. 2012;5:S35–40. https://doi.org/10.1016/j.jiph.2012.09.003.
Farwell MD, Pryma DA, Mankoff DA. PET/CT imaging in cancer: current applications and future directions. Cancer. 2014;120:3433–45. https://doi.org/10.1002/cncr.28860.
Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–8. https://doi.org/10.1016/j.tibs.2015.12.001.
Ruilong Z, Daohai X, Li G, Xiaohong W, Chunjie W, Lei T. Diagnostic value of 18F-FDG-PET/CT for the evaluation of solitary pulmonary nodules: a systematic review and meta-analysis. Nucl Med Commun. 2017;38:67–75. https://doi.org/10.1097/MNM.0000000000000605.
Lu Y-Y, Chen J-H, Liang J-A, Chu S, Lin W-Y, Kao C-H. 18F-FDG PET or PET/CT for detecting extensive disease in small-cell lung cancer: A systematic review and meta-analysis. Nucl Med Commun. 2014;35:697–703. https://doi.org/10.1097/MNM.0000000000000122.
Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157:849–55. https://doi.org/10.1001/archinte.1997.00440290031002.
Herder GJ, van Tinteren H, Golding RP, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest. 2005;128:2490–6. https://doi.org/10.1378/chest.128.4.2490.
Long NM, Smith CS. Causes and imaging features of false positives and false negatives on F-PET/CT in oncologic imaging. Insights Imaging. 2011;2:679–98. https://doi.org/10.1007/s13244-010-0062-3.
Deppen SA, Blume JD, Kensinger CD, et al. Accuracy of FDG-PET to diagnose lung cancer in areas with infectious lung disease: a meta-analysis. JAMA. 2014;312:1227–36. https://doi.org/10.1001/jama.2014.11488.
Verboom P, van Tinteren H, Hoekstra OS, et al. Cost-effectiveness of FDG-PET in staging non-small cell lung cancer: The PLUS study. Eur J Nucl Med Mol Imaging. 2003;30:1444–9. https://doi.org/10.1007/s00259-003-1199-9.
Sim AJ, Kaza E, Singer L, Rosenberg SA. A review of the role of MRI in diagnosis and treatment of early stage lung cancer. Clin Transl Radiat Oncol. 2020;24:16–22. https://doi.org/10.1016/j.ctro.2020.06.002.
Zhang Y, Qin Q, Li B, Wang J, Zhang K. Magnetic resonance imaging for N staging in non-small cell lung cancer: a systematic review and meta-analysis. Thorac Cancer. 2015;6:123–32. https://doi.org/10.1111/1759-7714.12203.
Taylor SA, Mallett S, Ball S, et al. Diagnostic accuracy of whole-body MRI versus standard imaging pathways for metastatic disease in newly diagnosed non-small-cell lung cancer: the prospective streamline L trial. Lancet Respir Med. 2019;7:523–32. https://doi.org/10.1016/S2213-2600(19)30090-6.
Koyama H, Ohno Y, Seki S, et al. Magnetic resonance imaging for lung cancer. J Thorac Imaging. 2013;28:138–50. https://doi.org/10.1097/RTI.0b013e31828d4234.
Raptis CA, McWilliams SR, Ratkowski KL, Broncano J, Green DB, Bhalla S. Mediastinal and pleural MR imaging: practical approach for daily practice. Radiographics. 2018;38:37–55. https://doi.org/10.1148/rg.2018170091.
Zhang X, Fu Z, Gong G, et al. Implementation of diffusion-weighted magnetic resonance imaging in target delineation of central lung cancer accompanied with atelectasis in precision radiotherapy. Oncol Lett. 2017;14:2677–82. https://doi.org/10.3892/ol.2017.6479.
Rami-Porta R, Call S, Dooms C, et al. Lung cancer staging: a concise update. Eur Respir J. 2018;51:1800190. https://doi.org/10.1183/13993003.00190-2018.
Korevaar DA, Crombag LM, Cohen JF, Spijker R, Bossuyt PM, Annema JT. Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer: a systematic review and meta-analysis. Lancet Respir Med. 2016;4:960–8. https://doi.org/10.1016/S2213-2600(16)30317-4.
Patrucco F, Gavelli F, Daverio M, et al. Electromagnetic navigation bronchoscopy: where are we now? Five years of a single-center experience. Lung. 2018;196:721–7. https://doi.org/10.1007/s00408-018-0161-3.
Gex G, Pralong JA, Combescure C, Seijo L, Rochat T, Soccal PM. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration. 2014;87:165–76. https://doi.org/10.1159/000355710.
Folch EE, Pritchett MA, Nead MA, et al. Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: one-year results of the prospective, multicenter NAVIGATE study. J Thorac Oncol. 2019;14:445–58. https://doi.org/10.1016/j.jtho.2018.11.013.
Yarmus L, Akulian J, Wahidi M, et al. A prospective randomized comparative study of three guided bronchoscopic approaches for investigating pulmonary nodules: The PRECISION-1 study. Chest. 2020;157:694–701. https://doi.org/10.1016/j.chest.2019.10.016.
Chen AC, Pastis NJ, Mahajan AK, et al. Robotic bronchoscopy for peripheral pulmonary lesions: a multicenter pilot and feasibility study (BENEFIT). Chest. 2021;159:845–52. https://doi.org/10.1016/j.chest.2020.08.2047.
Kumar A, Caceres JD, Vaithilingam S, Sandhu G, Meena NK. Robotic bronchoscopy for peripheral pulmonary lesion biopsy: evidence-based review of the two platforms. Diagnostics (Basel). 2021;11:1479. https://doi.org/10.3390/diagnostics11081479.
Hansen HJ, Petersen RH. Video-assisted thoracoscopic lobectomy using a standardized three-port anterior approach - the Copenhagen experience. Ann Cardiothorac Surg. 2012;1:70–6.
Wang L, Liu D, Lu J, Zhang S, Yang X. The feasibility and advantage of uniportal video-assisted thoracoscopic surgery (VATS) in pulmonary lobectomy. BMC Cancer. 2017;17:75.
Detterbeck FC, Lewis SZ, Diekemper R, Addrizzo-Harris D, Alberts WM. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:7s–37s.
Sihoe ADL, Hiranandani R, Wong H, Yeung ESL. Operating on a suspicious lung mass without a preoperative tissue diagnosis: pros and cons. Eur J Cardiothorac Surg. 2013;44:231–7.
Al-Ameri M, Bergman P, Franco-Cereceda A, Sartipy U. Video-assisted thoracoscopic versus open thoracotomy lobectomy: a Swedish nationwide cohort study. J Thorac Dis. 2018;10:3499–506.
Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17:836–44.
Lim EKS, Batchelor TJP, Dunning J, et al. Video-assisted thoracoscopic versus open lobectomy in patients with early-stage lung cancer: One-year results from a randomized controlled trial (VIOLET). J Clin Oncol. 2021;39:8504.
Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and oesophageal endosonography for the diagnosis and staging of lung cancer. Eur Respir J. 2015;46:40–60.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
No ethical approval needed as it is a review article.
Conflict of interest
All authors declare they have no conflicts of interest relevant to this article.
Informed consent
No patient consents were needed for this review study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Philip, B., Jain, A., Wojtowicz, M. et al. Current investigative modalities for detecting and staging lung cancers: a comprehensive summary. Indian J Thorac Cardiovasc Surg 39, 42–52 (2023). https://doi.org/10.1007/s12055-022-01430-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-022-01430-2