Abstract
Ebstein’s anomaly is a relatively rare congenital heart disorder characterised by downward displacement of septal and posterior leaflets of the tricuspid valve into the right ventricle cavity. The usual presenting symptoms are cyanosis, right-sided heart failure and arrhythmia. Progressive heart failure or tachyarrhythmia may worsen cyanosis. The acute coronary syndrome is rarely reported in Ebstein’s anomaly. We report a patient of undiagnosed Ebstein’s anomaly who was apparently asymptomatic but presented with the acute coronary syndrome. This case report deals with a rare combination of congenital heart disease (Ebstein’s anomaly) and coronary artery disease. Ebstein’s anomaly (EA) has a prevalence of 1% of all congenital heart diseases, and little evidence is reported in the literature where EA along with coronary artery disease (CAD) exists in individuals less than 45 years old. Therefore, this case report brings attention to the rarity of those pathologies, which individually are already considered rare. And in this case, the association turns this diagnosis exceptional and highlights the complexity of the treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ebstein’s anomaly is a rare syndrome characterised by the ventricular displacement of the tricuspid valve, leading to right atrium enlargement, right ventricle failure and, when it is diagnosed during early stages of life, death, which occurs commonly in childhood. Because of that, no evidence of an association between Ebstein’s syndrome and CAD has been found in the literature. We report a middle-aged adult with Ebstein’s anomaly presenting with severe CAD due to atherosclerotic coronary artery disease without traditional cardiovascular risk factors.
The CAD is the leading cause of deaths globally [1] and remains a huge burden to health systems over the world. Because of its prevalence, the disease path physiology has been extensively studied. Despite all knowledge accumulated over the decades about atherosclerosis and CAD, the causes of the disease in some cases are still uncertain, because they may not be clearly associated to any known cardiovascular risk factor [2,3,4,5], even after intensive investigation, or have strong association with a disease that had never been previously related with CAD.
Case report
A 44-year-old fisherman was previously asymptomatic presented with breathlessness and chest pain of sudden onset. He was haemodynamically stable with a pulse rate of 60/min. His electrocardiography (ECG) showed a T wave inversion and a left bundle branch block pattern.
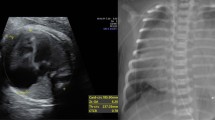
His transthoracic echocardiogram was done which showed a dilated and enlarged right atrium, and there was downward displacement of the tricuspid valve leaflet (Fig. 1). There was a small atrial septal defect (patent foramen ovale) on the left-to-right shunt. There was a mild tricuspid regurgitation but no tricuspid stenosis. There was a regional wall motion abnormality of the inferior wall; the septum and lateral wall were hypokinetic. There was a right ventricular (RV) dysfunction along with a mild left ventricular (LV) dysfunction.
His coronary angiography (Fig. 2a, b) showed severe triple vessel disease. His left anterior descending artery has 90% proximal lesion and 80% lesion in the mid left anterior descending (LAD) area, the circumflex artery had a proximal 90% long-segment lesion and the right coronary artery had mid and distal 80% lesions. The patient was referred to an early coronary artery bypass grafting (CABG). Before subjecting to surgery as per our protocol, the echocardiogram was reassessed by a senior cardiothoracic surgeon and cardiac anaesthetist who suspected an Ebstein’s anomaly, which was missed in routine pre-operative screening and later confirmed by a senior cardiologist. The patient’s lipid profile, thyroid profile, renal profile and other investigations were reported normal. The patient was posted for surgery; general anaesthesia was given and findings were confirmed by a transesophageal echocardiogram (intraoperative TEE). Since the Ebstein’s anomaly in the heart was larger than the normal ones and very irritable on manipulation, the aortic, superior vena cava (SVC) and inferior vena cava (IVC) purse string was taken and the aortic and bicaval cannulation was done and kept ready for the bypass in case of emergency; 2 deep stitches were taken from the posterior pericardium after gently lifting the heart. The CABG was done on a beating heart, three grafts were given to the left internal mammary artery (LIMA), LAD (Fig. 3a), saphenous vein graft (SVG), and major obtuse marginal (OM); and SVG was done to the posterior descending artery (PDA) on the beating heart (LIMA to LAD and PDA grafting was done without doing an on-pump to decrease the pump time). Then, the cardiopulmonary bypass and on-pump beating-heart OM graft were done. Since the anatomy was suitable for cone repair, which can be performed with low risk and provides a near anatomic repair, cone repair was done by mobilising the septal leaflet, placating the posterior leaflet and re-suturing in a cone-shaped fashion (Fig. 3b). Annuloplasty with the ring was not done as there was no need for it. Cone repair was perfect on TEE. The atrial septal defect was closed using the pericardial patch. The patient came off from bypass very well and was shifted to the ICU with minimal inotropic support. The patient was ventilated for the next 24 hours and was extubated the following day. The patient recovered well and was discharged on the 9th post-operative day. The post-surgery echocardiogram showed an improved RV and LV function. The patient is in a regular follow-up since the last 12 months and completely asymptomatic.
Discussion
The present case describes an atherosclerotic acute coronary syndrome in a young active adult presenting with undiagnosed Ebstein’s anomaly during the early ages of life, without any complications during childhood and any related symptoms in the adult age. This is an unusual evolution of that rare disease, and this association of CAD in the EA patient was not reported in the scientific literature. The echocardiogram clearly evidenced the association of two different pathophysiologic processes: the apical displacement of the tricuspid valve and the definite regional ischemic injury of the left ventricle. Although some Ebstein’s anomaly patients can present with a left ventricle dysfunction [6], the coronary angiogram confirmed the atherosclerotic origin of coronary obstructions. Pathophysiology mechanisms of the association between Ebstein’s anomaly and atherosclerosis are not stated, but maybe Ebstein’s anomaly might accelerate the development of atherosclerosis. The abnormalities of Ebstein’s anomaly in this case might allow the development of atherosclerotic injuries that were not observed in other severe patients. The absence of other cardiovascular risk factors also highlighted the association of both conditions. Ebstein’s anomaly is a rare congenital disease, occurring in one to five on every 200,000 live births, and 1% prevalence of all congenital heart diseases [7]. Besides its prevalence, the prognosis is poor when the disease is diagnosed in the first days or weeks of life, with 1- and 5-year survival rates estimates as 78.6% and 76.3%, respectively [8], most often requiring surgical treatment. In patients with an increased risk of arrhythmia, ablation procedures may be considered. Our patient had not presented any associated defects described in the literature that we quoted above or any symptoms due to Ebstein’s anomaly; the condition was only diagnosed after the acute coronary syndrome. The clinical presentation has occurred due to the acute coronary syndrome and relieved after the coronary artery bypass grafting and cone repair. Cone repair has excellent early and midterm results; consideration to earlier operative intervention may be given because this procedure can be performed with low risk and provides a near anatomic repair. Cone repair is widely used for Ebstein’s anomalies, and it is appreciated for the leaflet coaptation it provides and its creation of normal geometry of the tricuspid valve. The cone procedure has been performed around the world on patients ranging from newborns, a few days old, to adults aged 49, and is considered the “gold standard” for repairing Ebstein’s anomaly. Contraindication to cone repair are old age > 50 years, moderate pulmonary hypertension, significant LV dysfunction (ejection fraction < 30%), complete failure of delamination of septal and inferior leaflet with poor delamination of anterior leaflet (< 50% delamination of anterior leaflet), severe right ventricular enlargement, and severe dilation of the right atrioventricular junction (true tricuspid annulus). As this patient had none of these contraindications, we chose to do cone repair.
CAD in younger ages is commonly associated to strong risk factors or familial history, which were ruled out in our patient. As far as we are concerned, this can be considered as the first report of an association between EA and significant CAD atherosclerotic lesion in a young adult. Ebstein’s anomaly correction and CABG combined procedures and its risks were not described previously in the literature. Therefore, our case is one of the rare and first cases where combined CABG and cone repair of the tricuspid valve is done. Post-operatively, his RV and LV function has improved, and since last year, he is in a regular follow-up and doing well.
Conclusion
The present case describes an atherosclerotic acute coronary syndrome in a relatively young active adult with undiagnosed Ebstein’s anomaly, during the early life, without any complications during childhood and any related symptoms in the adult age. Ebstein’s anomaly correction by cone repair and CABG combined procedures was not described previously in the literature.
References
World Health Organization. Cardiovascular diseases [Internet]. [Cited 2016 Oct 12]. Available from: http://www.who.int/mediacentre/factsheets/fs317/en/.
Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 Countries (the INTERHEART Study): Case-control study. Lancet.2004;364:937-52.
Wang J C, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res.2012;111: 245-59.
Turhan H, Yasar AS, Basar N, Bicer A, Erbay AR, Yetkin E. High prevalence of metabolic syndrome among young women with premature coronary artery disease.” Coron Artery Dis.2005;16:37-40.
Panagiotakos DB, Rallidis LS, Pitsavos C, Stefanadis C, Kremastinos D. Cigarette smoking and myocardial infarction in young men and women: A case-control study. Int J Cardiol.2007;116:371-5.
Attenhofer Jost CH, Connolly HM, O’Leary PW, Warnes CA, Tajik AJ, Seward JB. Left heart lesions in patients with Ebstein anomaly. Mayo Clin Proc.2005;80:361-8.
Dearani JA, Danielson GK. Surgical management of Ebstein’s anomaly in the adult. Semin Thorac Cardiovasc Surg.2005;17:148-54.
Yu JJ, Yun TJ, Won HS, et al. Outcome of neonates with Ebstein’s anomaly in the current era. Pediatr Cardiol.2013;34:1590-96
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent has been taken from the patient to publish this case report. This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
Dr. Jaideep Kumar Trivedi is not a recipient of any research scholarship. The authors declare that they have no conflict of interest.
Funding
Nil
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Trivedi, J.K., Mahapatra, R.P., Gandham, R.K. et al. Ebstein’s anomaly presenting with the acute coronary syndrome—a rare combination. Indian J Thorac Cardiovasc Surg 36, 56–59 (2020). https://doi.org/10.1007/s12055-019-00840-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-019-00840-z