Abstract
Ebstein’s anomaly is an infrequent but fascinating congenital heart disorder accounting for <1% of all congenital heart disease. Since its depiction in 1866, dramatic developments in diagnosis and treatment have been made.
Ebstein’s anomaly is a complex congenital disorder with a broad pathologic-anatomical and also clinical spectrum and no two patients are identical. So, precise knowledge of the different anatomic and hemodynamic variables, related malformations and management choices are essential. Managing Ebstein’s anomaly patients is so complex. Thus, it is important that these patients are frequently seen by a cardiologist with expertise in congenital heart disease.
Here in we present a 24-year-old woman referred to our center due to frequent episodes of sudden-onset palpitation. He had a history of unknown congenital heart disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ebstein’s anomaly
- Sail sound
- Accessory conduction pathways
- Complex congenital disorder
- Right ventricular outflow tract obstruction
Clinical Presentation
A 24-year-old woman referred to our center due to frequent episodes of sudden-onset palpitation. She had a history of unknown congenital heart disease.
In physical exam, he was a well-developed young woman without distress, cyanosis, or clubbing. Blood pressure and pulses were normal. Jugular venous pressure was normal. PMI was in mid-clavicular line and, there was a widely split S1 with a loud tricuspid component (the “sail sound”), a widely split S2, a right-sided third heart sound, and a systolic murmur grade 2 in LSB (Fig. 4.1).
ECG findings: Sinus rhythm; Rate = 80 bpm; Left axis deviation; No voltage criteria infavour of LA or RA abnormalities; Short PR interval; Wide QRS complex with atypical RBBB pattern; Negative QRS and delta wave in V1; Q wave in inferior leads (II, III, AVF); ST elevation with bi-phasic T wave (coved or brugada pattern) in leads V1–3 and inferior leads; ST depression in I, aVl, V4–6
Diagnosis and differential diagnosis based on ECG:
-
DDX: right posteroseptal accessory pathway: Ebstein anomaly or brugada syndrome type 1
-
Dx: sinus rhythm, wide QRS complex, RBBB pattern, right posteroseptal accessory pathway: Ebstein anomaly (Fig. 4.2)
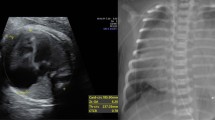
PA x-ray in full inspiration and normal KV from a woman. Mild deviation to the left; Increased cardiothoracic (CT) ratio infavour of cardiomegaly indicative of enlargement of right-sided chambers; Normal position of gastric bubble and carinal angle; Normal pulmonary vasculature; No tracheal deviation
-
DDX: “water bottle” or “box-shaped” heart
R/O Ebstein anomaly; or Severe RA enlargement, or Severe TR, or Massive pericardial effusion
Interestingly in this case the displacement of the posterior leaflet was more than the septal leaflet (5/2.2 cm) (Figs. 4.5, 4.6, 4.7 and 4.8).
Echocardiography allows accurate evaluation of the TV leaflets, the size of the right atrium, and size and function of both ventricles. The site and degree of TV regurgitation and the feasibility of valve repair are also assessed by echocardiography.
Associated anomalies with Ebstein’s include PFO or ASD in approximately 50% of patients; accessory conduction pathways in 25% (usually right-sided); and, occasionally, varying degrees of right ventricular outflow tract obstruction, VSD, aortic coarctation, PDA, or mitral valve disease.
Current Indications for Intervention in Ebstein Anomaly
-
Poor exercise capacity (NYHA class > II)
-
Right heart failure
-
Large heart size (cardiothoracic ratio > 64%)
-
Important cyanosis (resting oxygen saturation < 90%)
-
Severe symptomatic tricuspid regurgitation
-
Transient ischemic attack, or stroke due to a paradoxic embolus, sustained atrial flutter, or fibrillation and atrial arrhythmias secondary to an accessory pathway.
Management
Medical
Patients with mild forms of Ebstein’s anomaly may be followed medically for many years. Regular evaluation by a cardiologist with proficiency in congenital heart disease is suggested.
Endocarditis prophylaxis is commended in Ebstein’s anomaly, although the risk of endocarditis is low.
In patients with Ebstein’s anomaly and cardiac failure who are not candidates for surgery, we recommend standard heart failure treatment.
Heart transplantation is an alternative treatment option in select patients who are not candidates for standard surgical treatment.
Catheter Interventions
Most patients with symptomatic WPW syndrome, with or without EA, must undergo electrophysiological assessment and maybe radiofrequency ablation of the accessory pathway(s); also, all types of supraventricular tachyarrhythmia associated with EA can be successfully ablated by the electrophysiologist.
Surgery
Several types of procedures can be used to surgically treat Ebstein anomaly and associated defects:
-
Tricuspid valve repair.
-
Tricuspid valve replacement. ...
The goal of this surgery is to fix the defective valve between the right atrium and the right ventricle so that the leaflets open and close correctly.
When there is enough tissue present, the valve can be repaired. This is the preferred treatment because it uses your own tissue. When the existing valve cannot be repaired, it is possible to replace it with a mechanical valve or one made of biologic tissue.
References
Paranon S, Acar P. Ebstein’s anomaly of the tricuspid valve: from fetus to adult: congenital heart disease. Heart. 2008;94:237–43.
Ibrahim M, Tsang VT, Caruana M, et al. Cone reconstruction for Ebstein’s anomaly: patient outcomes, biventricular function, and cardiopulmonary exercise capacity. J Thorac Cardiovasc Surg. 2015;149:1144–50.
Attenhofer Jost CH, Connolly HM, Dearani JA, Edwards WD, Danielson GK. Ebstein’s anomaly. Circulation. 2007;115:277–85.
Yuan SM. Ebstein’s anomaly: genetics, clinical manifestations, and management. Pediatr Neonatol. 2017;58:211–5.
Kron IL, Roeser ME. Management of Ebstein’s anomaly. Ann Cardiothorac Surg. 2017;6:266–9.
Sadeghpour A, Alizadehasl A. The right ventricle: A comprehensive review from anatomy, physiology, and mechanics to hemodynamic, functional, and imaging evaluation. Review article. 2015;3(4):4–4. https://doi.org/10.5812/acvi.35717.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer-Verlag London Ltd., part of Springer Nature
About this chapter
Cite this chapter
Alizadehasl, A. (2021). Ebstein Anomaly. In: Maleki, M., Alizadehasl, A. (eds) Case-Based Clinical Cardiology. Springer, London. https://doi.org/10.1007/978-1-4471-7496-7_4
Download citation
DOI: https://doi.org/10.1007/978-1-4471-7496-7_4
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-7495-0
Online ISBN: 978-1-4471-7496-7
eBook Packages: MedicineMedicine (R0)