Abstract
MicroRNAs play important roles in the pathogenesis of human diseases by regulating target gene expression in specific cells or tissues. Previously, we identified microRNA 452 (MIR452), which was specifically upregulated in early stage of human colorectal cancer (CRC) tissues. Here, we show the biological role of MIR452 and general transcription factor IIE subunit 1 (GTF2E1) in colorectal cancer. A luciferase reporter system was used to confirm the effect of MIR452 on GTF2E1 expression. The expression levels of MIR452 and the target genes were evaluated by quantitative RT-PCR (qRT-PCR) and western blotting. We verified the association between MIR452 and the GTF2E1 expression and found that GTF2E1 transcripts were directly downregulated by MIR452. The mRNA and protein levels of GTF2E1 were also downregulated in CRC cells upon transfection with MIR452. GTF2E1 protein expression was decreased in CRC tissues compared to adjacent nontumour tissues. These results suggest that MIR452 might directly or indirectly regulate the genes transcription related to CRC by downregulating GTF2E1 expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
MicroRNAs (miRNAs or MiRs) endogenously synthesized small noncoding RNA molecules, involved in the regulation of crucial biological processes such as cell proliferation, differentiation, apoptosis and angiogenesis, by controlling the stability and translation of target mRNAs (Batel 2004; Serocki et al. 2018; Aydin et al. 2019). They also affect the pathogenesis of various types of cancers by functioning either as oncogenes or tumour suppressor genes (Lu et al. 2005; Braga et al. 2019). Accumulating evidence suggests that miRNAs are associated with several tumour types including cancers of lung (Wang et al. 2019; Zhang et al. 2019a, b), liver (Lu et al. 2019), gastric (Chen et al. 2019; Zhang et al. 2019a, b), and colorectal origin (CRC) (Mo et al. 2015, 2019; Alam et al. 2017).
MicroRNA 452 (MIR452, also known as hsa-miR-452) is encoded by the chromosomal region Xq28 in humans and clustered together with MIR224 within the gamma-aminobutyric acid A receptor epsilon subunit (GABRE) gene. Accumulating reports have shown the conflicting results of MIR452 expression levels in hepatocellular carcinoma (HCC) (Wang et al. 2012; Zheng et al. 2014). MIR452 has been shown to play diverse roles in distinct cancer types or tumourigenesis stage. In our previous study, MIR452 was upregulated in both early stage CRC tissues (Mo et al. 2015, 2019) and in dextran sulphate sodium salt (DSS)-induced colitis tissues of mice (Mo et al. 2016), as analysed by differential miRNA expression profiling.
In our previous study, the putative target genes of MIR452 were identified by comparing the mRNA microarray analysis of MIR452-overexpressing human CRC cells with the bioinformatics-predicted candidate target genes. We showed that MIR452 regulated cell proliferation, migration and angiogenesis by suppressing VEGFA expression in the early stage of CRC progression (Mo et al. 2019). We identified 27 putative target genes of MIR452 in our previous study (Mo et al. 2019; Mo and Chae, 2021). One of them, the general transcription factor IIE subunit 1 (GTF2E1, also known as TF2E1 and TFIIE-A) was a candidate target gene of MIR452. Since GTF2E1 is a general transcription factor, it was chosen to see that MIR452 can control the expression of other genes by controlling the general transcription factor.
In this study, we validated the expression levels of GTF2E1 in MIR452-transfected CRC cells, and showed that GTF2E1 expression was directly downregulated by MIR452. The expression levels of genes associated with GTF2E1, such as general transcription factor IIE subunit 2 (GTF2E2, also known as TTD6; TF2E2 and TFIIE-B), general transcription factor IIH subunit 1 (GTF2H1, also known as P62; BTF2; TFB1 and TFIIH), cyclin dependent kinase 7 (CDK7, also known as CAK1; HCAK; MO15; STK1; and CDKN), and RNA polymerase II subunit A (POLR2A, also known as RPB1; RPO2; POLR2 and POLRA) were also studied.
Material and methods
Patients and tissue samples
The tissue samples used in this study were provided by the Biobank of Wonkwang University Hospital, a member of the National Biobank of Korea. With approval from the institutional review board (WKIRB-201703-BR-010) and informed consent from patients. We obtained colon cancer tissues from four colon cancer patients (two males and two females) and rectal cancer tissues from six rectal cancer patients (four males and two females). The mean age of the colon cancer patients and rectal cancer patients was 64 years and 71 years, respectively. The human CRC tissue samples and matched normal colon tissue samples were used to analyse GTF2E1 protein expression.
Cell culture
The human CRC cell lines SW48, Caco2, SW480 and HT29 were obtained from the Korea Cell Line Bank (KCLB, Seoul, Korea) and American Type Culture Collection (ATCC, Manassas, USA), respectively. SW48, SW480 and HT29 cells were cultured in RPMI-1640 medium (HyClone, Logan, USA) supplemented with 10% FBS and incubated at 37°C in a humidified atmosphere containing 5% CO2. Caco-2 cells were cultured in Alpha-MEM (HyClone) supplemented with 20% FBS and incubated at 37°C in a humidified atmosphere containing 5% CO2.
RNA extraction and qRT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, USA), as described in our previous reports (Mo et al. 2019; Han et al. 2019). After digestion with DNase, RNA samples were quantified, aliquoted and stored at −80°C. The total RNA isolated from tissue samples and/or cultured cells was used as a template to synthesize cDNA for qRT-PCR analysis. The miRNA expression levels were validated by the TaqMan qRT-PCR assay (Applied Biosystems) for qRT-PCR. qRT-PCR with the SYBR Green dye (Applied Biosystems) was used to assess mRNA expression. RNU48 (for TaqMan qRT-PCR) and GAPDH served as endogenous controls for qRT-PCR of miRNA and mRNA, respectively. Primers for both qRT-PCR and TaqMan analyses are listed in table 1.
MIR452 transfection
SW48, SW480 and HT29 cells (3 × 105 cells per well) or Caco-2 cells (1.5 × 105 cells per well) were plated in 6-well or 10-cm culture plates and cultured as described above. MIR452 (hsa-miR-452, pre-miR miRNA Precursor AM17100, product ID: PM12509) was commercially synthesized (Ambion, Austin, USA) and transfected at a concentration of 50 nmol/L using Lipofectamine RNAiMAX (Invitrogen) or siPORT NeoFX transfection reagents (Ambion) according to the manufacturers’ recommendations. Cells were harvested at 48 h (for mRNA extraction) or 72 h (for protein extraction) after transfection.
Plasmid construct and luciferase assays
The wild type (WT) 3′ untranslated region (3′UTR) sequences of GTF2E1 containing the MIR452 binding site were PCR amplified from human genomic DNA. After amplification, the PCR products were cloned in the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega, Madison, USA). The corresponding mutant type (MT) 3′UTR sequences of GTF2E1 were prepared through the deletion of eight nucleotides in the seed region by using site directed mutagenesis PCR. All of the constructs were confirmed by DNA sequencing. The PCR primers used for luciferase assay are described in table 1. Analysis of the luciferase assay results was carried out according to our previously described method (Mo et al. 2019; Han et al. 2019). Briefly, cells (2 × 104 cells/well) were seeded onto 24-well plates and cotransfected with wild-type or mutant GTF2E1 constructs (500 ng/well) or with 50 nM of MIR452 or MIR1 (negative control) using Lipofectamine 2000 (Invitrogen) or siPORT NeoFX Transfection Agent (Ambion). Firefly and Renilla luminescence were measured 24 h after transfection using the Dual-Glo Luciferase Assay System (Promega) according to the manufacturer’s instructions. Untransfected cells were used for background subtraction, and the ratio of firefly reporter luminescence to Renilla reporter luminescence (control) was calculated. All experiments were performed in duplicate and repeated at least thrice.
Antibodies and western blot analysis
SW48, SW480, HT29 or Caco2 cells (2 × 105 cells/well) were seeded onto 6-well plates and cultured for 72 h. Whole cell lysates were prepared by culturing cells in RIPA buffer supplemented with a protease inhibitor mixture for 30 min at 4°C. Proteins were isolated by centrifugation at 12,000 rpm for 30 min at 4°C. Equal amounts of protein (50 μg; as determined by the Bradford assay) were boiled in Laemmli buffer, and separated by either 12.0% or 15.0% SDS-PAGE, and then transferred onto PVDF membranes. The membranes were incubated with blocking buffer (5% BSA in TBS containing 0.1% Tween-20 (TBS-T)) for 2 h at room temperature. Membranes were then incubated overnight at 4°C with primary antibodies to GTF2E1 (sc-374014), GTF2E2 (sc-238), GTF2H1 (sc-292), POLII (sc-899) and CDK7 (sc-7344; Santa Cruz Biotechnology), and washed thrice (10 min per wash) with TBS-T. Membranes were then incubated with HRP-conjugated rabbit or mouse IgG secondary antibodies for 1 h at room temperature. After washing thrice for 10 min in TBS-T, proteins were detected using ECL reagent (Millipore Corporation, Billerica, USA) and FluorChem E System (Cell Biosciences, Santa Clara, USA). After protein detection, some membranes were stripped using stripping buffer for 1 h at room temperature and reprobed with either GAPDH (sc-47724), which was used as a loading control. Protein expression was evaluated using ImageJ software (v. 1.44; http://rsbweb.nih.gov/ij/index.html).
Statistical analysis
We obtained consistent results from all experimental replicates. All data were represented as mean ± standard deviation (SD). Statistical differences were analysed by Student’s t-test or two-sided t-test, and results with P values less than 0.05 (P < 0.05) were regarded as statistically significant.
Results
GTF2E1 is direct targets of MIR452 in CRC cells
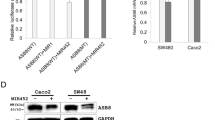
To determine whether MIR452 directly regulates GTF2E1 mRNA, we used a luciferase reporter system. The predicted MIR452 recognition sites in the GTF2E1 3′ UTRs were cloned into a pmirGLO Dual-Luciferase miRNA Target Expression Vector (pmirGLO-GTF2E1 WT). We also generated mutated (MT) MIR452 binding sites by site-directed mutagenesis (pmirGLO- GTF2E1 MT) (figure 1a). The base pairing between the MIR452 seed sites and the GTF2E1 mRNA targets is depicted in figure 1a. Subsequently, the vectors were cotransfected into Caco-2 cells with pre-MIR452 or pre-MIR1. MIR452 inhibited luciferase activity in cells transfected with pmirGLO-GTF2E1 WT (~32%, vs controls, P < 0.05) (figure 1b). In contrast, MIR452 did not reduce luciferase activity in cells transfected with pmirGLO-GTF2E1 MT vector construct (figure 1b).
MIR452 directly downregulates GTF2E1 expression. (a) Sequence alignment of MIR452 seed region and the putative binding sites in the GTF2E1 3′UTRs. The wild-type (WT) or mutant (MT) 3′UTR of the GTF2E1 gene containing the MIR452 target sequence was cloned into pmirGLO Dual-Luciferase miRNA Target Expression Vector. (b) Each luciferase reporter construct was cotransfected into Caco-2 cells with either MIR1 mimic as a negative control or MIR452 mimic. After 24 h, luciferase activity was measured in the cell extracts. Transfection with MIR452 mimic inhibited luciferase activity in the presence of WT GTF2E1 3′UTR (*P < 0.05; two-sided t-test). Inhibition was not observed when cells were transfected with reporter constructs containing the MT 3′UTR target sequences or with MIR1 (ns, not significant). (c) GTF2E1 mRNA levels in MIR452- or mock-transfected Caco-2 cells. Total RNA was isolated 48 h after transfection, and GTF2E1 mRNA levels were examined by qRT-PCR (*P < 0.05; two-sided t-test). (d) Western blot analysis of MIR452- or anti-MIR452 or mock-transfected Caco-2 cells. The GTF2E1 bands were quantified relative to the appropriate loading controls using ImageJ software and are shown relative to the protein level in mock-transfected cells. The GTF2E1 protein levels were reduced upon MIR452 transfection in Caco-2 cells (* P < 0.05). The data are representative of three independent experiments.
MIR452 regulates GTF2E1 expression in CRC cells
We investigated whether MIR452 regulated GTF2E1 mRNA and protein levels in Caco-2 cells. MIR452 mimic was transfected into Caco-2 cells, and qRT-PCR analysis was performed using cells harvested 48 h after transfection. GTF2E1 mRNA levels were significantly downregulated in Caco-2 cells transfected with MIR452 (figure 1c; P < 0.05). MIR452 mimic or anti-MIR452 were transfected into Caco-2 cells, and western blot analyses were performed using cells harvested 72 h after transfection. GTF2E1 protein expression levels were significantly downregulated in Caco-2 cells transfected with MIR452 (figure 1d; P < 0.05). On the contrary, GTF2E1 protein expression levels were upregulated in Caco-2 cells transfected with anti-MIR452 (figure 1d; P < 0.05). The ani-MIR452 used in this experiment was purchased for commercial use and could not see the perfect MIR452 suppression effect. These results suggest that the expression of GTF2E1 is directly regulated by MIR452 in CRC cells.
We also investigated GTF2E1 expression levels by M1R452 transaction using SW480 and HT29 cell lines. GTF2E1 protein expression levels were significantly downregulated in both SW480 and HT29 cells transfected with MIR452 (figure 1e; P < 0.05).
MIR452 regulates proteins related to GTF2E1
To determine the functional interaction between MIR452 and GTF2E1 in Caco-2 and SW48 cells, we analysed the expression levels of proteins associated with the functions of GTF2E1 by western blot analysis. GTF2E2 and POLR2A protein expression levels were reduced upon transfection with MIR452 mimic in both Caco-2 and SW48 cells, respectively (figure 2; P < 0.05). However, GTF2H1 and CDK7 protein expression levels were slightly upregulated when transfected with MIR452 mimic in both Caco-2 and SW48 cells, however, the results were not statistically significant (figure 2; ns, not significant). These results indicated that MIR452 regulates the expression of GTF2E1–GTF2E2–POLR2A axis in CRC cells.
MIR452 regulate GTF2E1 associated molecules in CRC cells. The expression levels of GTF2E2, GTF2H1, CDK7 and POLR2A in Caco-2 and Sw48 cells upon MIR452 mimic or mock- transfection. The results on the right indicate the relative expression levels of each protein. Five independent experiments were performed in duplicates, and the statistical differences were calculated using student’s t-test or two-sided t-test (ns, not significant; *P < 0.05).
MIR452 expression level in human CRC tissues
We have previously found that MIR452 is upregulated in human CRC tissues. To confirm this result, we compared the MIR452 levels in five human CRC tissue samples with those in matching healthy colon tissues by qRT-PCR. MIR452 levels were increased in CRC tissues (four out of five, figure 3a).
Endogenous MIR452 and GTF2E1 expression levels in human CRC tissues. (a) The expression of MIR452 was validated using 10 CRC tissue samples and matched with normal colon tissue samples. MiRNAs levels were normalized to colon specific RNU48. Values are presented as the fold-change in tumour tissue relative levels (ΔΔCT) to normal tissue. (b) GTF2E1 expression in 10 pairs of human CRC tissue samples and adjacent normal colon tissue samples. The GTF2E1 levels were quantified by ImageJ software (* P < 0.05; two-sided t-test). N and T indicate normal colorectal and tumour colorectal tissues, respectively. The data are representative of three independent experiments.
GFT2E1 expression levels in human CRC tissues
On the basis of the findings described above, we evaluated GTF2E1 expression in 10 human CRC tissues and their matched normal colorectal tissues by western blotting. GTF2E1 protein expression was decreased in CRC tissues (nine out of 10 pairs) compared to adjacent nontumourous tissues (P < 0.05; figure 3b).
Discussion
MiRNAs are important regulators of gene expression and their aberrant expression has been implicated in the development of various diseases (Bartel 2004; Lu et al. 2005; Garzon et al. 2006). Accumulating evidence suggests that miRNAs act as tumour suppressors or oncogenes by targeting genes involved in cell proliferation, survival, apoptosis and metastasis (Brennecke et al. 2003; Ma et al. 2007; Su et al. 2009). Therefore, characterization of the correlations between miRNAs and their target genes in colon cancer may have a substantial diagnostic, prognostic and therapeutic value. We identified MIR452 as an associated microRNA in both early stage human CRC tissues and DSS-induced colitis tissues of mice, and showed that MIR452 expression was upregulated in both these types of tissues compared to matched healthy CRC and normal mouse colon tissues, respectively (Mo et al. 2016, 2019). In this study, we validated the expression levels of GTF2E1 in CRC tissues and MIR452-transfected CRC cells to confirm that it indeed is the target gene of MIR452.
GTF2E1 is a subunit gene of GTF2E (also known as TFIIE) that is a member of the general transcription factors (GTFs). The transcription of protein-encoding genes in eukaryotes is performed by RNA polymerase II (also known as RNAP II and POL II) and GTFs (Roeder 1996; Bentley 2014). Five GTFs such as TFIIB, TFIID, TFIIE, TFIIF and TFIIH are required for transcription initiation by Pol II (Orphanides et al. 1996; Roeder 1996). GTF2E is composed of two subunits α (GTF2E1) and β (GTF2E2) with a molecular mass of 56 kDa and 34 kDa, respectively (Ohkuma et al. 1990). These two subunits interact with each other (Okamoto et al. 1998), and act as key factors to recruit the TFIIH kinase module (CAK) of GTF2H (Compe et al. 2019). Accumulating reports suggest that GTF2E plays a pivotal role in transcription by directly influencing the transition from initiation to elongation (Peterson et al. 1991; Watanabe et al. 2003). GTF2H1 and CDK7 are subunit genes of GTF2H (TFIIH), and POLR2A is a member of POL II.
We showed that GTF2E1 is a direct target of MIR452 in CRC cells (figure 1b). The GTF2E1 mRNA and protein expression levels were downregulated upon transfection with MIR452 mimic in Caco-2 cells (figure 1, c&d). On the contrary, GTF2E1 protein levels were upregulated in Caco-2 cells when transfected with anti-MIR452 construct (figure 1d). Thus, these results suggest that the upregulated levels of MIR452 in CRC cells may regulate GTF2E1 expression levels during colorectal carcinogenesis.
These results led us to study whether the expression of GTF2E1 reduced by MIR452 in CRC cells affected genes related to transcription initiation. The expression levels of GTF2E1, GTF2E2 and POLR2A were significantly downregulated when transfected with MIR452 mimic in the two CRC cell lines (figure 2). These results suggest that the expression level of GTF2E2 was directly downregulated by MIR452, while POLR2A was indirectly regulated by MIR452 transfection in CRC cells. Although GTF2H1 and CDK7 expression levels were slightly increased upon MIR452 mimic transfection in both CRC cell lines, there were no significant associations (figure 2). Although these results suggest that the functions of GTF2E and GTF2H might be differing from each other during transcription initiation due to MIR452 regulation, the reason for this difference is unclear at this point.
In this study, we also showed that the protein expression levels of GTF2E1 were downregulated in CRC tissues compared to adjacent nontumourous tissues (figure 3b). Thus, these results suggest that the upregulated expression of MIR452 in CRC tissues might directly regulate the transcription of other genes by inhibition of GTF2E1 during CRC pathogenesis. GTF2E1 is connected to the driver and tumour suppressor genes, and affect cancer-related functions such as transmembrane transport and transformation factors (Chang et al. 2020).
Although we did not study in great detail the signalling mechanisms of GTF2E1 mediated by the regulation of MIR452, our results in CRC cells suggest that GTF2E1 was a direct target gene of MIR452. Therefore, the upregulated expression of MIR452, as observed in CRC tissues, might regulate the expression levels of GTF2E1 during CRC carcinogenesis. Down regulated GTF2E1 by MIR452 during early CRC carcinogenesis plays an important role in transcription by directly influencing the transition from initiation to elongation. Our results could provide a valuable resource for further functional studies of genes associated with GTF2E1 in the pathogenesis of various organs.
References
Alam K. J., Mo J. S., Han S. H., Park W. C., Kim H. S., Yun K. J. et al. 2017 MicroRNA 375 regulates proliferation and migration of colon cancer cells by suppressing the CTGF-EGFR signaling pathway. Int. J. Cancer 141, 1614–1629.
Aydin Y., Kurt R., Song K., Lin D., Osman H., Youngquist B. et al. 2019 Hepatic stress response in HCV infection promotes STAT3-mediated inhibition of HNF4A-miR-122 feedback loop in liver fibrosis and cancer progression. Cancers (basel) 11, 10.
Bartel D. P. 2004 MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297.
Bentley D. L. 2014 Coupling mRNA processing with transcription in time and space. Nat. Rev. Genet. 15, 163–175.
Braga E. A., Fridman M. V., Loginov V. I., Dmitriev A. A. and Morozov S. G. 2019 Molecular mechanisms in clear cell renal cell carcinoma: role of miRNAs and hypermethylated miRNA genes in crucial oncogenic pathways and processes. Front. Genet. 10, 320.
Brennecke J., Hipfner D. R., Stark A., Russell R. B. and Cohen S. M. 2003 bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113, 25–36.
Chang Z., Miao X. and Zhao W. 2020 Identification of prognostic dosage-sensitive genes in colorectal cancer based on multi-omics. Front. Genet. 10, 1310.
Chen X. L., Hong L. L., Wang K. L., Liu X., Wang J. L., Lei L. et al. 2019 Deregulation of CSMD1 targeted by microRNA-10b drives gastric cancer progression through the NF-κB pathway. Int. J. Biol. Sci. 15, 2075–2086.
Compe E., Genes C. M., Braun C., Coin F. and Egly J. M. 2019 TFIIE orchestrates the recruitment of the TFIIH kinase module at promoter before release during transcription. Nat. Commun. 7, 2084.
Garzon R., Fabbri M., Cimmino A., Calin G. A. and Croce C. M. 2006 MicroRNA expression and function in cancer. Trends Mol. Med. 12, 580–587.
Han S. H., Mo J. S., Park W. C. and Chae S. C. 2019 Reduced microRNA 375 in colorectal cancer upregulates metadherin-mediated signaling. World J. Gastroenterol. 25, 6495–6507.
Lu J., Getz G., Miska E. A., Alvarez-Saavedra E., Lamb J., Peck D. et al. 2005 MicroRNA expression profiles classify human cancers. Nature 435, 834–838.
Lu Z., Li X., Xu Y., Chen M., Chen W., Chen T. et al. 2019 microRNA-17 functions as an oncogene by downregulating Smad3 expression in hepatocellular carcinoma. Cell Death Dis. 10, 723.
Ma L., Teruya-Feldstein J. and Weinberg R. A. 2007 Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 449, 682–688.
Mo J. S. and Chae S. C. 2021 MicroRNA 452 regulates ASB8, NOL8, and CDR2 expression in colorectal cancer cells. Genes Genomics 43, 33–41.
Mo J. S., Alam K. J., Kang I. H., Park W. C., Seo G. S., Choi S. C. et al. 2015 MicroRNA 196B regulates FAS-mediated apoptosis in colorectal cancer cells. Oncotarget 6, 2843–2855.
Mo J. S., Alam K. J., Kim H. S., Lee Y. M., Yun K. J. and Chae S. C. 2016 MicroRNA 429 regulates mucin gene expression and secretion in murine model of colitis. J. Crohns Colitis 10, 850–859.
Mo J. S., Park W. C., Choi S. C., Yun K. J. and Chae S. C. 2019 MicroRNA 452 regulates cell proliferation, cell migration, and angiogenesis in colorectal cancer by suppressing VEGFA expression. Cancers 11, 1613.
Ohkuma Y., Sumimoto H., Horikoshi M. and Roeder R. G. 1990 Factors involved in specific transcription by mammalian RNA polymerase II: purification and characterization of general transcription factor TFIIE. Proc. Natl. Acad. Sci. USA 87, 9163–9167.
Okamoto T., Yamamoto S., Watanabe Y., Ohta T., Hanaoka F., Roeder R. G. et al. 1998 Analysis of the role of TFIIE in transcriptional regulation through structure function studies of the TFIIEb subunit. J. Biol. Chem. 273, 19866–19876.
Orphanides G., Lagrange T. and Reinberg D. 1996 The general transcription factors of RNA polymerase II. Genes Dev. 10, 2657–2683.
Peterson M. G., Inostroza J., Maxon M. E., Flores O., Admon A., Reinberg D. et al. 1991 Structure and functional properties of human general transcription factor IIE. Nature 354, 369–373.
Roeder R. G. 1996 The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem. Sci. 21, 327–335.
Serocki M., Bartoszewska S., Janaszak-Jasiecka A., Ochocka R. J., Collawn J. F. and Bartoszewski R. 2018 miRNAs regulate the HIF switch during hypoxia: a novel therapeutic target. Angiogenesis 21, 183–202.
Su H., Yang J. R., Xu T., Huang J., Xu L., Yuan Y. et al. 2009 MicroRNA-101, down-regulated in hepatocellular carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer Res. 69, 1135–1142.
Wang Y., Toh H. C., Chow P., Chung A. Y. F., Meyers D. J., Cole P. A. et al. 2012 MicroRNA-224 is upregulated in hepatocellular carcinoma through epigenetic mechanisms. Faseb. J. 26, 3032–3041.
Wang Y. F., Ao X., Liu Y., Ding D., Jiao W. J., Yu Z. et al. 2019 MicroRNA-608 promotes apoptosis in non-small cell lung cancer cells treated with doxorubicin through the inhibition of TFAP4. Front. Genet. 10, 809.
Watanabe T., Hayashi K., Tanaka A., Furumoto T., Hanaoka F. and Ohkuma Y. 2003 The carboxy terminus of the small subunit of TFIIE regulates the transition from transcription initiation to elongation by RNA polymerase II. Mol. Cell. Biol. 23, 2914–2926.
Zhang W. C., Wells J. M., Chow K. H., Huang H., Yuan M., Saxena T. et al. 2019a miR-147b-mediated TCA cycle dysfunction and pseudohypoxia initiate drug tolerance to EGFR inhibitors in lung adenocarcinoma. Nat. Metab. 1, 460–474.
Zhang Z., Pi J., Zou D., Wang X., Xu J., Yu S. et al. 2019b microRNA arm-imbalance in part from complementary targets mediated decay promotes gastric cancer progression. Nat. Commun. 10, 4397.
Zheng Q., Sheng Q., Jiang C., Shu J., Chen J., Nie Z. et al. 2014 MicroRNA-452 promotes tumorigenesis in hepatocellular carcinoma by targeting cyclin-dependent kinase inhibitor 1B. Mol. Cell. Biochem. 389, 187–195.
Acknowledgements
The biospecimens for this study were provided by the Biobank of Wonkwang University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health and Welfare. This research was supported by Wonkwang University in 2019.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Murali Dharan Bashyam
Rights and permissions
About this article
Cite this article
Mo, J.S., Chae, S.C. MicroRNA 452 regulates GTF2E1 expression in colorectal cancer cells. J Genet 100, 62 (2021). https://doi.org/10.1007/s12041-021-01312-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12041-021-01312-3