Abstract
The deterioration of groundwater quality has become a serious problem for the safe drinking water supply in many parts of the world. Along coastal aquifers, the saline water moves landward due to several reasons even though significant rainfall is available. The objective of the present study is to investigate the impact of a combined recharge structure including a percolation pond and a recharge shaft in improving the groundwater quality of the surrounding area. The area chosen for this study is Andarmadam, Thiruvallur district of Tamil Nadu. As a part of the study, a suitable site was selected for the construction of a percolation pond based on preliminary field investigations in 2012. Three piezometers were also constructed near the percolation pond to investigate the impact of the structure on groundwater recharge. Further, a recharge shaft was added to this structure in 2013 to overcome the clogging issues at the pond bottom and to enhance the recharge. The impact of the percolation pond on groundwater was assessed by comparing the periodical groundwater level fluctuations with rainfall in the area. The fluctuations in groundwater level near the percolation pond show variations before and after the construction of recharge shaft. The amount of water recharged through the percolation pond during the water year 2012–2013 was estimated as 250–300 m3. The volume of recharge was calculated to be increased more than twice after the construction of recharge shaft inside the percolation pond, on the assumption that recharge through the pond surface remained almost same as before. The dilution of ionic concentration in water was three times higher after the construction of recharge shaft. The long-term groundwater quality in the surrounding area of the pond improves gradually with time. The total dissolved solids (TDS) decrease considerably with time due to the dilution of dissolved solids in water with the fresh water recharging into the aquifer. The Wilcox diagram of most of the water samples after the construction of the recharge structure fall in the excellent to good category, indicating improvement in irrigation water quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The deterioration of fresh water resources by saline intrusion (Schroter et al. 2005; Werner et al. 2013) has become a global issue which affects the coastal water quality and threatens the sustainable water supply to the coastal communities (Cassardo and Jones 2011; Gain et al. 2012). Even though several expensive technological solutions are available to deal with reduced freshwater availability along coastal aquifers (Shahid et al. 2000), the management of coastal water resources by natural or artificial recharge has been widely promoted all over the world (Shahid et al. 2000; Sener et al. 2005). Even though precipitation is the dominant source of natural recharge to aquifer systems, other possible sources such as surface water bodies, irrigation and induced recharge also attribute to subsurface recharge (Anbazhagan et al. 2005; Parimala and Elango 2013; Zuurbier and Stuyfzand 2016; Raicy and Elango 2014). The type of recharge process depends on the nature and source of water used for the aquifer recharge (Balke and Zhu 2008). The enhanced fresh water recovery (Maliva et al. 2005; Ward et al. 2007; Parimala and Elango 2013) and improved groundwater quality (Bakker 2010; Zuurbier et al. 2014) upon aquifer recharge has been reported all over the world. Different techniques of aquifer recharge such as aquifer storage and recovery, aquifer storage transfer and recovery, bank filtration, dune filtration, infiltration ponds, percolation tanks, rain water harvesting, soil aquifer treatment, sand dams, underground dams and recharge releases have been successfully used for groundwater storage for water-resource management in the USA, Europe, South Africa, India, China and Middle East (Shahid et al. 2000; Martin et al. 2002; Sener et al. 2005; Dillon et al. 2009; Zuurbier et al. 2015).
In India, the benefit of managed aquifer recharge is being exploited since the last few decades (Anbazhagan et al. 2005). Considering India’s present groundwater situation, aquifer recharge can be adopted further in about 14% of the area (CGWB 2005). Percolation ponds are one among the most economically feasible methods of aquifer recharge and have been implemented widely in India by the support of both Central government and international agencies (Farrington et al. 1999). A few examples are in Agrahara Valavanthi micro-watershed, Namakkal District (TWAD 1998), Nedardi (VIKSAT 2004) at Moti Rayan and Bhujpur area in Mandvi Kutch District (CGWB 2000).
Aquifer recharge through inverted shafts when water is available gain attention in recent years. These aquifer storages transfer and recovery (ASTR) methods involve several steps such as capture of water sources such as storm water, percolation of water into the underground aquifer, temporary storage of water in the aquifer and extraction of water for later use. The aquifer system can be managed in a better way by these combined recharge techniques to function in a much effective manner. Many countries and several US states such as Florida, Arizona, and California practice ASTR methods (Martin et al. 2002).
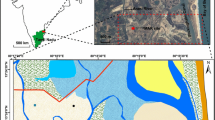
The efficacy of aquifer recharge structures in improving the groundwater quality in the nearby areas has not been explored much as compared to groundwater recharge. Hence, the present study was carried out to assess the feasibility of a percolation pond and a recharge shaft in improving the quality of groundwater in a saline aquifer, north of Chennai (figure 1). Seawater intrusion due to overpumping of groundwater during the last few decades along with marine deposits of Holocene age (Rao 1979) have led to very high salinity of groundwater in this area (Elango and Manickam 1987; UNDP 1987; Elango and Ramachandran 1991; Charalambous and Garratt 2009; Raicy et al. 2014; Rajaveni et al. 2015). The groundwater is saline also due to the recharge of saline water from the Buckingham canal and the backwaters from Pulicat estuary. The non-availability of usable water for the local community both for agriculture and for domestic use urge the immense need to convert the excess rainfall runoff into recharge. Hence, an economically feasible and easily practicable recharge structure was constructed in the area to investigate its effect in coping with saline water intrusion. Hence the objective of the present study is to assess the impact of a combined recharge structure including a percolation pond and a recharge shaft in improving the groundwater recharge and quality.
2 Methodology
2.1 Study area
The study area forms a part of Arani river basin, which is located at about 40 km north of Chennai, Tamil Nadu, India (figure 1). The area lies nearly 3.8 km west of Bay of Bengal and 0.15 km east of Arani river, which joins the sea at about 4 km north through Pulicat estuary. The Arani river is non-perennial and flows for short periods during the northeast monsoon (October–December). The average annual rainfall in the area is about 1200 mm. The average elevation of the area is 3 m msl. The area is mainly characterized by alluvial and coastal plains. The basement of this region comprises of gneissic and charnockite rocks of Proterozoic era which is overlain by Gondwana and Tertiary formations. Quaternary formation of about 45–60 m thickness, consisting of clay, silt, sand, gravel, their admixtures and sandstone, occupies the top of the sequence, which are underlined by Carbonaceous shales of Upper Gondwana. The quaternary formation functions as unconfined aquifer. The groundwater occurs at shallow depths. The depth to groundwater table varies from 2 to 3 m from ground surface. The groundwater flow direction is from west to east.
2.2 Field investigation
As a part of the study, field investigations were carried out to select suitable locations for recharge structures to achieve maximum performance. A percolation pond and three piezometers P1, P2 and P3 with depths 2, 4, and 6 m, respectively, were constructed at 0.5 m apart during May 2012. The bottom of the piezometers was perforated for a length of 0.5 m and were wrapped with wire mesh. The design of these structures are detailed in Raicy and Elango (2017). Even though this structure could collect maximum surface runoff water and could recharge the aquifer, its impact in improving the groundwater quality in the nearby area was less due to its small size and clogging of suspended particle at the pond bottom which reduced the recharge (Raicy et al. 2014). Hence, to directly recharge the aquifer, a recharge shaft was constructed at the center of the percolation pond by the method of hand auguring during August 2013. The schematic sketch of the combined recharge structure is shown in figure 2. The shaft includes two polyvinyl chloride (PVC) slotted pipes of 0.15 and 0.25 m diameter, in which the former is kept inside the latter. The space in between outer and inner pipes was packed with gravels. The bottom of recharge shaft was kept at maximum reachable depth where the formation is sandy. The length of the vertical recharge shaft is 9.5 m, beyond which hand auguring was difficult. The direct vertical entry of pond water into the gravel pack and subsequent clogging of suspended particles in the pore spaces inside the gravel pack were restricted by a concrete base made at the pond bottom surrounding the inner pipe. The same setup was also arranged as a continuation of the shaft above pond bottom. The outer pipe was coated by a dhoti cloth to filter the water containing suspended particles before entering the gravel pack. The inner pipe is sealed by a PVC cap at the top to restrict the direct entry of suspended pond water into the aquifer. When the pond is filled with water, it enters the outer pipe, wherein the dhoti cloth and gravels act as filtering mediums. The filtered water, entering the inner pipe, spread both horizontally and vertically due to the hydrostatic pressure inside the pipe. The horizontal movement of water is also aided by the secondary filtration through the gravel packing surrounding the inner pipe. The groundwater level and quality of the nearby area were monitored periodically to assess the impact of the induced recharge.
Rainfall in the area was monitored using an automatic weather station installed at an approximate distance of 100 m from the pond. Digital automatic water level recorders (Solinst 3001) were installed in piezometers as well as in the pond and were set to monitor the groundwater level. Water level in the pond was measured once in two weeks. Water samples from the pond and piezometers were collected in clean, inert 500 ml plastic bottles every two weeks. The alkalinity of water samples was measured by titration (Merck 1.11109.0001) and the electrical conductivity was measured using a conductivity meter (Eutech Cyber scan 600) immediately after sampling in the field. A default temperature of 25°C and a linear temperature coefficient of 2.1% per °C were selected in the conductivity meter to directly measure temperature corrected electrical conductivity. The water samples were brought to the laboratory immediately after collection and were analysed for the concentration of major and minor ions by an ion chromatograph (Metrohm Compact 861). Samples were filtered with 0.22 µm filter paper and were analysed after suitable dilution. The TDS in water were calculated by multiplying the electrical conductivity (EC) in µS/cm by a factor of 0.64.
As the pond water level and evaporation data were available, the groundwater recharge was estimated by water balance approach, which states that the water recharged is equal to the change in storage in the pond minus the volume of water lost due to evaporation. Water balance models, which estimate the balance between inflow and outflow, were developed in the 1940s by Thornthwaite (1948) and revised by Thornthwaite and Mather (1955). This method can be used to quantitatively evaluate the dynamic behaviour of water resources as well as the individual contribution of water sources over different time periods and to establish the degree of variation in water regime due to changes in components of the system (Weiss and Gvirtzman 2007). The basic concept of water balance is that storage of the system (over a period) is equal to the difference between change in input to the system and outflow from the system. The general components of computations of water balance are identification of significant components, evaluation/quantification of individual components and the presentation in the form of water balance equation. The approach for water balance, can be stated as:
3 Results and discussion
3.1 Improvements in groundwater level
As the water level in the percolation pond rises due to the surface runoff collected during precipitation, the groundwater level in the piezometers also shows sequential rise. Hence, rainfall events, which could produce enough surface run off to fill the percolation pond, were followed by an increase in groundwater level in the piezometers. The periodicity of rainfall events during the water year 2012–2013 was comparatively less and the pond water level started declining from January 2013 until it became dry by the end of May 2013 (figure 3). During the water year 2013–2014, the water level rose to the maximum capacity of the percolation pond by the end of September 2013 and started declining subsequently by January. The pond became dry by March 2014 (figure 3) and this rapid water level drop is attributed to the direct entry of water to the aquifer directly through the shaft, which makes the recharge process faster. This recharge process reoccurred annually and a water level of nearly 30 cm was sustained in the piezometers, after construction of recharge shaft, even when the pond became dry (figure 3).
3.2 Estimated recharge volume
The amount of water recharged into the aquifer through the percolation pond before and after the construction of recharge shaft was calculated by considering the temporal variation in water level and surface area of the water in the pond. As the horizontal to vertical side slope of the percolation pond was maintained as 1:1 on all sides, the surface area changes with respect to water level. The monthly volume of water recharged into the aquifer through the percolation pond from 2012 to 2016 is shown in figure 4. The volume of water recharged through the percolation pond during the water year 2012–2013 is estimated as 98 m3 (Raicy and Elango 2017). As the rainy season commenced during August 2012, suspended particles in pond water settles at the bottom, which slowed down the rate of recharge. As more and more particles settled, the thickness of clogged layers at the pond bottom increased which caused a gradual decline in recharge rate. However, after the construction of recharge shaft, groundwater recharge increased considerably (figure 4). The annual volume of water recharged from the combined structure during the water years 2013–2014, 2014–2015 and 2015–2016 were estimated as 356, 396 and 460 m3, respectively. This is two to three times of the amount of recharge through the percolation pond without recharge shaft (figure 5). The volume of recharge was higher during the water years 2014–2015 and 2015–2016 due to frequent intermittent rainfall events and subsequent recurrence of water level rise (figure 3).
3.3 Improvement in groundwater quality
The recharge from the percolation pond has enhanced the groundwater quality in the nearby piezometers. When the pond fills, the groundwater level in the nearby area rises and forms a fresh water plume, which dilutes the concentration of ions in groundwater. The long-term groundwater quality in the surrounding area of the pond shows a gradual improvement with time (figure 6). Even though there was water at the bottom of P1, it was difficult to pump or extract water when the water level was very close to the bottom surface. So we could not collect water samples from P1 during some months. The impact of the structure was assessed by comparing the groundwater quality before and after the construction of recharge shaft. EC of groundwater shows distinct variations before and after the construction of recharge shaft. The range EC of water in pond, P1, P2 and P3 during 2012–2013 were 552–1778, 555–2627, 33290–41212 and 58313–73640 µS/cm, respectively (figure 6). EC increases with depth and the groundwater is super-saline at a depth of 6 m. High EC of groundwater is attributed to the presence of marine sediments and nearby saline surface water bodies in the area (UNDP 1987). The groundwater quality improved significantly after the construction of the recharge shaft. However, the range of EC of water in pond, P1, P2 and P3 during the water year 2013–2014 were reduced to 590–1217, 876–1835, 34547–19201 and 63569–1773 µS/cm, respectively, after the construction of the recharge shaft. Figure 6 shows EC of water in pond, P1, P2 and P3 before (2012–2013) and after (2013–2016) the construction of recharge shaft. The dilution of ionic concentration in groundwater during rainfall recharge is comparably less before construction of recharge shaft, whereas dilution is around three times more after the construction of recharge shaft. This is evidenced by the drastic decrease in EC during 2013–2014. Though the recharge takes place only for about five months every year, the EC of groundwater in P3 remains comparatively less throughout the year due to the direct horizontal recharge through the sandy layer.
3.4 Effect of recharge on concentration of ions
The variation in concentration of hydrochemical components was investigated to understand the impact of percolation pond in improving the groundwater quality in the vicinity. Even though the concentration of Na+ and Cl− were reduced after rainfall, the concentration of ions increases gradually due to leaching from in-situ sediments in the area. However, the EC and concentration of ions sustain to a reduced level after drying up of the pond. As the volume of water recharged into the aquifer is more after the construction of recharge shaft, the rate of dilution is also high, which is indicated by a sharp decrease in EC and ionic concentrations during August 2013. Hence, the grade of groundwater quality primarily depends on the level of contamination of groundwater in the area and volume of water recharged into the aquifer.
The order of dominance of major and minor ions in the water in pond, P1, P2 and P3 are Cl– > Na+ > Mg2+ > SO42− > Ca2+ > HCO3–, Cl– > Mg2+ > Na+ > Ca2+ > SO42– > HCO3–, Na+ > Cl– > Mg2+ > SO42– > Ca2+ > HCO3– and Cl– > Na+ > SO42– > Mg2+ > Ca2+ > HCO3–, respectively, before the construction of recharge shaft. The dilution of ions in P1 was more as compared to P2 and P3 during 2012–2013 (table 1) (figure 6) due to the greater interaction between pond water and groundwater at shallower depths. The reduction in the concentration of major ions in water is due to the recharge of relatively fresh rain water collected in the pond. The mean concentrations of major ions in water in the pond, P1, P2 and P3 before and after the construction of recharge shaft are shown in table 1. The ionic concentrations in water decrease immediately after the rainfall recharge due to the dilution of ions by the fresh runoff water and after that gradually increases with time as evident from table 1. The concentrations of cations and anions show a decreasing trend over the past 3 years and this indicates that the continuous recharge of fresh rainfall and runoff from the surrounding area to the aquifer can dilute the salinity of groundwater in due course of time. However, most of the cation and anion concentration in P1 increases during the water year 2014–2015. During this time, even though the rainfall was commenced by July 2014, the pond was getting filled only by the end of August. The considerably long gap between the rainy season in 2013 and 2014, along with the high mean daily temperatures caused accumulation of evaporated residues of salts in the near surface. As P1 is shallow, effect of evaporate residue will be more in comparison to deeper piezometers. When the pond was filled during August 2014, the dissolution of salts causes higher concentration of ions in P1. The same process happens in pond also. However, as the clogged sediment layer at the pond bottom was removed before the commencement of monsoon, the effect is less in pond water. The ionic concentration of water in P2 and P3 did not vary significantly as compared to pond and P1. In P2 and P3, the fresh groundwater recharged from the pond stays at the top of saturated zone due to lower density and the dense saline groundwater at deeper zones slows down the rate of dilution. The faster dilution in P1 was due to the rapid recharge and interaction between pond water and P1 due to the proximity of P1 to the pond. Also, as the bottom of P1 is at 2 m, even small rainfall events which could bring run off water to the pond, could dilute the ionic concentrations of water in P1. However, the infiltration rate is rather slow below P1 due to the presence of low permeable sandy silt and silt layer and hence the improvement in groundwater quality in P2 and P3 is less during the water year 2012–2013. This is evident by the high concentration of Na+ as compared to Ca2+ and Mg2+ in the water in P2 and P3. After the construction of recharge shaft, the concentration of major ions in P3 becomes less than that of P2 (table 1), indicating that the perforated zone of P3 is very much benefitted due to the recharge through the shaft bypassing a silty layer of about 1 m thickness present just beneath P2. This is due to the comparatively higher hydraulic conductivity of this zone, which could directly benefit the water in P3 (figure 6). The order of dominance of ions in the pond and the piezometers after the construction of recharge shaft also remained same for pond, P1 and P2.
3.5 Reduction in TDS
The suitability of water for domestic use mainly depends on TDS, which is the organic/inorganic salts in water, generally deriving from natural seepages, urban/agricultural runoff industrial waste water, etc. The palatability of drinking water has been classified into excellent for less than 300 mg/l, good for 300–600 mg/l, fair 600–900 mg/l, poor 900–1200 mg/l and unacceptable for greater than 1200 mg/l based on TDS level (WHO 2004). The minimum and maximum of TDS before and after construction of recharge shaft is shown in table 2. Almost all the samples were categorised as unsuitable for drinking purpose except few of the pond water samples. The minimum TDS values increased from 2012 to 2016 which may be attributed to the continuous reoccurring of precipitation and evaporation, that can accumulate high concentrations of water soluble minerals and ions overtime at the pond bottom. Also, runoff coming from agricultural fields may carry organic sources such as grass and animal decays which contribute soluble minerals. However, the maximum TDS values considerably decreased every year due to the dilution of dissolved solids in water with the fresh water recharged into the aquifer.
3.6 Improvement in irrigation water quality
The water quality for irrigation purpose has been a concern during recent decades due to groundwater deterioration. The very high EC and elevated levels of Na+ and Cl– ions in groundwater along the coastal aquifers make them unsuitable for irrigation. The irrigation water quality of the water samples was assessed by calculating the concentration of sodium, which is expressed by sodium percentage (Todd and Mays 2005). High level of sodium in groundwater affects the soil permeability by clogging. The sodium percentage in water can be obtained by the relation
where all hydrogeochemical parameters are given in meq/l.
Based on the percentage of sodium, water is classified into excellent, good, permissible, doubtful and unsuitable categories with respective sodium percentage values below 20, 20–40, 40–60, 60–80 and more than 80 (Wilcox 1955). Wilcox (1955) diagram of water samples collected during the water year 2012–2013 shows that water in pond and P1 are classified in the excellent to good category, P2 in the doubtful to unsuitable category and P3 in the unusable category as illustrated in figure 7. However, after the construction of the recharge shaft, the irrigation water quality was improved as shown in figure 7. Most of the pond water samples from 2012 to 2013 fall in the permissible to doubtful category, even though the source water for recharge is rainfall. The groundwater quality in P2 shows a gradual shift towards doubtful to unsuitable category from the unsuitable category after the construction of recharge shaft. Similarly, the irrigation water quality in P3 shifted to excellent to good category from the unsuitable category. The benefits from the recharge structure were more in P3 as compared to that in P2 because of the direct recharge from the shaft at greater depths through the sandy layer, which enables more recharge and higher rate of dilution.
4 Conclusion
Providing drinking water supply for rural community has become a major concern in areas, where the groundwater is extremely saline. The present study investigated the effect of recharge from a small percolation pond with a recharge shaft at its centre, in saline groundwater. The study site is in the northeastern part of the Arani Korattalaiyar river basin, where the groundwater is saline and the local community have not been supplied with adequate safe drinking water. The electrical conductivity of groundwater in the area is about 70000 µs/cm. The recharge from the percolation pond could not improve the groundwater quality significantly in the deeper high salinity zones as the quantity of recharge was not enough to dilute the brackish groundwater. However, the combined recharge structure could recharge on an annual average of 376 m3 of water from 2012 to 2016 which is 2.5–3 times more of the recharge by the percolation pond without recharge shaft. This could improve the groundwater quality of the nearby area significantly. Maximum dilution of ionic species could be observed in P3, which is more benefitted by the recharge shaft through the permeable sandy layer. Even though, the combined structure could not enhance the drinking water quality to permissible limits, the irrigation water quality was improved from unsuitable category to excellent to good category in P3 and doubtful to unsuitable category in P2. The EC and concentration of ions sustains to a reduced level throughout the year after the construction of recharge shaft. The periodic maintenance and cleaning of the structure can improve the performance. The impact of combined structure could have been more in areas where the depth to water table is more than 3 m and the background salinity of groundwater is not very high.
References
Anbazhagan S, Ramasamy S M and Das Gupta S 2005 Remote sensing and GIS for artificial recharge study, runoff estimation and planning in Ayyar basin, Tamil Nadu, India; Environ. Geol. 48 158–170.
Bakker M 2010 Radial Dupuit interface flow to assess the aquifer storage and recovery potential of saltwater aquifers; Hydrogeol. J. l18 107–115, https://doi.org/10.1007/s10040-009-0508-1.
Balke K D and Zhu Y 2008 Natural water purification and water management by artificial groundwater recharge; J. Zhejiang Univ. Sci. 9 221–226.
Cassardo C and Jones J A J 2011 Managing water in a changing world; Water 3 618–628.
Central Ground Water Board (CGWB) 2000 ‘Guide on Artificial recharge to Groundwater’ Government of India Ministry of Water Resources’, http://cgwb.gov.in/documents/ArtificialRecharge-Guide.pdf.
Central Ground Water Board (CGWB) 2005 Master plan for artificial recharge to groundwater in India; Ministry of Water Resources Government of India.
Charalambous A N and Garratt P 2009 Recharge–abstraction relationships and sustainable yield in the Arani–Koratalaiyat groundwater basin India; Quart. J. Eng. Geol. Hydrogeol. 42 39–50.
Dillon P, Pavelic P, Page D, Beringen H and Ward J 2009 Managed aquifer recharge: An introduction waterlines; Report No. 13, National Water Commission.
Elango L and Manickam S 1987 Hydrogeochemistry of the Madras aquifer India: Spatial and temporal variation in chemical quality of groundwater; Geol. Soc. Hong Kong Bull. 3 525–534.
Elango L and Ramachandran S 1991 Major ion correlations in groundwater of a coastal aquifer; Indian Water Res. Soc. 1 54–57.
Farrington J, Carney D, Ashley C and Turton C 1999 Sustainable livelihoods in practice: Early application of concepts in rural areas; Nat. Resour. Perspect. 42, London: Overseas Development Institute.
Gain A K, Giupponi C and Renaud F G 2012 Climate change adaptation and vulnerability assessment of water resources systems in developing countries: A generalized framework and a feasibility study in Bangladesh; Water 4 345–366.
Maliva R G, Guo W and Missimer T M 2005 Hydrogeology of aquifer storage and recovery system performance Gulf Coast; Assoc. Geol. Soc. Trans. 55 474–485.
Martin J B, Wicks C M and Sasowsky I D 2002 Hydrogeology and biology of post-Paleozoic carbonate aquifers; Karst Waters Institute Special Publication, Charles Town, 7.
Parimala R S and Elango L 2013 Impact of recharge from a check dam on groundwater quality and assessment of suitability for drinking and irrigation purposes; Arab. J. Geosci. 7 3119–3129.
Raicy M C and Elango L 2017 Percolation pond as a method of managed aquifer recharge in a coastal saline aquifer: A case study on the criteria for site selection and its impacts; J. Earth Syst. Sci. 126 66.
Raicy M C and Elango L 2014 An integrated approach to understand the lake water groundwater interaction in coastal part of Arani–Koratalaiyar River basin, Tamil Nadu, India; Disaster Adv. 7 32–38.
Raicy M C, Parimalarenganayaki S, Schneider M and Elango L 2014 Groundwater responses to managed aquifer recharge structures: Case studies from Chennai, Tamil Nadu, India; In: Natural Water Treatment Systems for Safe and Sustainable Water Supply in the Indian Context (eds) Wintgens T, Nattorp A, Elango L and Asolekar S R, IWA Publishing, 5 99–112.
Rajaveni S P, Indu S Nair and Elango L 2015 Finite element modelling of a heavily exploited coastal aquifer to assess the response of groundwater level to the changes in pumping and rainfall variation due to climate change; Hydrol. Res. 2 111–118.
Rao N J 1979 Studies on the Quaternary formations of the coastal plains flanking Pulicat lake; Geol. Surv. India, Misc. Publ. 45 231–33.
Schroter D, Zebisch M and Grothmann T 2005 Climate change in Germany – vulnerability and adaptation of climate-sensitive sectors Klimastatusbericht Report on the State of the Climate; Deutscher Wetterdienst (German Meteorological Service: Offenbach).
Sener E, Davraz A and Ozcelik M 2005 An integration of GIS and remote sensing in groundwater investigations: A case study in Burdur Turkey; Hydrogeol. J. 13 826–834.
Shahid S, Nath S and Roy J 2000 Groundwater potential modelling in a soft rock area using a GIS; Int. J. Remote Sens. 21 1919–1924.
Thornthwaite C W and Mather J R 1955 The Water Balance’ Publication in Climatology 8 Thornthwaite and Associates, Centerton, New Jersey.
Thornthwaite C W 1948 A new and improved classification of climates; Geogr. Rev. 38 55–94.
Todd D K and Mays L W 2005 Ground-water hydrology (3rd edn); Wiley, New York.
TWAD 1998 Influence of percolation pond in Agrahara Valavanthi micro-watershed; TWAD.
UNDP 1987 Hydrogeological and artificial recharge studies Madras; Technical report UNDP Report number DP/UN/IND-78-029/2.
VIKSAT 2004 Ensuring drinking water security through rainwater harvesting; UNESCO.
Ward J D, Simmons C T and Dillon P J 2007 A theoretical analysis of mixed convection in aquifer storage and recovery: How important are density effects? J. Hydrol. 343 169–186, https://doi.org/10.1016/j.jhydrol.2007.06.011.
Weiss M and Gvirtzman H 2007 Estimating ground water recharge using flow models of perched karstic aquifers; Ground Water 45 761–773.
Werner A D, Bakker M, Post V E A, Vandenbohede A, Lu C, Ataie-Ashtiani B, Simmons C T and Barry D A 2013 Seawater intrusion processes investigation and management: Recent advances and future challenges; Adv. Water Res. 51 3–26.
WHO 2004 Guidelines for drinking-water quality; 3rd edn, Recommendations World Health Organization Geneva 1.
Wilcox L V 1955 Classification and use of irrigation water; USDA Circular, Washington DC, 19p.
Zuurbier K G, Zaadnoordijk W J and Stuyfzand P J 2014 How multiple partially penetrating wells improve the freshwater recovery of coastal aquifer storage and recovery (ASR) systems: A field and modeling study; J. Hydrol. 509 430–441.
Zuurbier K G, Kooiman J W, Groen M M A, Maas B and Stuyfzand P J 2015 Enabling successful aquifer storage and recovery of freshwater using horizontal directional drilled wells in coastal aquifers; J. Hydrol. Eng. https://doi.org/10.1061/(asce)he.1943-5584.0000990.
Zuurbier K G and Stuyfzand P J 2016 Consequences and mitigation of saltwater intrusion induced by short-circuiting during aquifer storage and recovery (ASR) in a coastal subsurface; Hydrol. Earth Syst. Sci., https://doi.org/10.5194/hess-2016-343.
Acknowledgement
Co-funding of this study under the ‘Saph Pani’ project of the European Commission within the Seventh Framework Program (Grant Agreement No. 282911) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Rajib Maity
Rights and permissions
About this article
Cite this article
Raicy, M.C., Elango, L. Percolation pond with recharge shaft as a method of managed aquifer recharge for improving the groundwater quality in the saline coastal aquifer. J Earth Syst Sci 129, 63 (2020). https://doi.org/10.1007/s12040-019-1333-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12040-019-1333-0