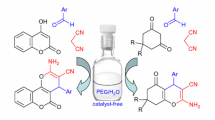
Abstract
A series of 1-carbonyl-1H-indoles were prepared by 2-iodoanilines and calcium carbide in a one-pot reaction catalyzed by recyclable10% Pb/C, resulting in the corresponding substituted indoles in good yields. This protocol offers several advantages, including the utilization of sustainable, low-cost calcium carbide, an easy-to-handle acetylene source, and recyclable Pb/C catalysts.
Graphic abstract
The synthesis of a series of 1-carbonyl-1H-indoles were prepared by 2-iodoanilines and calcium carbide in a one-pot reaction catalyzed by 10% Pb/C, resulting in the corresponding substituted indoles in good yields. This protocol offers several advantages, including the utilization of sustainable, low-cost feedstock, an easy-to-handle acetylene source, and recyclable Pb/C catalysts.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Indole, the most widely employed nitrogen-containing hetero ring in medicinal chemical compounds and natural products (Scheme 1), was initially obtained by the reduction of indigo in 1866 by Adolf von Baeyer. Indole compounds find diverse applications in chemical, material, pesticide, and other fields, particularly in biomedicine, where indole derivatives exhibit structural diversity and serve as crucial sources of bioactive molecules and lead compounds. In order to make drugs active, an abundance of indole ring structures are introduced in drug design, which enriches the development of a wide range of synthetic methods.1 The Fischer indole synthesis is the most common method for indole synthesis. However, this approach needs prefabricated arylhydrazine raw materials and reacts under strong acidic conditions.2,3,4,5,6,7,8 In order to explore the synthesis of key heterocycles for indole development, modern chemical research has shifted its focus to the use of transition metal catalytic methods, which can selectively catalyze the synthesis of the required indole rings, while tolerating a variety of functional groups. Among the transition metal catalysts, palladium is the most well-studied and employed metal for the construction of indole skeletons.9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28
Alkyne compounds are used in the synthesis of indole because of their rich variety and versatile reactivities.29,30,31,32,33 Among the established synthetic methods, Larock indole synthesis12,34,35,36,37 (Scheme 2A) is a common method used in the laboratory, which generates the Larock indole skeletons from 2-haloanilines and internal alkynes catalyzed by palladium catalyst.38,39,40,41,42,43,44,45 Although, this method greatly expands the synthesis of indole structures, however, there is a need of prefunctionalized terminal alkynes. In 2008, Lebel’s team successfully developed a multi-component one-pot synthesis of 2, 3-disubstituted indoles by using cheap, readily available and stable 2-iodobenzoic acid as the starting material (Scheme 2B).46,47 However, this protocol has limitations, with the main drawback being the use of explosive sodium azide as the aminating agent. In 2013, Zhang’s group27 (Scheme 2C) used palladium and copper as co-catalysts to catalyze the reaction of N-arylhydroxamic acids/N-aryl-N-hydroxycarbamates and a variety of alkynes to synthesize indole. In 2018, Hu’s group22 (Scheme 2D) synthesized the indole ring structure, using tert-butyl ((2-iodobenzoyl) oxy) carbamate as the initial raw material coupled with alkynes, and further decarboxylated/cyclized. In 2019, Hoarau’s group28 (Scheme 2E) achieved the synthesis of indoles via the intermolecular cyclization framework from ortho iodoallenamide incorporated with alkynyl carboxylic acids. However, the reactions of Zhang’s group, Hu’s group, and Hoarau’s group could be limited in scope due to the preparation of commercially unavailable substrates and their uneconomical nature. Additionally, an acetylene source is required for these reactions. Acetylene, a gas at atmospheric pressure, which is inflammable, explosive and difficult to handle, was used as the original source for pre-synthesis. In 2022, Sarkar and his team reported (Scheme 2F) gold(I)-catalyzed synthesis of heterocycles via allene oxide from propargylic alcohols. This method offered significant advantages such as being acetylene-free, and the substrate itself having an acetylene unit. However, substrate synthesis still requires the use of carbide, as well as the use of the precious metal gold.28
Calcium carbide is a sustainable, cost-effective, readily available, and safe-to-store solid material with stable non-toxic properties, making it easy to handle in experiments. Historically, it has primarily served as a raw material for producing hydrolyzed acetylene gas in the chemical industry. Over the last decade, there has been an increasing number of reports on the direct utilization of calcium carbide as a substitute for acetylene in various synthetic transformations.48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70 Our research group71,72 made great efforts to investigate the direct application of calcium carbide in organic synthesis, and reported the results of one-pot synthesis of unsymmetrical 1,3-butadiyne derivatives by using calcium carbide instead of acetylene raw materials, and direct synthesis of unsymmetrical 1,3-butadiynes from calcium carbide and aryl iodides. In 2022, Li’s group73 reported an efficient method for the construction of 1-sulfonyl-1H-indoles by the reactions of N-(2-iodoaryl)sulfonamides with calcium carbide. This protocol uses inexpensive and easy-to-handle solid alkyne source instead of flammable and explosive gaseous acetylene, cheap and readily available starting materials. However, this method uses expensive Pd2(dba)3 as the catalyst, and requires prefabricated raw material, which increases the operation procedure.
Palladium on carbon (Pd/C), a heterogeneous catalyst system for the hydrogenation reaction, various kinds of carbon–carbon,74,75,76,77 carbon–nitrogen78,79 bond forming reactions have recently been explored. Among them, 2-substituted indole can be synthesized from monosubstituted alkynes using commercial heterogeneous palladium as a catalyst.80,81,82 Pd/C, as an air-stable, recoverable, recyclable, cheaper than other traditional Pd-complexes and salt, easily separable (from the product), and convenient to store and handle, has drawn our attention to extrapolate the course of our ongoing research work. As an extension of our group's research on calcium carbide as an alternative acetylene source, we try to directly synthesize 1-carbonyl-1H-indoles by using calcium carbide as an acetylene source, recycled Pb/C as the catalyst, 2-iodoaniline, Boc2O as starting materials through a one-pot procedure to try a new method of "upgraded Larock indole synthesis".
2 Experimental
2.1 Materials and methods
All chemicals and solvents were obtained from the commercial providers (Aladdin and Bokachem, China) and used without further purification. Reactions were monitored by Agilent GC Series 6890N and GCMS 7890A. Infra-red spectra were recorded on a Perkin-Elmer Spectrum One FT-IR. Flash column chromatography was performed on silica gel 60 (particle size 200–400 mesh ASTM, purchased from Aladdin, China). Melting points were determined using Fisatom 430D equipment. All compounds were further characterized by 1H, and 13C NMR spectra were measured with a Bruker ACF400 in CDCl3.
2.2 General synthetic procedure
2-iodoaniline (0.5 mmol), N(iPr)2H (1.0 ml) and Boc2O (0.6 mmol) in EtOH (5ml) was gradually added to the three-neck round-bottom reaction flask, then stirred at 25°C for 2 h. The reaction was monitored by GC. After the reaction was complete, without any processing, CaC2 (1.5 mmol), H2O (2.0 mmol), 10% Pd/C (0.05 mmol), (4-MeO-Ph)3P (0.10 mmol), CuI (0.05 mmol) and NMP (3.0 ml) were added to the reaction flask again, and the reaction temperature was increased to reflux. Then 2.0 mmol of water was added again after reflux to maintain the reaction for 4 hours, and the reaction temperature was maintained for 2 hours. After the reaction was complete, the resulting mixture was cooled down to room temperature and added to water. The resulting mixture was filtered to remove the Pd/C (no processing, can be recycled), and the liquor was extracted with ethyl acetate (3×30 ml), and washed with saturated brine (3×30 ml). The resulting organic phase was dried with anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was isolated by flash column chromatography using petroleum ether/ethyl acetate as eluent to give the pure products.
2.3 Activation of palladium–carbon catalyst
First, stir with 5 times the amount (weight/volume) 2 N hydrochloric acid at room temperature for 30 min; stew for 30 min and then filter; filter cake was washed with purified water to neutral (pH = 7); drain and dry at low temperature. Then, reflux with 5 times the amount (weight/volume) ethanol for 2 hours and then dry for the second time.
3 Results and discussion
Initially, we examined the catalytic activity of the different Pd catalysts for the synthesis of N-tert-butoxycarbonyl-indoles starting from 2-iodoaniline (0.5 mmol), Boc2O (0.5 mmol) and CaC2 (1.0 mmol). The same reaction conditions {H2O (4.0 mmol), 10% Pd/C (0.02 mmol), (4-MeO-Ph)3P (0.04 mmol), CuI (0.02 mmol), TEA (1.0 ml), DMF (8.0 ml), 100°C, 5 hours} were applied for all substrates. The results are summarized in Table 1.
The initial attempt using Pd(PPh3)4 as the palladium catalyst and triethylamine as the base yielded the corresponding product N-tert-butoxycarbonyl-indoles (2a) in 25% yield (Table 1, entry 1). Pd2(PPh3)2Cl2 showed high catalytic activities (Table 1, entry 2), gave a mixture of indole (3a) and N-acetyl-2-(1-hexynyl)aniline (4a). Pd(OAc)2 as catalyst affording N-tert-butoxycarbonyl-indoles (2a) in 48% yield (Table 1, entry 3) along with an amount of the competing N-acetyl-2-(1-hexynyl)aniline product (4a). 10% Pd/C with (4-MeO-Ph)3P phosphate ligand played a key catalytic role in the reaction, and the reaction gave 41% yield of product 2a (Table 1, entry 4). The optimum effect was achieved when the ratio of catalyst to ligand was increased to 0.05 to 0.10 (Table 1, entries 4–6). Various bases, such as TEA, N(iPr)2H, DBU, DABCO, KOAc, NaOAc, K2CO3, Na2CO3 and Cs2CO3 were also tested for the reaction (Table 1, entries 6–14). Replacing triethylamine by N(iPr)2H afforded satisfactory results (Table 1, entry 7), while other organic bases and all inorganic bases showed poor reactivity (Table 1, entries 8–14).
A systematic increase in the equivalent amount of Boc2O resulted in an enhanced yield of 2a (Table 2, entries 1–3). Similar yields were obtained with Boc2O amounts ranging from 0.5 to 0.7 equivalents, whereas 0.4 equivalents of Boc2O yielded sluggish results (Table 2, entry 1). In addition, the choice of solvents was also quite important. It was observed that calcium carbide nearly did not participate in the reaction under PhMe, EtOH, and THF because of the poor solubility for calcium carbide (Table 2, entries 8–10). In contrast, DMF, DMA, DMSO, NMP and DMPU (Table 2, entries 2, 4–7) were practicable solvents, and the ratio of mixed solvent NMP to EtOH is 3 to 5 and was proved to be the best choice in this reaction system, which could provide 2a in 83% yield (Table 2, entry 12). Moreover, the amount of calcium carbide and water could also affect the yield of the reaction (Table 2, entries 14–16), and a total of 3 equivalents of calcium carbide and 8 equivalents of water based on 1a is an appropriate amount for the reaction. Any increase or decrease in the amounts of calcium carbide and water led to a drop in yield (Table 2, entry 15). Finally, we also investigated the effects of reaction temperature and reaction time. On rising the temperature to 120°C, the yield actually showed an improvement to 90% (Table 2, entry 17). Lowering of the temperature to 80°C, however, resulted in a drop in yield. The optimal time was determined to be 8h.
Under the optimized reaction conditions, we tried to use various 2-iodoanilines as the reaction substrate to test the universality and scope of application of this methodology, and the research results were summarized in Table 3. In the process of the experiment, it was found that the effect of two-phase reaction and reaction in the same reaction pot after two steps was unexpectedly good, a variety of 2-iodoanilines were used as raw materials to generate a series of corresponding1-carbonyl-1H-indoles products, and the yield was very high. Both electron donating groups or electron withdrawing groups were well tolerated. The R2 could be 2-iodoaniline groups optionally substituted, such as electron donating groups Me (entries 2b–2e), OMe (entries 2f and 2g), or electron withdrawing groups F (entry 2h), Cl (entry 2i), CF3 (entry 2j). The R2 could also be functional groups such as ether (entries 2k, 2l and 2s), cyanide (entries 2n and 2p), ester (entry 2m), ketone (entries 2o and 2q) etc., or heterocyclic group (2r). Finally, we extended the N-substituent group to the acetyl group, obtaining N-acetyl-indoles in an 82% yield.
In addition to the use of readily available CaC2 as an alternative raw material for acetylene, another important finding is that Pd/C catalysts can be reused in this study. The Pd/C catalyst at the end of the reaction was separated by funnel filter and then washed with EtOH, and activated (see the supporting information for details). The results show that the activated catalyst has better reaction activity, shorter reaction time and higher yield than the unactivated catalyst. Although the catalyst can still be used after four cycles, the reaction time should be significantly extended if satisfactory yield is to be obtained.
The experimental results of reuse were summarized in Table 4. While the catalytic activity of the recovered Pd/C catalyst was inferior to that of the fresh catalyst, it still exhibited good activity for the indole ring synthesis reaction. However, it should be noted that the drawback is the longer reaction time compared to that of the fresh Pd/C catalyst. The more times the catalyst was recovered, the longer the reaction time was required to achieve satisfactory catalytic yield.
To explore the possible mechanism, the reaction was initiated with the acylation of 2-iodoaniline, forming the initial compound A. Meanwhile, it was followed by the formation of aryl palladium iodides B by the oxidative addition of A to Pd(0), generated by the reaction of Palladium/carbon with (4-MePh)3P83 (Scheme 3). At the same time, calcium carbide was gradually and slowly hydrolyzed to form calcium acetylene hydroxide C,84 which then reacted with the intermediate B, and the resulting product D underwent reducing to eliminate the loss of palladium (0) and converted to calcium arylacetylene hydroxides E, whose subsequent hydrolysis provided aryl acetylenes F.85,86 Aryl acetylenes reacted with CuI to produce Cu(I) N-tert-butylcarbonyl indole complexes G, which finally produced the target N-tert-butylcarbonyl indole H and released the Cu(I) species.
4 Conclusion
In summary, an effective method was successfully developed to obtain indole and its derivatives by the reaction of the various of 2-iodoanilines with calcium carbonate in a Pd/C catalytic system. This protocol facilitates the easy synthesis of essential indole units under laboratory conditions. The use of recycled Pd/C catalyst, along with the utilization of cheap calcium carbide as a sustainable and economical acetylene gas replacement, contributes to making this a highly attractive reaction.
References
Humphrey G R and Kuethe J T 2006 Practical methodologies for the synthesis of indoles Chem. Rev. 106 2875
Fischer E and Jourdan F 1883 Ueber die hydrazine der brenztraubensäure Ber. Dtsch. Chem. Ges. 16 2241
Fischer E and Hess O 1884 Synthesis of indole-derivatives Ber. Dtsch. Chem. Ges. 17 559
Chen C Y, Senanayake C H, Bill T J, Larsen R D, Verhoeven T R and Reider P J 1994 Improved Fischer indole reaction for the preparation of N, N-Dimethyltryptamines: synthesis of L-695,894, a potent 5-HT1D receptor agonist J. Org. Chem. 59 3738
Haag B A, Zhang Z G, Li J S and Knochel P 2010 Fischer-Indolsynthese mit Organozinkreagentien Angew. Chem. 122 9703
Mller S, Webber M J and List B 2011 The catalytic asymmetric Fischer indolization J. Am. Chem. Soc. 133 18534
Inman M, Carbone A and Moody C J 2012 Two-step route to indoles and analogues from haloarenes: a variation on the Fischer indole synthesis J. Org. Chem. 77 1217
Kuznetsov A, Makarov A, Rubtsov A E, Butin A V and Gevorgyan V 2013 Brönsted acid-catalyzed one-pot synthesis of indoles from o-aminobenzyl alcohols and furans J. Org. Chem. 78 12144
Zeni G and Larock R C 2004 Synthesis of heterocycles via palladium π-olefin and π-alkyne chemistry Chem. Rev. 104 2285
Nakamura I and Yamamoto Y 2004 Transition-metal-catalyzed reactions in heterocyclic synthesis Chem. Rev. 104 2127
Cacchi S and Fabrizi G 2005 Synthesis and functionalization of indoles through palladium-catalyzed reactions Chem. Rev. 105 2873
Zeni G and Larock R C 2006 Synthesis of heterocycles via palladium-catalyzed oxidative addition Chem. Rev. 106 4644
Jia Y and Zhu J 2006 Palladium-catalyzed, modular synthesis of highly functionalized indoles and tryptophans by direct annulation of substituted o-haloanilines and aldehydes J. Org. Chem. 71 7826
McLaughlin M, Palucki M and Davies I W 2006 Efficient access to azaindoles and indoles Org. Lett. 8 3307
Trost B M and McClory A 2007 Rhodium-catalyzed cycloisomerization: formation of indoles, benzofurans, and enol lactones Angew. Chem. Int. Ed. 46 2074
Fang Y Q, Karisch R and Lautens M 2007 Efficient syntheses of KDR kinase inhibitors using a Pd-catalyzed tandem C-N/suzuki coupling as the key step J. Org. Chem. 72 1341
Nagamochi M, Fang Y Q and Lautens M 2007 A general and practical method of alkynyl indole and benzofuran synthesis via tandem Cu-and Pd-catalyzed cross-couplings Org. Lett. 9 2955
Ackermann L and Althammer A 2007 Domino N-H/C-H bond activation: Palladium-catalyzed synthesis of annulated heterocycles using dichloro(hetero)arenes Angew. Chem. Int. Ed. 46 1627
Jensen T, Pedersen H, Bang-Andersen B, Madsen R and Jørgensen M 2008 Palladium-catalyzed aryl amination-heck cyclization cascade: a one-flask approach to 3-substituted indoles Angew. Chem. Int. Ed. 47 888
Hodgkinson R C, Schulz J and Willis M C 2009 Tandem copper-catalysed aryl and alkenyl amination reactions: the synthesis of N-functionalised indoles Org. Biomol. Chem. 7 432
Baxter C A, Cleator E, Alam M, Davies A J, Goodyear A and O’Hagan M 2010 A novel approach to 3-methylindoles by a heck/cyclization/isomerization process Org. Lett. 12 668
Lee W, Jung J W, Sim J, An H C and Suh Y G 2013 Microwave-assisted synthesis of 3-substituted indoles via intramolecular arene–alkene coupling of o-iodoanilino enamines Tetrahedron 69 7211
Gati W, Couty F, Boubaker T, Rammah M M, Rammah M B and Evano G 2013 Intramolecular carbocupration of N-aryl-ynamides: a modular indole synthesis Org. Lett. 15 3122
Hovey M T, Check C T, Sipher A F and Scheidt K A 2014 N-heterocyclic-carbene-catalyzed synthesis of 2-aryl indoles Angew. Chem. Int. Ed. 53 9603
Thirupathi N, Kumar Y K, Kant R and Reddya M S 2014 Selective 5-exo-dig cyclization of in situ synthesized N-Boc-2-aminophenyl ethoxyethynyl carbenols: Synthesis of multifunctional indoles and their derivatives Adv. Synth. Catal. 356 1823
Ma N, Li P, Wang Z, Dai Q P and Hu C W 2018 Synthesis of indoles from aroyloxycarbamates with alkynes via decarboxylation/cyclization Org. Biomol. Chem. 16 2421
Hedouin J, Carpentier V, Renard R M Q, Schneider C, Gillaizeau I and Hoarau C 2019 Regioselective Pd-catalyzed carbopalladation/decarboxylative allylic alkynylation of ortho-iodoallenamides with alkynyl carboxylic acids J. Org. Chem. 84 10535
Bera N, Lenka B S, Bishi S, Samanta S and Sarkar D 2022 Gold (I)-catalyzed synthesis of heterocycles via allene oxide from propargylic alcohols J. Org. Chem. 87 9729
Bandini M 2011 Gold-catalyzed decorations of arenes and heteroarenes with C-C multiple bonds Chem. Soc. Rev. 40 1358
Cho S H, Kim J Y, Kwak J and Chang S 2011 Recent advances in the transition metal-catalyzed twofold oxidative C-H bond activation strategy for C–C and C–N bond formation Chem. Soc. Rev. 40 5068
Yamamoto Y 2014 Synthesis of heterocycles via transition-metal-catalyzed hydroarylation of alkynes Chem. Soc. Rev. 43 1575
Fang G and Bi X 2015 Silver-catalysed reactions of alkynes: recent advances Chem. Soc. Rev. 44 8124
Boyarskiy V P, Ryabukhin D S, Bokach N A and Vasilyev A V 2016 Alkenylation of arenes and heteroarenes with alkynes Chem. Rev. 116 5894
Larock R C, Yum E K and Refvik M D 1991 Synthesis of indoles via palladium-catalyzed heteroannulation of internal alkynes J. Am. Chem. Soc. 113 6689
Larock R C, Yum E K and Refvik M D 1998 Synthesis of 2, 3-disubstituted indoles via palladium-catalyzed annulation of internal alkynes J. Org. Chem. 63 7652
Phetrak N, Rukkijakan T, Sirijaraensre J, Prabpai S, Kongsaeree C and Chuawong P 2013 Regioselectivity of larock heteroannulation: a contribution from electronic properties of diarylacetylenes J. Org. Chem. 78 12703
Pan X and Bannister T D 2014 Sequential Sonagashira and Larock indole synthesis reactions in a general strategy to prepare biologically active β-carboline-containing alkaloids Org. Lett. 16 6124
Sakai N, Annaka K, Fujita A, Sato A and Konakahara T 2008 InBr3-Promoted Divergent Approach to Polysubstituted Indoles and Quinolines from 2-Ethynylanilines: Switch from an Intramolecular Cyclization to an Intermolecular Dimerization by a Type of Terminal Substituent Group J. Org. Chem. 73 4160
Cacchi S and Fabrizi G 2011 Update 1 of: Synthesis and Functionalization of Indoles Through Palladium-Catalyzed Reactions Chem. Rev. 111 PR215
Herrero M T, de Sarralde J D, SanMartin R, Bravo L and Domínguez E 2012 Cesium Carbonate-Promoted Hydroamidation of Alkynes: Enamides, Indoles and the Effect of Iron (III) Chloride Adv. Synth. Catal. 354 3054
Abbiati G, Marinelli F, Rossi E and Arcadi A 2013 Synthesis of Indole Derivatives from 2-Alkynylanilines by Means of Gold Catalysis Isr. J. Chem. 53 856
Huang L, Arndt M, Gooßen K, Heydt H and Gooßen L J 2015 Late transition metal-catalyzed hydroamination and hydroamidation Chem. Rev. 115 2596
Li YL, Li J, Ma A L, Huang Y N and Deng J 2015 Metal-free synthesis of indole via NIS-mediated cascade C-N bond formation/aromatization J. Org. Chem. 80 3841
Mizukami A, Ise Y, Kimachi T and Inamoto K 2016 Rhodium-catalyzed cyclization of 2-ethynylanilines in the presence of isocyanates: approach toward indole-3-carboxamides Org. Lett. 18 748
Watanabe T, Mutoh Y and Saito S 2017 Ruthenium-catalyzed cycloisomerization of 2-alkynylanilides: synthesis of 3-substituted indoles by 1, 2-carbon migration J. Am. Chem. Soc. 139 7749
Dai Q, Li P, Ma N and Hu C 2016 Palladium-catalyzed decarboxylative synthesis of arylamines Org. Lett. 18 5560
Li P, Ma N, Li J, Wang Z, Dai Q and Hu C 2017 Regioselective synthesis of 2-vinylanilines using O-aroyloxycarba-mates by sequential decarboxylation/amination/heck reaction J. Org. Chem. 82 8251
Yang Q, Jiang Y and Kuang C 2012 Facile one-pot synthesis of monosubstituted 1-aryl-1H-1,2,3-triazoles from arylboronic acids and Prop-2-ynoic acid (=propiolic acid) or calcium acetylide (=calcium carbide) as acetylene source Helv. Chim. Acta. 95 448
Lin Z, Yu D, Sum Y N and Zhang Y 2012 Synthesis of functional acetylene derivatives from calcium carbide ChemSusChem 5 625
Sum Y N, Yu D and Zhang Y 2013 Synthesis of acetylenic alcohols with calcium carbide as the acetylene source Green Chem. 15 2718
Thavornsin N, Sukwattanasinitt M and Wacharasindhu S 2014 Direct synthesis of poly (p-phenyleneethynylene) s from calcium carbide Polym. Chem. 5 48
Hosseini A, Seidel D, Miska A and Schreiner P R 2015 Fluoride-assisted activation of calcium carbide: a simple method for the ethynylation of aldehydes and ketones Org. Lett. 17 2808
Kaewchangwat N, Sukato R, Vchirawongkwin V, Vilaivan T, Sukwattanasinitt M and Wacharasindhu S 2015 Direct synthesis of aryl substituted pyrroles from calcium carbide: An underestimated chemical feedstock Green Chem. 17 460
Rodygin K S and Ananikov V P 2016 An efficient metal-free pathway to vinyl thioesters with calcium carbide as the acetylene source Green Chem. 18 482
Matake R, Adachi Y and Matsubara H 2016 Synthesis of vinyl ethers of alcohols using calcium carbide under superbasic catalytic conditions (KOH/DMSO) Green Chem. 18 2614
Teong S P, Yu D, Sum Y N and Zhang Y 2016 Copper catalysed alkynylation of tertiary amines with CaC2 via sp3 C-H activation Green Chem. 18 3499
Teong S P, Chua A Y H, Deng S, Li X and Zhang Y 2017 Direct vinylation of natural alcohols and derivatives with calcium carbide Green Chem. 19 1659
Rodygin K S, Werner I and Ananikov V P 2017 A green and sustainable route to carbohydrate vinyl ethers for accessing bioinspired materials with a unique microspherical morphology ChemSusChem 11 292
Werner G, Rodygin K S, Kostin A A, Gordeev E G, Kashin A S and Ananikov V P 2017 A solid acetylene reagent with enhanced reactivity: fluoride-mediated functionalization of alcohols and phenols Green Chem. 19 3032
Turberg M, ArdilaFierro K J, Bolm C and Hernandez J G 2018 Altering copper-catalyzed A3 couplings by mechanochemistry: one-pot synthesis of 1,4-diamino-2-butynes from aldehydes, amines, and calcium carbide Angew. Chem. Int. Ed. 57 10718
Fu R and Li Z 2018 Direct synthesis of 2-methylbenzofurans from calcium carbide and salicylaldehyde p-tosylhydrazones Org. Lett. 20 2342
Ledovskaya M S, Rodygin K S and Ananikov V P 2018 Calcium-mediated one-pot preparation of isoxazoles with deuterium incorporation Org. Chem. Front. 5 226
Hosseini A and Schreiner P R 2019 Synthesis of exclusively 4-substituted β-lactams through the Kinugasa reaction utilizing calcium carbide Org. Lett. 21 3746
Rodygin K S, Vikenteva Y A and Ananikov V P 2019 Calcium-based sustainable chemical technologies for total carbon recycling ChemSusChem 12 1483
Gao L, Liu Z, Ma X and Li Z 2020 Direct synthesis of propen-2-yl sulfones through cascade reactions using calcium carbide as an alkyne source Org. Lett. 22 5246
Lu H and Li Z 2020 Synthesis of 1,2,3-triazolyl-based ketoximes direct using calcium carbide as an acetylene source Eur. J. Org. Chem. 7 845
Rodygin K S, Ledovskaya M S, Voronin V V, Lotsman K A and Ananikov V P 2021 Calcium carbide: versatile synthetic applications, green methodology and sustainability Eur. J. Org. Chem. 2021 43
Liu Z and Li Z 2021 Synthesis of 1,3-diynes using calcium carbide as an alkyne source Eur. J. Org. Chem. 2021 302
Metlyaeva S A, Rodygin K S, Lotsman K A, Samoylenko D E and Ananikov V P 2021 Biomass- and calcium carbide-based recyclable polymers Green Chem. 23 2487
Fu R, Lu Y, Yue G, Wu D, Xu L, Song H, et al. 2021 Direct synthesis of 3-coumaranones with calcium carbide as an acetylene source Org. Lett. 23 3141
Zhao Z C 2023 One-pot synthesis of unsymmetrical 1,3-butadiyne derivatives B. Korean Chem. Soc. 44 865
Zhao Z C 2023 Direct synthesis of unsymmetrical 1,3-butadiynes from calcium carbide and aryl iodides Russ. J. Org. Chem. 59 1436
Chen W, Li G R, Wen F, Wang Q and Li Z 2022 Concise construction of 1-sulfonyl-1H-indoles using solid calcium carbide as a surrogate of gaseous acetylene Chemistry Select 8 e202203855 (1 of 5)
Felpin F X, Ayad T and Mitra S 2006 Pd/C: an old catalyst for new applications-its use for the Suzuk-Miyaura reaction Eur. J. Org. Chem. 2679
Ajiki H S, Kurita T, Kozaki A, Zhang G, Kitamura Y, Maegawa T J and Hirota K 2004 Ligand-free Suzuki-Miyaura reaction catalysed by Pd/C at room temperature J. Chem. Res. 593
Kitamura Y, Sako S, Udzu T, Tsutui A, Maegawa T, Monguchi Y and Sajiki H 2007 Ligand-free Pd/C-catalyzed Suzuki-Miyaura coupling reaction for the synthesis of heterobiaryl derivatives Chem. Commun. 47 5069
Mori S, Yanase T, Aoyagi S, Monguchi Y and Maegawa Tand Sajiki H 2008 Ligand-free Sonogashira coupling reactions with heterogeneous Pd/C as the catalyst Chem.-Eur. J. 14 6994
Sajiki S, Ikawa T and Hirota K 2004 Reductive and catalytic monoalkylation of primary amines using nitriles as an alkylating reagent Org. Lett. 6 4977
Monguchi Y, Kitamoto K, Ikawa T, Maegawa T and Sajiki H 2008 Evaluation of aromatic amination catalyzed by Palladium on carbon: a practical synthesis of triarylamines Adv. Synth. Catal. 350 2767
Monguchi Y, Mori S, Aoyagi S, Tsutsui A, Maegawaa T and Sajiki H 2010 Palladium on carbon-catalyzed synthesis of 2- and 2,3-substituted indoles under heterogeneous conditions Org. Biomol. Chem. 8 3338
Ahmed A, Ghosh M, Sarkar P and Ray J K 2013 ZnCl2 and Pd/C catalyzed synthesis of 2-substituted indoles Tetrahedron Lett. 54 6691
Prasanna G L, Suresh N, Rao B M V and Pal M 2019 An ultrasound-based approach for the synthesis of indoles under Pd/C catalysis Arab. J. Chem. 12 5370
Havashi T, Kubo A and Ozawa F 1992 Catalytic asymmetric arylation of olefins Pure Appl. Chem. 64 421
Polynski M V, Sapova M D and Ananikov V P 2020 Understanding solubilization of Ca acetylide with a new computational model for ionic pairs Chem. Sci. 11 13102
Hosseini A, Pilevar A, Hogan E, Mogwitz B, Schulze A S and Schreiner P R 2017 Calcium carbide catalytically activated with tetran-butyl ammonium fluoride for Sonogashira cross coupling reactions Org. Biomol. Chem. 15 6800
Lu H and Li Z 2019 Palladium-catalyzed one-pot four-component synthesis of βCyano-α, β-unsaturated ketones using calcium carbide as an acetylene source and potassium hexacyanoferrate(II) as an eco-friendly cyanide source Adv. Synth. Catal. 361 4474
Acknowledgements
The author gratefully acknowledges financial support from Hebei Chemical & Pharmaceutical College.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Z. Recycled Pb/C-catalyzed one-pot synthesis of 1-carbonyl-1H-indoles from 2-iodoanilines and calcium carbide. J Chem Sci 136, 52 (2024). https://doi.org/10.1007/s12039-024-02286-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-024-02286-2